Hong Kong Med J 2021 Apr;27(2):140–1 | Epub 7 Apr 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Emergency cricothyroidotomy and conversion
tracheostomy in a patient with COVID-19: a case report
YF Lau, MRCSEd; Fergus KC Wong, FRCSEd (ORL); Peter KC Kwan, FRCSEd (ORL)
Department of Ear, Nose and Throat, Pamela Youde Nethersole Eastern Hospital, Hong Kong
Corresponding author: Dr YF Lau (lauyukfai@gmail.com)
Case report
In June 2020, a 56-year-old man with ankylosing
spondylitis, fixed flexion cervical spine deformity,
and restrictive lung disease was admitted to
our hospital with symptoms of pneumonia. A
diagnosis of coronavirus disease 2019 (COVID-19)
was confirmed by positive polymerase chain
reaction test results from throat swab and sputum
samples. The patient was treated with a triple
combination of interferon beta-1b, lopinavir and
ritonavir with clofazimine, in accordance with
local recommendations.1 The patient developed
respiratory failure with upper airway obstruction,
requiring endotracheal intubation and mechanical
ventilation. Intubation attempts failed and the
patient developed oxygen desaturation. Bedside
cricothyroidotomy was performed immediately with
a surgical scalpel. A Portex Blue Line size 6.0 cuffed
tracheostomy tube (Smiths Medical) was inserted.
Full personal protective equipment (PPE), including
N95 respirator, water-proof gown, face shield, and
goggles, was worn by the resuscitation team during
the bedside procedure.
On the same day, after the patient’s oxygen saturation stabilised, conversion tracheostomy was
performed in a negative pressure operating theatre.
Full PPE was worn by the operating team. Because
of the patient’s flexed cervical spine deformity, three
pillows were needed to support the head. A ‘barrier
box’ was set up over the head and neck using two
horizontal anaesthetic screen support bars at the
cephalic and caudal ends of the surgical field. Sterile
clear acrylic (450 mm × 350 mm) was placed on
top of the bars, and four Steri-Drape (3M) plastic
drapes were attached, one on each side (Fig 1). Local
anaesthetic (2% lidocaine with 1:80 000 epinephrine;
Xylestesin-A [3M]) was injected around the incision
site. Electrocautery was avoided and only cold steel
instruments were used for dissection. Haemostasis
was achieved by temporary local application of
1:20 000 adrenaline gauze. Before the trachea
incision, a muscle relaxant was given and ventilation
was stopped. A Portex Blue Line Ultra size 7.5 cuffed
tracheostomy tube (Smiths Medical) was inserted
and the cuff was inflated. Ventilation resumed only
after closed ventilatory circuit was achieved. The
cricothyroidotomy tube was then removed and the
wound was repaired (Fig 2).
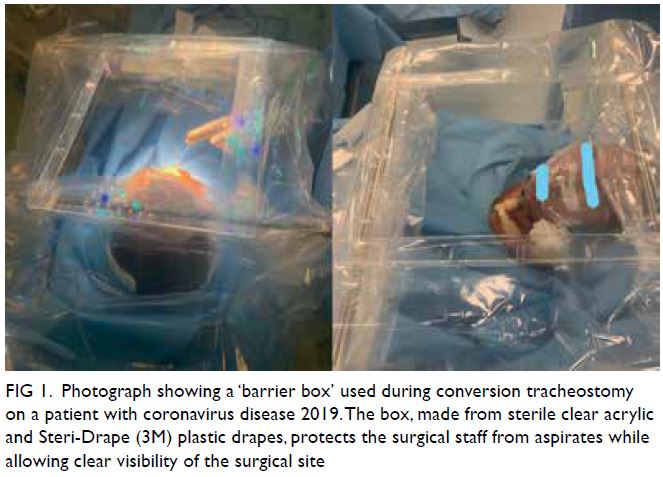
Figure 1. Photograph showing a ‘barrier box’ used during conversion tracheostomy on a patient with coronavirus disease 2019. The box, made from sterile clear acrylic and Steri-Drape (3M) plastic drapes, protects the surgical staff from aspirates while allowing clear visibility of the surgical site
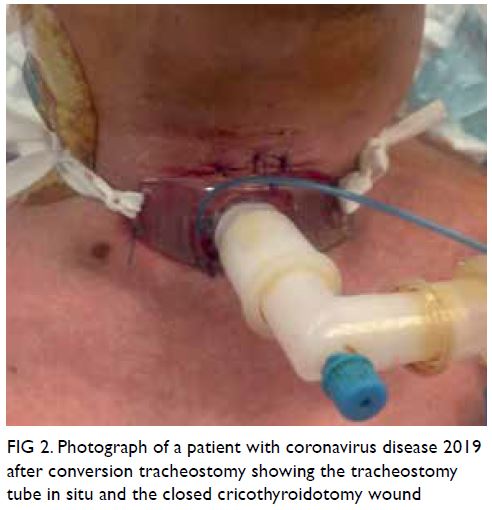
Figure 2. Photograph of a patient with coronavirus disease 2019 after conversion tracheostomy showing the tracheostomy tube in situ and the closed cricothyroidotomy wound
The patient remained stable after the surgical
procedures. There were no surgical complications.
None of the healthcare workers involved in the
cricothyroidotomy or tracheostomy tested positive
for COVID-19 within 14 days after the procedures.
Discussion
Up to 20% of patients with COVID-19 have severe
or critical cases, and a significant proportion
required mechanical ventilation.2 Tracheostomy is
seldom needed as these critically ill patients either
succumb or recover after weeks of mechanical
ventilation, without the need for prolonged
intubation.3 However, some of these patients may
require emergency surgical airway control. Aerosol-generating
procedures, including endotracheal
intubation, cricothyroidotomy, and tracheostomy,
are leading causes of viral transmission and pose a
substantial risk of viral infection to the healthcare
workers despite appropriate PPE.4
In an airway emergency involving a patient
with COVID-19, cricothyroidotomy rather than
tracheostomy should be done at the bedside if the
patient is unfit to be transferred to the operating
theatre. Cricothyroidotomy is a simple and quick
bedside procedure; however, it still poses an infection
risk for healthcare providers. Limiting the number of
healthcare personnel involved in the procedure may
reduce the risk of transmission. Placing a portable
local exhaust ventilation unit next to the patient and
pausing ventilation during the incision, until the
closed ventilation circuit is formed, can lower the risk
further. Scalpel rather than needle cricothyroidotomy
is recommended, because jet ventilation is required
after needle cricothyroidotomy, increasing the risk
of airborne infection. Conversion tracheostomy can
be done more safely after airway control, with staff
and theatre better prepared.
Wei et al5 previously described safety
precautions for performing tracheostomy in patients
with severe acute respiratory syndrome, including
using PPE, performing tracheostomy in a negative-pressure
room, and completely paralysing the patient
during the procedure. We took extra precautions to
further minimise the risk of COVID-19 infection to
medical staff. First, we used a sterile ‘barrier box’
to minimise aerosol exposure. Second, we used a
concentrated local anaesthetic and vasoconstriction
to create a bloodless surgical field, lessening the
need for aerosol-generating electrocautery. Third,
we paused ventilation before trachea incision until the tracheostomy tube was inserted and the
closed ventilatory system is formed, to prevent the
dissemination of the virus.
This case report highlights critical procedural
safety precautions during tracheostomy, including
the use of a ‘barrier box’, avoidance of electrocautery,
and pausing ventilation before incising the trachea,
which can minimise infection risks to healthcare
workers.
Author contributions
Concept or design: All authors.
Acquisition of data: YF Lau.
Analysis or interpretation of data: YF Lau.
Drafting of the manuscript: YF Lau.
Critical revision of the manuscript for important intellectual content: FKC Wong, PKC Kwan.
Acquisition of data: YF Lau.
Analysis or interpretation of data: YF Lau.
Drafting of the manuscript: YF Lau.
Critical revision of the manuscript for important intellectual content: FKC Wong, PKC Kwan.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration
of Helsinki. The patient provided informed consent for all
treatments and procedures, and consent for publication.
References
1. Hung IF, Lung KC, Tso EY, et al. Triple combination of
interferon beta-1b, lopinavir-ritonavir, and ribavirin in the
treatment of patients admitted to hospital with COVID-19:
an open-label, randomised, phase 2 trial. Lancet
2020;395:1695-704. Crossref
2. World Health Organization. Report of the WHO-China
Joint Mission on coronavirus disease 2019 (COVID-19).
28 Feb 2020. Available from: https://www.who.int/docs/
default-source/coronaviruse/who-china-joint-mission-on-covid-19---final-report-1100hr-28feb2020-11mar-update.pdf. Accessed 7 Jul 2020.
3. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes
of critically ill patients with SARS-CoV-2 pneumonia
in Wuhan, China: a single-centered retrospective,
observational study. Lancet Respir Med 2020;8:475-81. Crossref
4. McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy
in the COVID-19 era: global and multidisciplinary
guidance. Lancet Respir Med 2020;8:717-25. Crossref
5. Wei WI, Tuen HH, Ng RW, Lam LK. Safe tracheostomy
for patients with severe acute respiratory syndrome.
Laryngoscope 2003;113:1777-9. Crossref

