Hong Kong Med J 2021 Aug;27(4):312–3 | Epub 24 Jun 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
LETTER TO THE EDITOR
Serological response to mRNA and inactivated
COVID-19 vaccine in healthcare workers in Hong Kong: preliminary results
Jonpaul ST Zee, FRCPath, FHKAM (Medicine)1,2; Kristi TW Lai, MMedSc (HKU)1; Matthew KS Ho, MMedSc (HKU)1; Alex CP Leung, MMedSc (HKU)1; Queenie WL Chan, BScN, FHKAN (Medicine-Infection Control)2; Edmond SK Ma, MD (HK), FRCPath1; KH Lee, MMedSc (HKU), FHKAM (Community Medicine)3; CC Lau, MB, BS, FHKAM (Emergency Medicine)3; Raymond WH Yung, MB, BS, FHKCPath1,2,3
1 Department of Pathology, Hong Kong Sanatorium and Hospital, Hong Kong
2 Infection Control Team, Hong Kong Sanatorium and Hospital, Hong Kong
3 Hospital Administration, Hong Kong Sanatorium and Hospital, Hong Kong
Corresponding author: Dr Jonpaul ST Zee (jonpaul.st.zee@hksh.com)
To the Editor—Healthcare workers (HCWs) in
Hong Kong are among the priority groups to
receive coronavirus disease 2019 (COVID-19)
vaccination. We recruited HCWs who enrolled
for COVID-19 vaccination from 22 February to 30
April 2021 for serial measurement of their anti-spike
immunoglobulin M (IgM)/immunoglobulin G (IgG)/total antibody and surrogate neutralising antibody
using Abbott SARS-CoV-2 IgM/IgG II Quant assay;
Roche Elecsys® Anti-SARS-CoV-2 S, and GenScript
cPass SARS-CoV-2 Surrogate Virus Neutralization
Test Kit. The key exclusion criteria were history of
polymerase chain reaction–confirmed COVID-19 or
positive test for severe acute respiratory syndrome
coronavirus 2–specific IgG or IgM in the serum. The clinical trial protocol was approved by the Research
Ethics Committee of Hong Kong Sanatorium and
Hospital Medical Group (Ref: RC-2021-07).
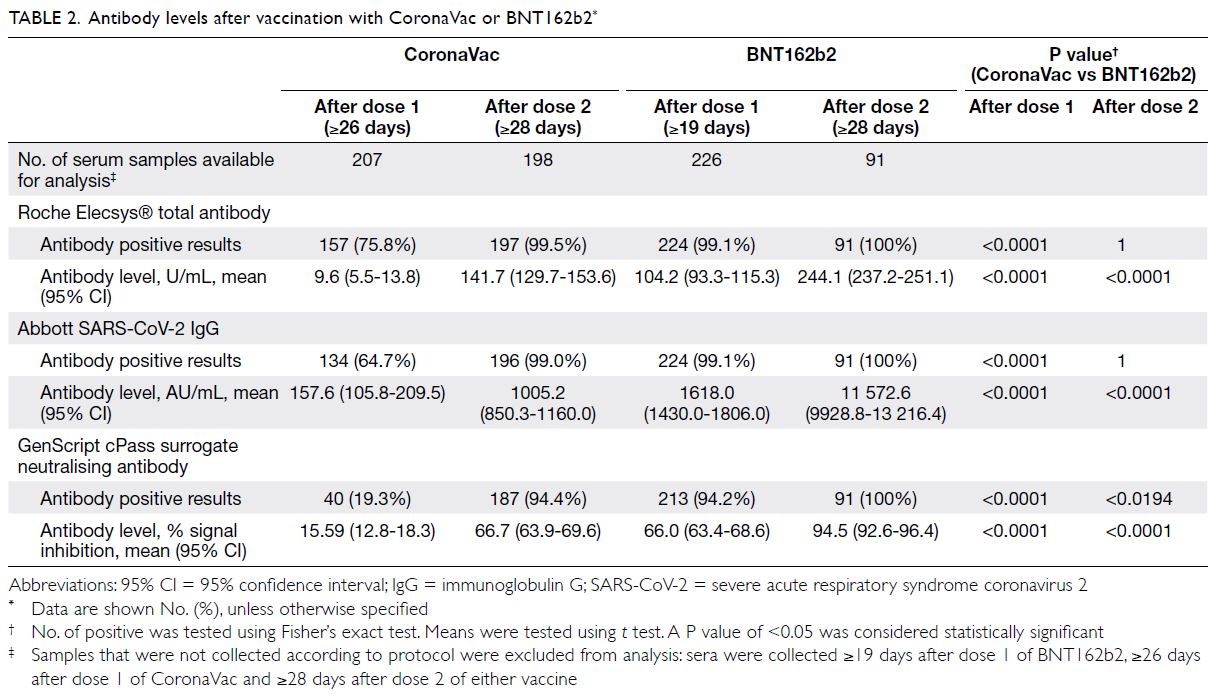
Of the 457 HCWs recruited, 220 (48.1%)
selected an inactivated vaccine (CoronaVac; Sinovac
Life Sciences, Beijing, China) and 237 (51.9%)
selected an mRNA vaccine (BNT162b2/Comirnaty;
Fosun-BioNTech Pharma), based on their personal
preference. The CoronaVac arm was older (mean
age=49.11 vs 44.06 years; P<0.0001) and had a
higher prevalence of having at least one medical co-morbidity
(31.6% vs 22.22%; P=0.0318) [Table 1].
At the time of writing, 210 participants have
received two doses of CoronaVac and 92 have
received two doses of BNT162b2. After dose 1,
more BNT162b2 recipients had positive anti-spike
IgG than did CoronaVac recipients (99.1% vs 64.7%;
P<0.0001). However, the majority developed anti-spike
IgG after dose 2 with no significant difference
between the two arms (100% vs 99%; P=1) [Table 2].
Of 289 samples taken after receiving dose 2, only
two were negative for anti-spike IgG. These two
non-responders were both immunocompromised,
one with psoriatic arthritis receiving methotrexate
treatment, and the other with chronic lymphocytic
leukaemia. The IgG and total antibody levels induced
by BNT162b2 were higher than those induced by
CoronaVac after dose 1 (P<0.0001) and after dose
2 (P<0.0001) [Fig]. After dose 2, more BNT162b2
recipients had positive surrogate neutralising
antibody (100% vs 94%; P<0.0194).
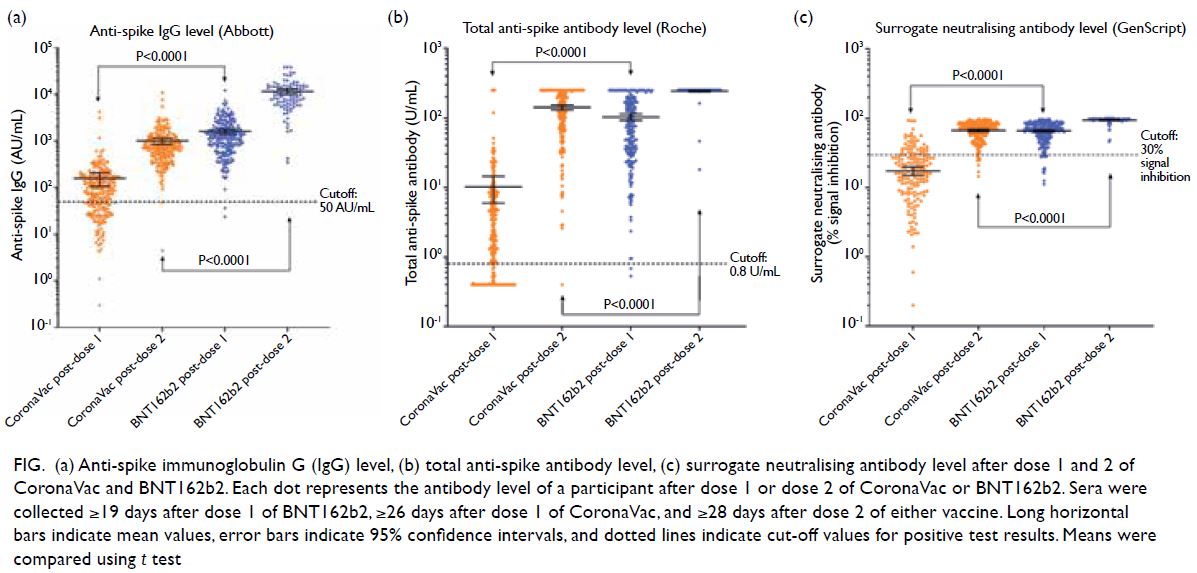
Figure. (a) Anti-spike immunoglobulin G (IgG) level, (b) total anti-spike antibody level, (c) surrogate neutralising antibody level after dose 1 and 2 of CoronaVac and BNT162b2. Each dot represents the antibody level of a participant after dose 1 or dose 2 of CoronaVac or BNT162b2. Sera were collected ≥19 days after dose 1 of BNT162b2, ≥26 days after dose 1 of CoronaVac, and ≥28 days after dose 2 of either vaccine. Long horizontal bars indicate mean values, error bars indicate 95% confidence intervals, and dotted lines indicate cut-off values for positive test results. Means were compared using t test
Both CoronaVac and BNT162b2 are
immunogenic in these HCWs. Our findings
underscore the importance of maintaining social
distancing and other infection control measures
until 4 weeks after completing the two-dose regimen.
Although most vaccine recipients developed
antibodies after the second dose, the level of antibody
or neutralising activity required to confer protection
against future infection is currently not well defined.
More research is needed for a better understanding
of serology after vaccination. Data collection is
ongoing and new findings will be published when
available.
Author contributions
JST Zee drafted the letter. All authors contributed to the
concept or design of the study, acquisition of the data, analysis
or interpretation of the data, and critical revision of the letter
for important intellectual content. All authors had full access
to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.