Hong Kong Med J 2021 Apr;27(2):118–26 | Epub 15 Apr 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Potential effects of COVID-19 on reproductive
systems and fertility; assisted reproductive technology guidelines and considerations: a review
WY Lee, MB, ChB1; Alex Mok, MB, ChB1; Jacqueline PW Chung, FHKCOG, FHKAM (Obstetrics and Gynaecology)2
1 Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong
2 Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Prof Jacqueline PW Chung (jacquelinechung@cuhk.edu.hk)
Abstract
Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) employs the angiotensin-converting
enzyme 2 (ACE2) receptor in the renin-angiotensin
system for viral entry. The ACE2 receptor is
present in both female and male reproductive
systems, and reports of multi-organ involvement
have led to uncertainty regarding its effects on the
reproductive system and fertility. We review the
existing literature regarding the function of ACE2
and the renin-angiotensin system in the female and
male reproductive systems to postulate the possible
implications of SARS-CoV-2 regarding fertility.
Because of the presence of ACE2 in the ovaries,
SARS-CoV-2 infection may disrupt ovarian function
and hence oocyte quality. Higher expression of ACE2
in the endometrium with age and during the secretory
phase raises concern about increased susceptibility
to infection during periods of high ACE2 expression.
The possibility of vertical transmission and the
presence of ACE2 in the placenta and during
pregnancy are also discussed. The presence of
SARS-CoV-2 RNA in semen is controversial, but
impaired semen quality has been found in men
with moderate coronavirus disease 2019 infection. Evidence of orchitis and hormonal changes seen in
male coronavirus disease 2019 infection may lead
to infertility. The implications of these effects on
assisted reproductive technology (ART) outcomes
are also explored. The ART guidelines from different
fertility societies for the management of patients
treated with ART are provided. The importance
of prioritising ‘time-sensitive’ patients for ART,
counselling patients about the uncertainty and risks
of ART, and pregnancy during the pandemic is
discussed. Recommendations are also provided for
infection control and safe regulation of ART centres
and laboratories.
Introduction
Coronavirus disease 2019 (COVID-19) is a
serious respiratory disease caused by severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2)
infection. The SARS-CoV-2 employs the angiotensin-converting
enzyme 2 (ACE2) receptor in the renin-angiotensin
system (RAS) for viral entry.1 The ACE2
receptor is present in the reproductive system, and
reports of multi-organ involvement have led to
uncertainty regarding COVID-19’s effects on the
reproductive system and fertility.2 We reviewed the
existing literature regarding the function of ACE2
and RAS in the reproductive system. Our aim was to
postulate and understand the effects of SARS-CoV-2
infection on fertility and assisted reproductive
technology (ART) outcomes through RAS, so as
to prompt further quantitative research. We also
discuss guidelines on the management of patients
treated with ART and safe regulation of ART centres/laboratories to improve infection control during the pandemic.
Relationships between severe
acute respiratory syndrome
coronavirus, angiotensin-converting
enzyme 2 receptor, and
renin-angiotensin system
The SARS-CoV-2 virus enters the body via binding
to ACE2 expressed on target host cells.1 The spike
protein of SARS-CoV-2 attaches to this receptor,
similarly to SARS-CoV-1, to facilitate endocytosis
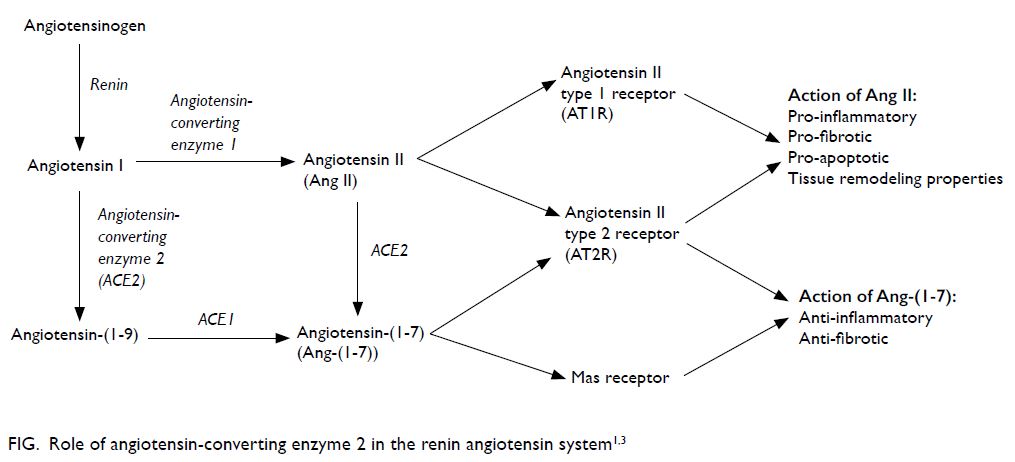
and cellular infection.1 The Figure illustrates the role
of ACE2 in RAS.1 3
The angiotensin II (Ang II) and Ang-(1-7)
hormones have opposing effects. Whereas Ang II is
pro-inflammatory, pro-fibrotic, and pro-apoptotic
with tissue remodelling properties, Ang-(1-7) is anti-inflammatory and anti-fibrotic.3 In other words,
ACE1 and ACE2 counteract each other, and their
roles are essential in balancing RAS.
Infection with SARS-CoV-2 causes reduced
ACE2 activity and downregulation. This increases
circulating Ang II in patients with SARS-CoV-2
infection.1 4 5 This explains the inflammatory and
fibrotic effects seen in COVID-19 lung injury.4 5
There are increasing reports of multi-organ
involvement, with SARS-CoV-2 found in blood,
stools, urine, and saliva.2 3 6 This suggests that
ACE2 in different organ systems may contribute to
the pathophysiology of COVID-19 dissemination
through viraemia.1 2 3 As ACE2 is also present in
the testes and female reproductive system, it is
speculated that the reproductive system may also
be affected by SARS-CoV-2.7 8 We analysed the local RAS and its function in the reproductive system
to postulate how COVID-19 may affect female and
male steroidogenesis, germ cells, and reproductive
health.8 9
Influence of coronavirus disease
2019 on the female reproductive system
Ovarian and follicular development
Although ACE2 is ubiquitous in the female
reproductive system, it is found mostly in the
ovaries.8 9 10 All other components of RAS are also
found in the ovaries, making them potential targets
for damage by SARS-CoV-2.8 9 10
Angiotensin II, found predominantly in
granulosa cells, regulates follicular development,
oocyte maturation, and ovulation.8 9 It is involved
in sex hormone secretion, follicular atresia, and
ovarian and corpus luteum angiogenesis.8 10
Angiotensin-(1-7), presenting in theca-interstitial
cells, is involved in steroidogenesis, oocyte meiosis
resumption, follicular development, atresia, and
enhancing ovulation.8 9 The ACE2, Ang-(1-7), and
Mas-receptor are found in all stages of follicular
development, and studies in rats demonstrated
that their expression was altered by gonadotropin,
implicating the pathway’s potential role in fertility.8 9
Furthermore, Ang-(1-7) levels in follicular fluid
collected during ovarian stimulation for in vitro
fertilisation positively correlate with the proportion
of mature oocytes.11 Combined with animal studies
proving a causal relationship between Ang-(1-7)
and oocyte maturation, the evidence indicates that
Ang-(1-7) may be a human oocyte maturation
factor.11
The downregulation of ACE2 by SARS-CoV-2
may cause alterations in normal ovarian physiology, such as follicular development and oocyte maturation, impacting oocyte quality and fertility.
Oxidative stress is also increased by Ang II as it exerts
pro-inflammatory effects.9 This may be detrimental
to reproductive ability. Further investigations ought
to be done to demonstrate whether increased
Ang II/Ang II type 1 receptor (AT1R) signalling in
SARS-CoV-2 cases affects ovarian physiology and
fertility.
Uterus and fallopian tubes
The RAS is present in the uterus, mostly confined to the
epithelial and stromal cells of the endometrium.8 10 12
Thus, if COVID-19 damages endometrial epithelial
cells, it may affect early embryo implantation.13
Little research has been done to analyse RAS
function in the uterus, but the expression of RAS
components fluctuates during the cycle.10 The
ACE2, Ang-(1-7), and Mas receptor expression
are higher in the secretory than the proliferative
phase, whereas Ang II and AT1R expression are
higher in the proliferative than the secretory
phase.8 10 12 This raises concern about whether the
endometrium is more susceptible to SARS-CoV-2
during the secretory phase. Nevertheless, a study
by Henarejos-Castillo et al14 revealed low overall endometrial susceptibility to SARS-CoV-2. They
also reported a positive correlation between age and
SARS-CoV-2–related gene expression (ACE2),
suggesting increased susceptibility to endometrial
infection in older women.14 15
A normal level of Ang II expression is crucial
to maintaining regular menstrual cycles and
endometrial activity, as it facilitates regeneration
of blood vessels and the endometrium and initiates
menstruation.8 Endometrial and myometrial
activities including endometrial regeneration,
proliferation, fibrosis, and stromal proliferation
are regulated by the intricate balance of Ang II and
Ang-(1-7) in the uterus, ie, stimulated by Ang II and
inhibited by Ang-(1-7).8 Infection of the uterus with
SARS-CoV-2 may severely disrupt such balance.
Disruption of Ang II levels has been found to be
related to dysfunctional uterine bleeding associated
with hyperplastic endometrium.8 Whether this has
any correlation to altered blood flow and increased
risk of miscarriage is unknown and requires further
quantitative research.
In the fallopian tubes, Ang II has been found in
the endothelium and stroma.10 Both AT1R and AT2R
are present in the epithelium.8 10 12 Similarly to Ang II/AT1R expression fluctuation in the uterus, AT1R
expression also changes throughout the cycle (ie,
higher in the proliferative and lower in the secretory
phase).12 The function of Ang II remains unclear, but
one study reported that it stimulates the ciliary beat
frequency in epithelial cells.8 10 12
Placenta and pregnancy
Studies regarding vertical transmission are
controversial, and there is insufficient evidence to
confirm transplacental COVID-19 infection. One
study detected SARS-CoV-2 in the placental and fetal
membranes, but the infants tested negative in the
first 5 days of life.16 Possible contamination sources
include maternal blood, vaginal secretions, and
amniotic fluid.16 Nevertheless, the risk of placental/amniotic sac COVID-19 infection still cannot be
ruled out, warranting further research.
Expression of ACE2 is higher in the placenta
than in the lungs,8 further substantiating the risk of placental SARS-CoV-2 infection. Low placental
ACE2 and Ang-(1-7) have been reported to be
associated with intrauterine growth restriction, an
outcome that has also been seen in pregnant patients
with COVID-19.8 This signifies that placental
COVID-19 infection may have severe implications
for pregnancy outcomes.
Local RAS expression has been identified
in the placenta and cell lines as early as 6 weeks of
gestation, but its function remains ambiguous.10
One study reported possible RAS involvement in
trophoblast invasion and angiogenesis and suggested
that local RAS alteration may contribute to abnormal
uteroplacental perfusion, leading to pre-eclampsia.10
The maternal decidua and pericytes of
endometrial spiral arteries also contain Ang II.
Angiotensin II type 1 receptor is found in maternal
decidua, cytotrophoblasts, syncytiotrophoblasts,
and fetal capillaries,10 and Ang-(1-7) and ACE2 are
localised in syncytiotrophoblasts, cytotrophoblasts,
and the endothelium and vascular smooth muscle
of primary and secondary villi.8 10 Angiotensin-converting
enzyme 2 is also localised in invading
and intravascular trophoblasts and in decidual cells
of maternal stroma.8 In the umbilical cord, ACE2
is localised in smooth muscles and the vascular
endothelium.8 All of these serve as potential
SARS-CoV-2 entry points to the placenta.
In addition, RAS expression fluctuates
throughout pregnancy.10 Whereas AT1R expression
increases during gestation and peaks at the end,
ACE2 peaks early in gestation.8 10 Whether this causes
increased susceptibility to placental SARS-CoV-2
infection during early gestation is unknown. The
expression of ACE2 also differs in location throughout
pregnancy: it appears in the primary and secondary
decidual zones, the luminal zone, and the glandular
epithelium during early gestation and in the labyrinth
placenta and the epithelium of the amniotic and yolk
sac during late gestation.8
Implications on outcomes of assisted
reproductive technology
Whereas COVID-19 has not yet been reported to damage female fertility, its potential detrimental
effects cannot be ignored. If patients who recover
from COVID-19 undergo ART, it is unknown
whether their oocyte quality, quantity, and other
parameters will be affected, nor is the duration of
abnormality. Future research should be conducted
to assess these parameters.
Influence of coronavirus disease
2019 on the male reproductive system
Angiotensin-converting enzyme 2 receptor in
the male reproductive system
Some parts of the testis have been found to contain
ACE2 (the spermatogonia, Leydig cells, and Sertoli
cells), rendering them potential SARS-CoV-2
targets.9 17 The Leydig cells, Sertoli cells, and
seminiferous tubules also contain Ang-(1-7) and
Mas receptor.9 17
Infertile men with severely impaired
spermatogenesis have lower ACE2, Ang-(1-7), and
Mas receptor levels compared with fertile men.9
Men with non-obstructive azoospermia were found
to have absence of Ang-(1-7) and Mas receptor
in the seminiferous tubules.17 As Leydig cells are
responsible for steroidogenesis and secretion,
particularly testosterone, ACE2, Ang-(1-7) and Mas
expression in Leydig cells strongly suggests their
potential roles in the regulation of steroidogenesis
and secretion, spermatogenesis, and hence
their influence on male fertility.9 17 Therefore,
ACE2 downregulation in COVID-19 may impair
spermatogenesis and male fertility. Nevertheless,
ACE2 knockout mice demonstrated no reduction
in fertility, suggesting the possibility of other rescue
mechanisms that may compensate for ACE2 loss.9 17
Angiotensin II in the testes inhibits Leydig cells
and testosterone production and regulates anion and
fluid secretion from the epididymis.18 The increase
of Ang II induced by COVID-19 may hypothetically
affect these functions.
Positive severe acute respiratory syndrome
coronavirus 2 in semen
Presence of SARS-CoV-2 RNA in semen has been
controversial among studies. A cross-sectional
observational study by Pan et al19 was unable to
identify SARS-CoV-2 in semen samples among 34
confirmed cases 1 month after diagnosis. Another
study by Li et al20 revealed six cases that were
positive for SARS-CoV-2 in semen: four during the
acute infection and two during the recovery phase.
This raises concern about sexual transmission
during the acute and particularly the recovery phase
of infection. This may have negative implications on
fertility, assisted reproduction, vertical transmission,
and fetal development. Abstinence and condoms should be used to reduce the potential risk of sexual
transmission until more evidence is available.20
Orchitis in coronavirus infection
Multiple studies have reported a high risk of male
patients with COVID-19 developing orchitis-like
symptoms, suggesting viral orchitis.17 A histological
study of 12 testes of deceased patients with
COVID-19 revealed characteristics of viral orchitis,
lymphocytic infiltration, seminiferous tubular
injury, reduced numbers of Leydig cells, vascular
congestion, and extensive germ cell destruction.21
As COVID-19 is associated with coagulopathy, the
orchitis could have resulted from vasculitis.22
The possibility of orchitis leading to infertility
as a complication of infection with coronaviruses
such as SARS-CoV-1 is widely accepted.23 24 Similar
to the case in COVID-19, pathology revealed
focal testicular atrophy, germ cell destruction
with decreased number of spermatozoa, and
inflammatory cell infiltrates.17 23 24 Interestingly,
SARS-CoV-1 was not identified in the testis; instead,
high immunoglobulin G precipitation was detected
in the seminiferous epithelium, suggesting that
an immune-mediated response was causing the
testicular damage, rather than direct testicular
infection.17 23 24 Male patients with COVID-19 and
high immunoglobulin G titre might also have adverse
reproductive effects, possibly caused by anti-sperm
antibodies such as antiphospholipid antibodies,
which interfere with fertilisation.25
Inflammatory infiltration may disrupt
spermatogenesis, impede steroidogenesis, and
destroy cells in seminiferous tubules.26 Moreover,
SAR-CoV-2 induces oxidative stress via inflammatory
responses, which might disrupt the process of
spermiogenesis and lead to spermatozoa having
poorly remodelled chromatin.27 Cytokine release
activates a secondary autoimmune response and
production of antibodies within the seminiferous
tubules, leading to autoimmune orchitis and the
presence of antibodies in semen.25 26 The cytokine
response may also suppress the hypothalamic-pituitary-gonadal axis, leading to reduction of
testosterone and sperm production.25 This is
consistent with studies that have revealed reduced
serum testosterone in patients with COVID-19.25
Semen analysis and follow-up of patients with orchitis
during COVID-19 infection should be conducted to
evaluate their reproductive functioning.
Hormonal changes in patients with
coronavirus disease 2019: signs of
hypogonadism
Multiple studies have revealed significant increases
in serum luteinising hormone (LH) and prolactin
levels among male patients with COVID-19.28 29 A
significant decrease in testosterone to LH ratio and follicle-stimulating hormone to LH ratio were also
reported.29 It is postulated that the LH increase
in COVID-19 resulted from the early stage of
impaired testosterone production and was caused
by reduction of Leydig cells. This could have caused
negative feedback that stimulated Leydig cells to
temporarily increase testosterone production.28
There may be a risk of clinical hypogonadism as
the disease progresses.28 It is therefore important to
perform follow-up with post-recovery patients for at
least 3 to 6 months, with serum LH and testosterone-to-LH ratio serving as clinical indicators of primary
hypogonadism.29
Implications on the outcomes of assisted
reproductive technology
Infection and viral-mediated immune response
to SARS-CoV-2 may disrupt steroidogenesis
and spermatogenesis and destroy cells of the
seminiferous tubules.17 26 A systematic review on
semen analysis by Khalili et al30 revealed significantly
impaired semen quality in patients with moderate
active COVID-19 infection compared with mild
active infection and a control group. Semen samples
of patients with moderate SARS-CoV-2 infection
were shown to have significantly lower sperm
concentration (P<0.05), lower total number of sperm
per ejaculation, lower total number of motile sperm,
and lower total number of progressively motile
sperm than normal patients.30 In combination with
the risk of sexual transmission, the consideration
of deferring conception in recovered patients until
more evidence is available should be taken seriously.
Sperm donation/cryopreservation of active/recovered COVID-19 patients should be avoided, as many viruses remain viable and infectious when
cryopreserved.31
Assisted reproductive technology
recommendations for patients
with coronavirus disease 2019
and the general public during the
pandemic
Coronavirus disease 2019 infection and
possible outcomes of assisted reproductive
technology
In consideration of the lack of legitimate evidence
and the fact that the available data are mostly
derived from studies with small sample sizes,
the risk of serious implications of COVID-19 on
fertility cannot be ruled out. Furthermore, fever
is common in SARS-CoV-2 infection. In female
patients who are undergoing ovarian stimulation for
in vitro fertilisation, fever negatively affects follicular
development and ovarian oestradiol production.32
In male patients, fever transiently impairs spermatogenesis and sperm parameters (count,
motility, and DNA integrity) for 50 to 70 days.17 33
Male patients with SAR-CoV-2 may also develop
cytokine storm syndrome, which may disrupt
testicular function.21 Therefore, patients treated with
ART, gamete donors, and gestation carriers with
acute/recovered SARS-CoV-2 infection should avoid
participation in any fertility programmes until more
research is conducted.34
Guidelines on assisted reproductive
technology procedures
Infertility is a time-sensitive disease: the longer it
is left untreated, the lower the patient’s chances of
becoming a biological parent. Previously, fertility
societies recommended cessation of all reproductive
care except urgent cases.35 However, as countries
around the world begin to successfully mitigate
the spread of COVID-19, a new joint statement
was released on 29 May 2020 by the American
Society for Reproductive Medicine, the European
Society of Human Reproduction and Embryology
(ESHRE), and the International Federation of
Fertility Societies. The statement sanctioned gradual
resumption of full reproductive care in areas where
COVID-19 has been well controlled.35 Recognition
of the importance of fertility care provides relief for
infertile patients whose reproductive time is running
out.
Risk assessment should take place within
ART centres to access practices before restarting
services.36 Staff should closely monitor the local
COVID-19 situation for updated epidemiological
data and changes to governmental regulations.36 The
ESHRE/local triage questionnaires, and if feasible,
COVID-19 testing should be done in all patients
and partners before starting ART.37 In Hong Kong,
testing is also performed before ovarian retrieval
and embryo transfer, as test results are only valid for
72 hours under local guidelines.
Specific protocols should be enforced regarding
screening and management of patients treated
with ART during the pandemic.38 Table 1 provides
such guidelines by different fertility societies.38 39 40 41 42 43
The ESHRE guideline is used as a reference point.
Other societies’ recommendations that are the
same as those of ESHRE are omitted, ie, only extra
information is added for other societies. Because of
the ever-changing nature of this pandemic and the
variability of cases between countries, there may
be future changes to ART regulations. The most
updated country-specific regulations should be
followed.
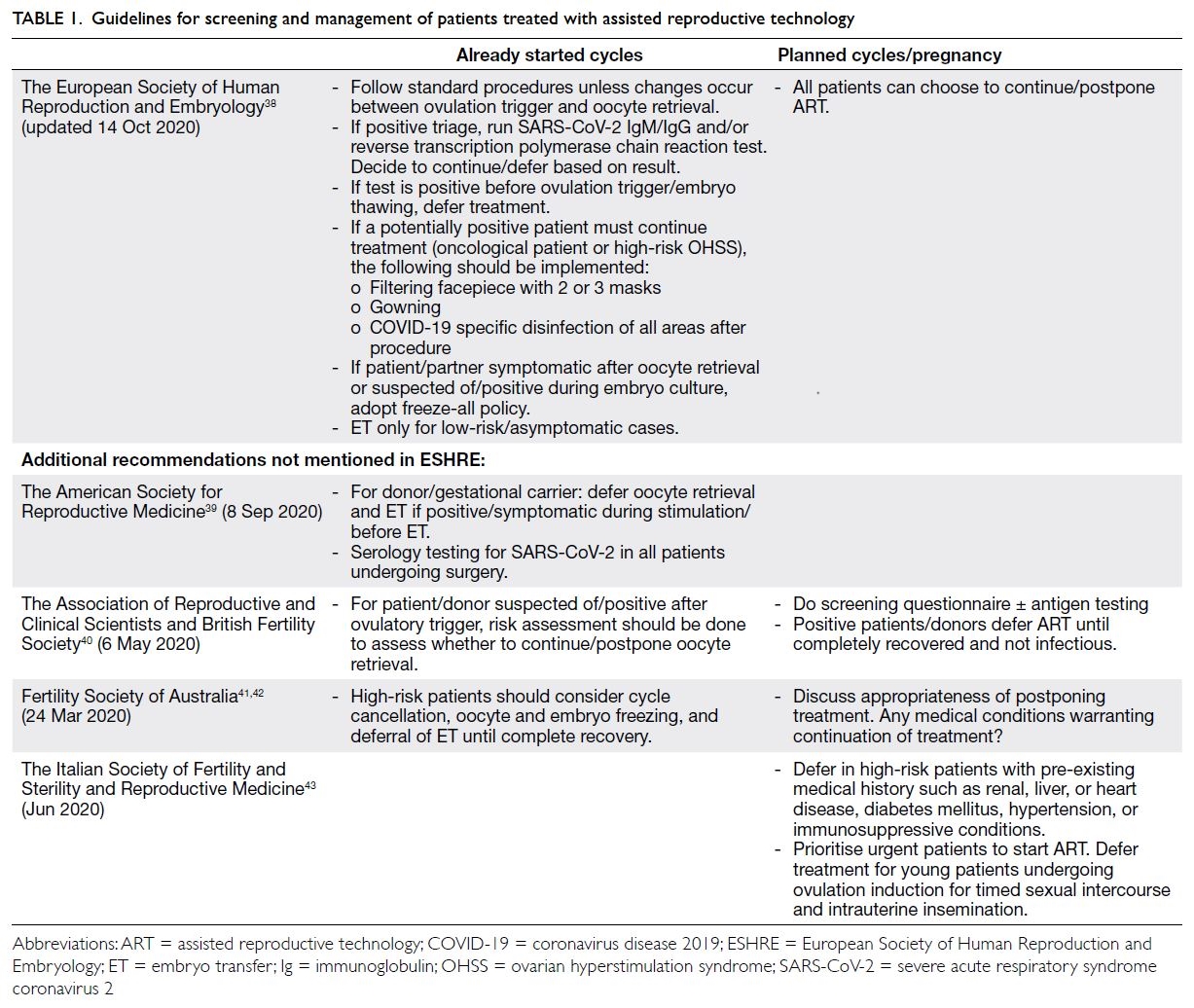
Table 1. Guidelines for screening and management of patients treated with assisted reproductive technology
There is an increased risk of lung and kidney
complications if patients with COVID-19 develop
ovarian hyperstimulation syndrome during ovarian
stimulation.44 An individualised approach should
be adopted. Anti-Müllerian hormone and antral follicle count should be used to assess ovarian
reserve and guide the dosage of gonadotrophins.
The gonadotrophin-releasing hormone (GnRH)
antagonist protocol (with GnRH agonist triggering
oocyte maturation and elective cryopreservation of
embryos) is extremely effective at minimising the risk
of ovarian hyperstimulation syndrome.43 Moreover,
the risk of coagulopathy in COVID-19 may augment
the risk of thromboembolic complications during
ovarian stimulation.45 Other than using GnRH
agonist in high responders/patients with COVID-19,
suggested solutions to reduce thromboembolic risk
include segmenting the in vitro fertilisation cycle
and administering prophylactic low-molecular-weight
heparin.45
Infection control in assisted reproductive
technology centres and laboratories
Table 2 lists recommendations for infection control
in ART centres and laboratories to help reduce the
spread of COVID-19.36 38 43 44 46 47 48 49
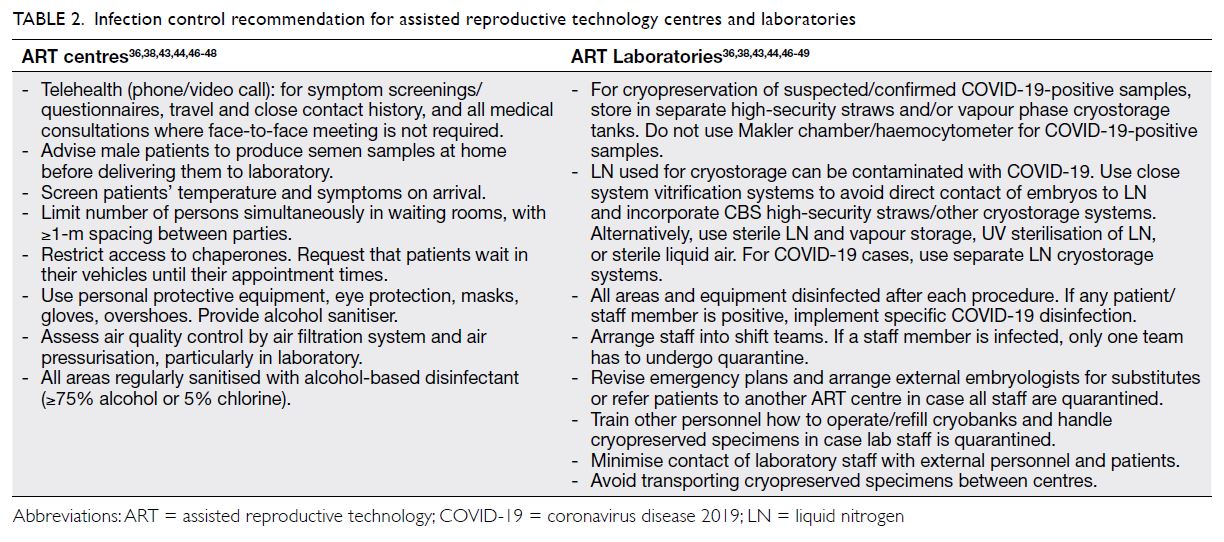
Table 2. Infection control recommendation for assisted reproductive technology centres and laboratories
As SARS-CoV-2 can be present in semen,
strict protective protocols should be implemented
in specimen handling to avoid spillage/exposure.50
If the operator becomes infected, cryopreserved
semen samples handled by the operator should be
tested via polymerase chain reaction.48 The viral
titre of COVID-19-positive semen should be kept
at the lowest possible level.51 For gametes/embryos,
repeated washing should be done to dilute out any
viral contaminants.51
Identification of ‘time-sensitive’ patients for
assisted reproductive technology
With the gradual resumption of reproductive
services, it is crucial to identify and prioritise
patients who have a low prognosis of ART success
and whose fertility potential deteriorates rapidly.43 47
Stratifying patients according to Poseidon groups,
patients in Poseidon groups 2 and 4 (advanced
maternal age with normal/reduced ovarian reserve)
should be prioritised, followed by group 3 (age
<35 years but with reduced ovarian reserve).47 In
male patients who undergo medical treatment to
improve sperm quality and quantity, their ‘fertility
window’ is short and transient. Sperm analysis
and banking should be done as soon as possible to
increase their prospects of biological parenthood.
Regarding fertility preservation, patients with
cancer and inflammatory and autoimmune diseases
should be given priority, as their treatments are
gonadotoxic.43 47 48 Fertility preservation can only
be done during the ‘remission window’, which is
achieved after temporary discontinuation of therapy
for 3 to 4 months.47 48 If those patients’ remission
window coincided with the pandemic, they would
have to either forego this ART opportunity and start
gonadotoxic drugs again, meaning reduced ART
success in future attempts as they age, or go without
drugs for an extended period of time in the hopes
of resuming fertility care. This would cause them to
bear the risk of their medical conditions flaring up.48
Furthermore, the COVID-19 pandemic should be a
novel indication for fertility preservation, especially
in Poseidon groups 2 and 4.52
Considerations for members of the general
public who wish to undergo in vitro
fertilisation
Because of the lack of data and knowledge about
SARS-CoV-2, it is imperative to discuss the
uncertainties of COVID-19’s effects on fertility and
ART with patients. Well-documented informed
consent should be signed before commencing ART
treatment. Patients should understand all the risks
involved, including the risk of exposure at the ART
clinic during treatment. In addition, it is important
to counsel patients about the available options, from
postponing to resuming treatment. Balancing should
be done between the risks of deferring treatment
in patients with low ART prognosis and those of
undergoing treatment on fertility and pregnancy.47
The unknown effects of COVID-19 on
pregnancy outcomes must also be discussed.
Although there is no clear evidence of vertical
transmission, it still cannot be ruled out.2 16 43 46 53 54
Immunosuppression and hormonal fluctuation
during pregnancy leave women more vulnerable
to respiratory pathogens and severe pneumonia.46
Nevertheless, a study revealed no higher
susceptibility to COVID-19 in pregnant women than
non-pregnant women.36 Further, pregnant women
with COVID-19 do not have more severe symptoms
than non-pregnant women.46 53 55
Despite the unclear pathogenesis of COVID-19
in pregnancy, it is associated with more maternal
and fetal complications.46 These include preterm
birth (most common), fetal distress, intrauterine
growth restriction, and increase in Caesarean sections.46 54 56 57 Miscarriages and neonatal and
maternal deaths have been reported, but no
evidence has suggested that they are caused directly
by COVID-19.46 54 56 57 Pre-existing co-morbidity
in pregnant women with COVID-19 is associated
with increased severity, higher intensive care unit
admission, invasive ventilation, and neonatal unit
admission of their newborns.58 These data are from
women infected during the third trimester, and
COVID-19’s effects during the first trimester are
unknown.46 53 59
Patients with infertility face a high amount of
stress, from fear of ART failure to uncertainty about
the pandemic.43 Clinical and psychological support
should be provided to advocate for patients’ well-being
and to reduce treatment dropout.
Conclusion
Coronavirus disease 2019 has affected every
part of the world, and it is likely to persist in the
coming years. The potential risk of SARS-CoV-2
infection in the reproductive system and its effects
on reproductive parameters and fertility cannot be
ignored and warrant further quantitative research.
Shared decisions between doctors and patients
should be made regarding fertility care. Patients’
autonomy allows them to decide whether to resume
or postpone treatment, but it is their doctors’
responsibility to counsel them on all the risks and
benefits involved. Individualisation of patients’ ART
treatment is the key to safe practice during this
ongoing pandemic.
Author contributions
Concept or design: All authors.
Acquisition of data: WY Lee, A Mok.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: JPW Chung.
Acquisition of data: WY Lee, A Mok.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: JPW Chung.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, JPW Chung was not involved in the peer review process. Other authors have disclosed no
conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Sriram K, Insel PA. A hypothesis for pathobiology and
treatment of COVID-19: The centrality of ACE1/ACE2
imbalance. Br J Pharmacol 2020;177:4825-4. Crossref
2. Li Y, Zhou W, Yang L, You R. Physiological and pathological
regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol
Res 2020;157:104833. Crossref
3. Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting
enzyme 2 (ACE2), SARS-CoV-2 and the
pathophysiology of coronavirus disease 2019 (COVID-19).
J Pathol 2020;251:228-48. Crossref
4. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The
pivotal link between ACE2 deficiency and SARS-CoV-2
infection. Eur J Intern Med 2020;76:14-20. Crossref
5. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin
converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med 2005;11:875-9. Crossref
6. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020;323:1843-4. Crossref
7. Fu J, Zhou B, Zhang L, et al. Expressions and significances
of the angiotensin-converting enzyme 2 gene, the receptor
of SARS-CoV-2 for COVID-19. Mol Biol Rep 2020;47:4383-92. Crossref
8. Yan J, Li RQ, Wang HR, et al. Potential influence of
COVID-19/ACE2 on the female reproductive system. Mol
Hum Reprod 2020;26:367-73. Crossref
9. Pan PP, Zhan QT, Le F, Zheng YM, Jin F. Angiotensinconverting enzymes play a dominant role in fertility. Int J Mol Sci 2013;14:21071-86. Crossref
10. Herr D, Bekes I, Wulff C. Local renin-angiotensin system in the reproductive system. Front Endocrinol (Lausanne)
2013;4:150. Crossref
11. Cavallo IK, Dela Cruz C, Oliveira ML, et al.
Angiotensin-(1-7) in human follicular fluid correlates with
oocyte maturation. Hum Reprod 2017;32:1318-24. Crossref
12. Vinson GP, Saridogan E, Puddefoot JR, Djahanbakhch O. Tissue renin-angiotensin systems and reproduction. Hum
Reprod 1997;12:651-62. Crossref
13. Li R, Yin T, Fang F, et al. Potential risks of SARS-CoV-2
infection on reproductive health. Reprod Biomed Online
2020;41:89-95. Crossref
14. Henarejos-Castillo I, Sebastian-Leon P, Devesa-Peiro A,
Pellicer A, Diaz-Gimeno P. SARS-CoV-2 infection risk
assessment in the endometrium: viral infection-related
gene expression across the menstrual cycle. Fertil Steril
2020;114:223-32. Crossref
15. Abhari S, Kawwass JF. Endometrial susceptibility to SARS
CoV-2: explained by gene expression across the menstrual
cycle? Fertil Steril 2020;114:255-6. Crossref
16. Penfield CA, Brubaker SG, Limaye MA, et al. Detection of
severe acute respiratory syndrome coronavirus in placental
and fetal membrane samples. Am J Obstet Gynecol MFM
2020;2:100133. Crossref
17. Younis JS, Abassi Z, Skorecki K. Is there an impact
of the COVID-19 pandemic on male fertility? The
ACE2 connection. Am J Physiol Endocrinol Metab
2020;318:E878-80. Crossref
18. Leung PS, Sernia C. The renin-angiotensin system and male reproduction: new functions for old hormones. J Mol
Endocrinol 2003;30:263-70. Crossref
19. Pan F, Xiao X, Guo J, et al. No evidence of severe acute
respiratory syndrome-coronavirus 2 in semen of males
recovering from coronavirus disease 2019. Fertil Steril
2020;113:1135-9. Crossref
20. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 2020;3:e208292. Crossref
21. Singh B, Gornet M, Sims H, Kisanga E, Knight Z, Segars J.
Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) and its effect on gametogenesis and early
pregnancy. Am J Reprod Immunol 2020;84:e13351. Crossref
22. Corona G, Baldi E, Isidori AM, et al. SARS-CoV-2
infection, male fertility and sperm cryopreservation: a
position statement of the Italian Society of Andrology and
Sexual Medicine (SIAMS) (Società Italiana di Andrologia
e Medicina della Sessualità). J Endocrinol Invest
2020;43:1153-7. Crossref
23. Segars J, Katler Q, McQueen DB, et al. Prior and novel
coronaviruses, Coronavirus Disease 2019 (COVID-19),
and human reproduction: what is known? Fertil Steril
2020;113:1140-9. Crossref
24. Xu J, Qi L, Chi X, et al. Orchitis: a complication of
severe acute respiratory syndrome (SARS). Biol Reprod
2006;74:410-6. Crossref
25. Huang C, Ji X, Zhou W, et al. Coronavirus: a possible cause
of reduced male fertility. Andrology 2021;9:80-7. Crossref
26. Paoli D, Pallotti F, Colangelo S, et al. Study of SARS-CoV-2
in semen and urine samples of a volunteer with positive
naso-pharyngeal swab. J Endocrinol Invest 2020;43:1819-22. Crossref
27. Sengupta P, Dutta S. Does SARS-CoV-2 infection cause
sperm DNA fragmentation? Possible link with oxidative
stress. Eur J Contracept Reprod Health Care 2020;25:405-6. Crossref
28. Illiano E, Trama F, Costantini E. Could COVID-19 have an impact on male fertility? Andrologia 2020;52:e13654. Crossref
29. Verma S, Saksena S, Sadri-Ardekani H. ACE2 receptor
expression in testes: implications in coronavirus disease
2019 pathogenesis. Biol Reprod 2020;103:449-51. Crossref
30. Khalili MA, Leisegang K, Majzoub A, et al. Male fertility
and the COVID-19 pandemic: systematic review of the
literature. World J Mens Health 2020;38:506-20. Crossref
31. Yakass MB, Woodward B. COVID-19: should we continue to cryopreserve sperm during the pandemic? Reprod
Biomed Online 2020;40:905. Crossref
32. Awwad J, Ghazeeri G, Toth T, Hannoun A, Abdallah MA, Farra C. Fever in women may interfere with follicular
development during controlled ovarian stimulation. Int J
Hyperthermia 2012;28:742-6. Crossref
33. Sergerie M, Mieusset R, Croute F, Daudin M, Bujan L. High
risk of temporary alteration of semen parameters after
recent acute febrile illness. Fertil Steril 2007;88:970.e1-7. Crossref
34. Cardona Maya WD, Du Plessis SS, Velilla PA. SARS-CoV-2
and the testis: similarity with other viruses and routes of
infection. Reprod Biomed Online 2020;40:763-4. Crossref
35. Veiga A, Gianaroli L, Ory S, Horton M, Feinberg E,
Penzias A. Assisted reproduction and COVID-19: a joint
statement of ASRM, ESHRE and IFFS. Hum Reprod Open
2020;2020:hoaa033. Crossref
36. American Society for Reproductive Medicine. Patient
management and clinical recommendations during the
coronavirus (COVID-19) pandemic. Update #3 (April
24, 2020 through May 11, 2020). Available from: https://www.asrm.org/globalassets/asrm/asrm-content/news-and-publications/covid-19/covidtaskforceupdate3.pdf. Accessed 11 May 2020.
37. European Society of Human Reproduction and
Embryology. Safe ART services during the third phase of
the COVID-19 pandemic. Available from: https://www.eshre.eu/Home/COVID19WG. Accessed 14 Oct 2020.
38. European Society of Human Reproduction and
Embryology. ESHRE guidance on recommencing ART
treatments. Available from: https://www.eshre.eu/-/media/sitecore-files/Guidelines/COVID19/ESHRE-Guidance-on-Recommencing-ART-treatments_update-04052020.pdf. Accessed 3 Dec 2020.
39. American Society for Reproductive Medicine. Patient
management and clinical recommendations during the
Coronavirus (COVID-19) pandemic. Update #4 (May
11, 2020 through June 8, 2020). Available from: https://www.asrm.org/globalassets/asrm/asrm-content/news-and-publications/covid-19/covidtaskforceupdate4.pdf. Accessed 3 Dec 2020.
40. The Association of Reproductive and Clinical
Scientists, British Fertility Society. Best practice
guidelines for reintroduction of routine fertility
treatments during the COVID-19 pandemic. Available
from: https://www.britishfertilitysociety.org.uk/wp-content/uploads/2020/05/ARCS-BFS-COVID-19-guideline-v1.1-1.pdf. Accessed 3 Dec 2020.
41. The Fertility Society of Australia. Statement of the
COVID-19 FSA Response Committee (19 March 2020).
Available from: https://www.fertilitysociety.com.au/wp-content/uploads/20200319-COVID-19-Statement-FSA-Response-Committee.pdf. Accessed 3 Dec 2020.
42. The Fertility Society of Australia. Updated Statement of
the FSA COVID-19 Response Committee. Available from:
https://www.fertilitysociety.com.au/home/fsa-statement-covid-19/. Accessed 3 Dec 2020.
43. Vaiarelli A, Bulletti C, Cimadomo D, et al. COVID-19 and
ART: the view of the Italian Society of Fertility and Sterility
and Reproductive Medicine. Reprod Biomed Online
2020;40:755-9. Crossref
44. La Marca A, Niederberger C, Pellicer A, Nelson SM. COVID-19: lessons from the Italian reproductive medical
experience. Fertil Steril 2020;113:920-2. Crossref
45. Fabregues F, Peñarrubia J. Assisted reproduction and thromboembolic risk in the COVID-19 pandemic. Reprod
Biomed Online 2020;41:361-4. Crossref
46. Monteleone PA, Nakano M, Lazar V, Gomes AP, de H Martin, Bonetti TC. A review of initial data on pregnancy
during the COVID-19 outbreak: implications for assisted
reproductive treatments. JBRA Assist Reprod 2020;24:219-25. Crossref
47. Alviggi C, Esteves SC, Orvieto R, et al. COVID-19 and
assisted reproductive technology services: repercussions
for patients and proposal for individualized clinical
management. Reprod Biol Endocrinol 2020;18:45.Crossref
48. Esteves SC, Lombardo F, Garrido N, et al. SARS-CoV-2
pandemic and repercussions for male infertility patients:
a proposal for the individualized provision of andrological
services. Andrology 2021;9:10-8. Crossref
49. Arav A. A recommendation for IVF lab practice in light of
the current COVID-19 pandemic. J Assist Reprod Genet
2020;37:1543. Crossref
50. Kashi AH. COVID-19 and semen: an unanswered area of research. Urol J 2020;17:328.
51. Pascolo L, Zito G, Zupin L, et al. Renin angiotensin system,
COVID-19 and male fertility: any risk for conceiving?
Microorganisms 2020;8:1492. Crossref
52. Geber S, Prates N, Sampaio M, Valle M, Meseguer M. COVID-19 should be a novel indication for fertility preservation. JBRA Assist Reprod 2020;24:233-4. Crossref
53. Souza M, Nakagawa H, Taitson PF, Cordts EB, Antunes RA.
Management of ART and COVID-19: infertility in times of
pandemic. What now? JBRA Assist Reprod 2020;24:231-2. Crossref
54. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effects
of coronavirus disease 2019 (COVID-19) on maternal,
perinatal and neonatal outcomes: a systematic review.
Ultrasound Obstet Gynecol 2020;56:15-27. Crossref
55. Elshafeey F, Magdi R, Hindi N, et al. A systematic scoping
review of COVID-19 during pregnancy and childbirth. Int
J Gynaecol Obstet 2020;150:47-52. Crossref
56. Dashraath P, Wong JL, Lim MX, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet
Gynecol 2020;222:521-31. Crossref
57. Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019
(COVID-19) and pregnancy: a systematic review. J Matern
Fetal Neonatal Med 2020 Apr 30. Epub ahead of print. Crossref
58. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations,
risk factors, and maternal and perinatal outcomes of
coronavirus disease 2019 in pregnancy: living systematic
review and meta-analysis. BMJ 2020;370:m3320. Crossref
59. Rodriguez-Wallberg KA, Wikander I. A global
recommendation for restrictive provision of fertility
treatments during the COVID-19 pandemic. Acta Obstet
Gynecol Scand 2020;99:569-70. Crossref