© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Acute flaccid paralysis associated with enterovirus D68 infection: a case report
Wilson YK Chan, FHKAM (Paediatrics)1,2; Stella HY Chim, FHKAM (Paediatrics)2; Donald ML Tse, FHKAM (Radiology)3; PL Ho, FHKAM (Pathology)4
1 Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong
2 Department of Paediatrics and Adolescent Medicine, Queen Mary Hospital, Hong Kong
3 Department of Diagnostic Radiology, Queen Mary Hospital, and St Teresa’s Hospital, Hong Kong
4 Department of Microbiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong
Corresponding author: Dr Wilson YK Chan (wykchan@hku.hk)
Case report
In July 2017, a 28-month-old boy presented to
a private outpatient clinic with a 2-day history
of fever and coryzal symptoms (Table). He had
enjoyed good past health and his family history
was unremarkable. On clinical examination, he was
noted to have respiratory distress and tachycardia.
Plain radiograph of the chest (CXR) showed perihilar
haziness but no consolidation. He was transferred
to Queen Mary Hospital, Hong Kong, to exclude
myocarditis in view of elevated serum troponin in
blood taken in the private clinic. On admission, he
was noted to have diffuse crepitations and wheeze
suggestive of pneumonitis. Nebulised salbutamol,
hypertonic saline, and intravenous cefotaxime
were administered. Complete blood count revealed
neutrophilia, normal liver and renal function, and
normal creatine kinase level. Venous blood gas
showed no acidosis. Troponin was high initially
but then gradually normalised. Echocardiogram
showed no features of myocarditis. Nasopharyngeal
aspirate and throat swab test results were positive
for enterovirus (EV)/rhinovirus ribonucleic acid
(RNA) using reverse transcriptase-polymerase
chain reaction (RT-PCR) detection method. EV71
RNA was not evident. Nasopharyngeal aspirate
for mycoplasma, throat swab and blood culture
were all negative. He then developed progressive
respiratory distress with increased cough and high
fever up to 40°C on day 7 of illness and CXR showed
worsening of bilateral perihilar haziness. In view
of the progressive respiratory failure, the child was
admitted to the paediatric intensive care unit 2 days
later (day 9).
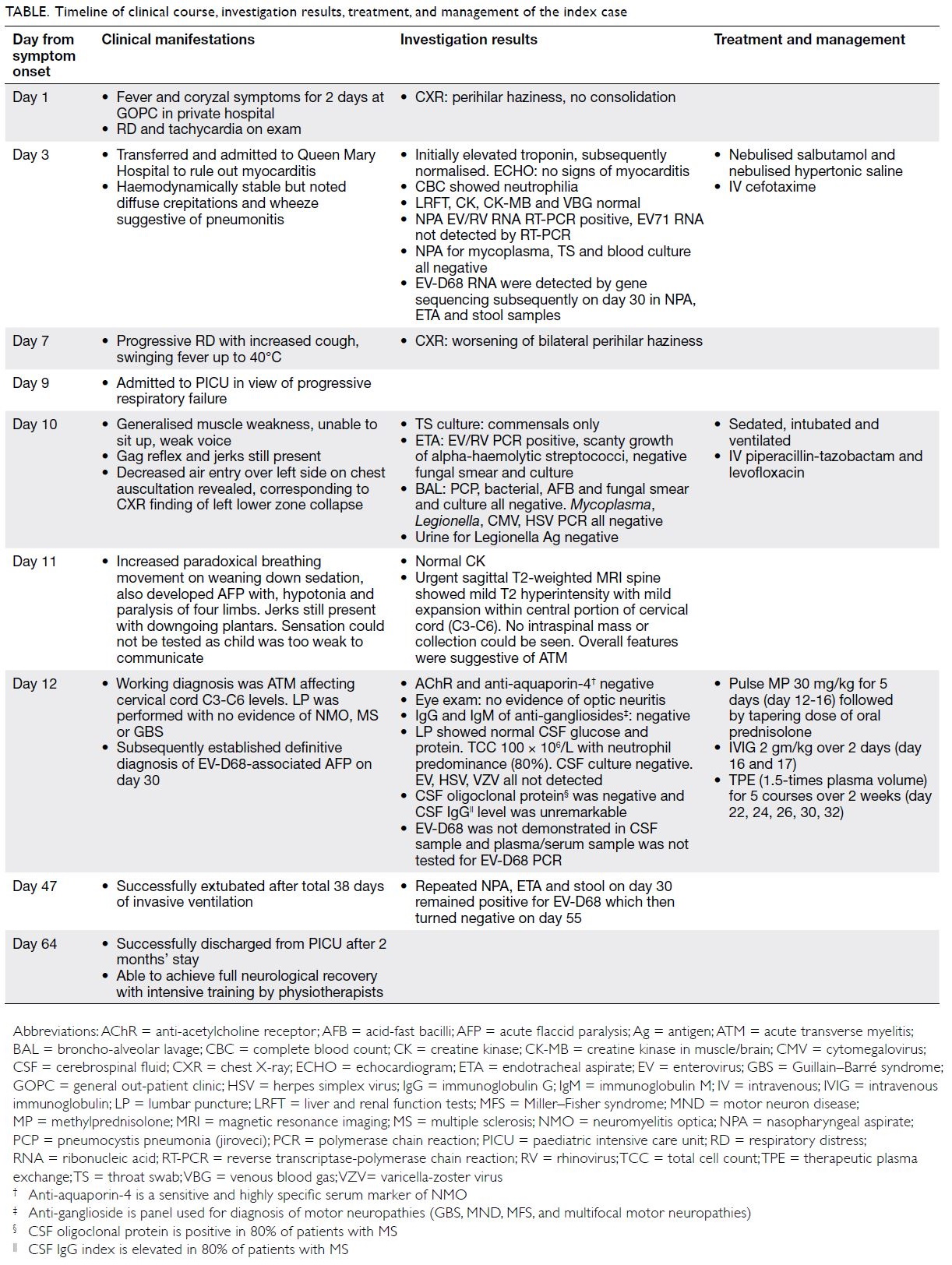
Table. Timeline of clinical course, investigation results, treatment, and management of the index case
On admission to paediatric intensive care unit,
he was noted to have generalised muscle weakness
with a weak voice and an inability to sit up. Gag reflex
and jerks were preserved. He was then intubated and
ventilated under sedation. On reassessment, chest
auscultation revealed decreased air entry over the left
side, corresponding to the left lower zone collapse
evident on CXR. He was prescribed piperacillin-tazobactam and levofloxacin. On day 10, throat swab
culture grew only commensals while endotracheal
aspirate revealed scanty growth of alpha-haemolytic
streptococci with negative fungal smear and culture,
and RT-PCR identified EV/rhinovirus. Broncho-alveolar
lavage was also performed but results were
unremarkable: Pneumocystis jiroveci (carinii), smear
and culture for bacteria, fungus and acid-fast bacilli,
and RT-PCR for cytomegalovirus, herpes simplex
virus, Mycoplasma, Legionella, were all negative.
Urine culture was negative for Legionella antigen.
On day 11, the patient exhibited paradoxical
breathing on weaning of sedation along with
hypotonia and paralysis of four limbs. Urgent sagittal
T2-weighted magnetic resonance imaging of spine
(day 11) showed mild T2 hyperintensity with mild
expansion within the central portion of the cervical
cord from C3 to C6 (Fig). No intraspinal mass or
collection could be seen. Neurology examination
was performed on day 12. Creatine kinase was
normal and anti-acetylcholine receptor and anti-aquaporin-4 were negative. Eye examination the
following day showed no evidence of optic neuritis.
Immunoglobulin G and immunoglobulin M of anti-gangliosides
were all negative. Lumbar puncture
revealed normal cerebrospinal fluid level of glucose
and protein. Total cell count was 100 × 106/L with
predominantly (80%) neutrophils. Cerebrospinal
fluid culture was negative for viruses, including
EV, herpes simplex virus or varicella-zoster virus.
Cerebrospinal fluid levels of oligoclonal protein
and immunoglobulin G were unremarkable. The
working diagnosis was transverse myelitis affecting
cervical cord C3 to C6. He was prescribed pulse
methylprednisolone 30 mg/kg for 5 days (day 12 to
16) followed by a tapering oral dose of prednisolone
together with intravenous immunoglobulin 2 g/kg
over 2 days (day 16 and 17). He was also treated with
therapeutic plasma exchange with 1.5-times plasma
volume for five courses over 2 weeks (day 22, 24, 26,
30, 32). His condition gradually improved and he was
extubated (day 47) after 38 days of invasive ventilation. The child was successfully discharged from the
paediatric intensive care unit after 2 months (day
64) and achieved full neurological recovery with
intensive training by physiotherapists. Subsequent
review by a microbiologist using gene sequencing
of initial specimens obtained on day 3 of admission
to Queen Mary Hospital revealed EV-D68 RNA
in nasopharyngeal aspirate, endotracheal aspirate
and stool samples. Samples remained positive for 4
weeks (day 30, during acute deterioration warranting
intensive care unit admission) and were negative
after 6 weeks (day 55). The definitive diagnosis
was EV-D68-associated acute flaccid paralysis
(AFP) although EV-D68 was not present in the
cerebrospinal fluid and plasma/serum samples were
not tested for EV-D68 by RT-PCR.
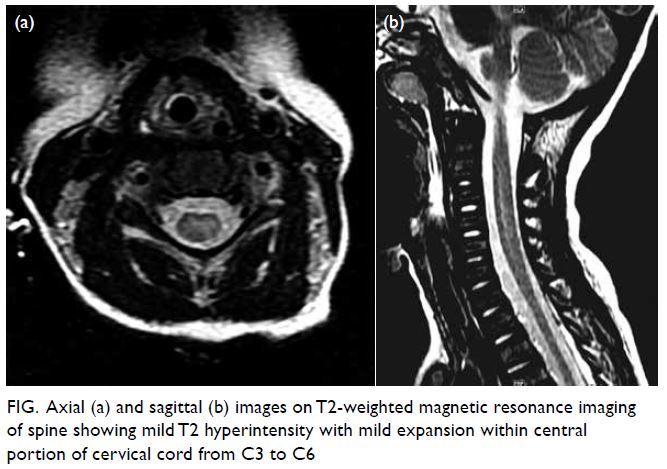
Figure. Axial (a) and sagittal (b) images on T2-weighted magnetic resonance imaging of spine showing mild T2 hyperintensity with mild expansion within central portion of cervical cord from C3 to C6
Discussion
Acute flaccid paralysis is defined by the World
Health Organization as a clinical syndrome of
diverse aetiology characterised by acute-onset
limb weakness or paralysis with varying degrees of
autonomic and somatic nervous system dysfunction
that reaches maximum severity over a period of days
or weeks in a child younger than 15 years of age.1 It
is a diagnosis of exclusion. In 1962, a new strain of
EV, EV-D68, was identified in Berkeley, California.
In 2014, EV-D68 outbreaks were reported in
20 countries including the United States, Canada,
Europe, and Asia with a total of over 2000 cases. This
corresponded to an increased global incidence of
AFP.2 A casual association between EV-D68 and AFP
is supported by Bradford Hill criteria.3 Despite public
health attempts in 1988 to eliminate AFP through the
Global Polio Eradication Initiative4 and roll-out of
the oral polio vaccine5 to prevent vaccine-associated
poliomyelitis, the emergence of EV-D68-associated
AFP has become a significant cause of neurological
deficits in children since 2014. Owing to its impact
on the healthcare system, a comprehensive literature
review and further detailed studies are warranted.
This is the first case encountered in our department.
It is important for clinicians in Hong Kong to be
alert for the disease.
As a newly emerging disease manifestation,
a high index of suspicion and clinical awareness
is advocated to facilitate earlier recognition and
diagnosis through appropriate investigations, and
presumably, improved clinical outcomes. The
optimum treatment strategy has yet to be defined
and preventive strategies are still being developed.
Local and international notification systems as well
as comprehensive surveillance are suggested since
disease outbreaks may occur at any time and may
have a serious impact on affected children.
Author contributions
Acquisition of data: WYK Chan, SHY Chim, DML Tse.
Analysis or interpretation of data: WYK Chan, DML Tse, PL Ho.
Drafting of the manuscript: WYK Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Analysis or interpretation of data: WYK Chan, DML Tse, PL Ho.
Drafting of the manuscript: WYK Chan.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The parents of the patient gave consent for publication.
References
1. Bitnun A, Yeh EA. Acute flaccid paralysis and enteroviral infections. Curr Infect Dis Rep 2018;20:34. Crossref
2. Holm-Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: a systematic review. Lancet
Infect Dis 2016;16:e64-75. Crossref
3. Messacar K, Asturias EJ, Hixon AM, et al. Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for
causality. Lancet Infect Dis 2018;18:e239-47. Crossref
4. World Health Organization. Global Polio Eradication Initiative. 2018. Available from: http://polioeradication.org/where-we-work/polio-endemic-countries/. Accessed 24 Mar 2018.
5. World Health Organization. Global Polio Eradication
Initiative. Circulating vaccine-derived poliovirus. 2018. Available from: http://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/. Accessed 24 Mar 2018.

