Hong Kong Med J 2021 Feb;27(1):35–45 | Epub 30 Sep 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Clinical manifestations and outcomes of
COVID-19 in the paediatric population: a systematic review
Maha Jahangir, Marrium Nawaz, Deedar Nanjiani, Mishal S Siddiqui
Dow Medical College, Dow University of Health Sciences, Karachi, Pakistan
Corresponding author: Ms M Jahangir (jahangirmaha@yahoo.com)
Abstract
Objective: Coronavirus disease 2019 (COVID-19),
the respiratory illness caused by severe acute
respiratory syndrome coronavirus 2, has affected
hundreds of thousands of people. We aim to report
the distribution of cases, prevalence, and clinical,
radiological, and laboratory signs and outcomes
of COVID-19 in paediatric patients. Moreover,
we intend to evaluate neonatal clinical outcomes.
Hence, our age range of interest is 0 to 19 years.
Methods: A systematic literature review was
conducted using the Medline database to identify
papers published between 1 December 2019 and
9 April 2020 on COVID-19.
Results: The search identified 27 relevant scientific
papers and letters. The review showed that the
prevalence of COVID-19 in the paediatric population
accounts for a small percentage of patients, whose
clinical signs and symptoms are often milder than
those of adults. Despite better prognosis and low
mortality in children, the disease can progress to
severe pneumonia in some cases, especially in the
presence of co-morbidities. Children are likely to
become a hidden source of infection because of their atypical presentation, and they may play a role in
community transmission, leading to unfavourable
outcomes. There is little evidence about intrauterine
vertical transmission. As no vaccine or specific
antiviral is currently available, management plans
include supportive treatment.
Conclusion: As compared with that in adults, the
presentation of COVID-19 in children is mild and
has a better prognosis. Sufficient evidence regarding
the probability of intrauterine vertical transmission
could not be found, and further studies need to be
conducted to establish this relationship.
Introduction
Severe acute respiratory system coronavirus-2
(SARS-CoV-2) outbreak emerged as a series of
idiopathic cases of severe pneumonia in early
December 2019, with the first report made to the
regional office of the World Health Organization
on 30 December 2019.1 The epidemiological curve
of affected individuals rose steeply worldwide, thus
leading to the outbreak being declared as a public
health emergency of international concern on
30 January 20201 and subsequently a pandemic on
11 March 2020.2 As of 30 April 2020, 3 090 445 cases
have been reported worldwide,3 with a mortality
rate of 3.4%,4 culminating in the deaths of 217 769 individuals.3
Like other members of the Coronaviridae
family, SARS-CoV-2, which causes coronavirus
disease 2019 (COVID-19), is a positive-sense single-stranded
RNA virus with an icosahedral capsid
that primarily affects the respiratory tract.5 Other
members of the family have caused pandemics
with similar clinical presentations, such as Middle East respiratory syndrome–related coronavirus
(MERS-CoV) and SARS-CoV. However, COVID-19
outnumbers both of the others in terms of cases and
deaths, despite its lower mortality rate compared
with SARS-CoV and MERS-CoV.6
The COVID-19 outbreak primarily affects
adults, and the severity of disease increases in an
age-dependent fashion. Additional risk factors
include male sex and co-morbidities including
diabetes, hypertension, and previous respiratory
impairments.7 Children and adolescents comprise
a relatively minor proportion of patients who have
tested positive for the virus. Moreover, the paediatric
body responds to the disease differently from the
adult body. This leads to heterogeneous clinical
presentation, disease severity, and mortality rates
across the age spectrum.
review of 72 314 cases by the Chinese Centre
for Disease Control and Prevention reported that
the age-groups of 10 to 19 years and under 10 years
contributed 1% each to the total disease burden.8
Children aged under 18 years have been reported to comprise 1.2% of the total cases in Italy,9 whereas in
the US and Madrid, Spain, the contribution of this
age-group has been reported as 1.7%10 and 0.8%11, respectively. Statistics from Pakistan reveal that
children aged 10 to 19 years comprise 7.25% of the
total number of cases, and this age-group’s mortality
has been approximated as 0.52%.12 Although the
outbreak appears to be stabilising or declining in
certain areas of the world, many regions are still
witnessing an upward trend or even a resurgence.
Increased incidence of asymptomatic carriage
and milder symptoms may lead to a decreased
need for testing in the paediatric population,
especially in already burdened healthcare systems.
Hence, this age-group may remain as a source of
continued transmission, the magnitude of which
remains unexplored. Thus, we aimed to review the
characteristics and presentation of COVID-19 among
children and describe any subtle characteristics that
may strengthen the clinical suspicion of infection
and prompt further testing. This may help to break
the chain of transmission and effectively decrease
the global burden of the pandemic.
Methods
Considering the date of the earliest confirmed report
of COVID-19, we searched Medline for studies
published from 1 December 2019 until 9 April 2020,
with no language restriction and with a combination
of the key search terms “coronavirus” OR “COVID-19”
or “2019-nCoV” OR “SARS-CoV-2” AND “baby” OR “babies” OR “pediatric” OR “paediatric” OR
“newborn” OR “neonate” OR “adolescent” OR “child”
OR “children” OR “infant” OR “boy” OR “girl” OR
“teenage”. We used a comprehensive search strategy
to identify the relevant studies. The screening process
was conducted by two independent reviewers (MJ
and MN), and a third reviewer (DN) was consulted
in the event of discrepancies. The articles were
screened on the basis of title and abstract to assess
their relevance to the aims of our study, followed
by full-text screening. For each retrieved full-text
article, we hand-searched and examined the citation
chain for additional studies. The PubMed search
identified 325 articles. After excluding 229 irrelevant
articles, 96 full-text articles were reviewed, of which
only 27 were included in this review.10 11 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37
This systematic review was conducted in
accordance with the PRISMA statement.38 An
inclusive approach to eligibility assessment was
taken. Studies were deemed eligible if they provided
clinical data on COVID-19 in the paediatric
population (ie, aged 0-19 years). Therefore, a few
letters that provided original data were also included
in the study. We excluded letters, case reports,
editorials, opinion pieces, or studies from which no
data from paediatric patients could be extracted,
focused on other coronaviruses than COVID-19,
epidemiological studies that provided no clinical
findings, and those in other languages with no
English translation. The Figure shows PRISMA chart outlining the search strategy.
Results and Discussion
The clinical manifestations and outcomes of
COVID-19 in the paediatric population are
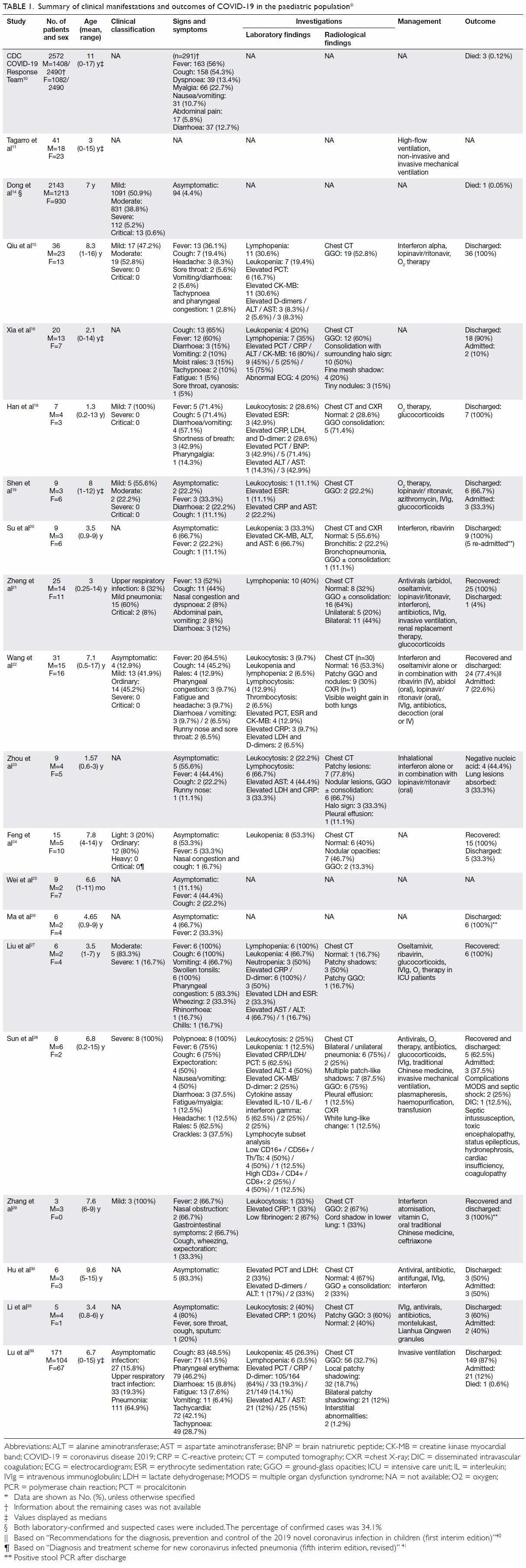
summarised in Table 1.10 11 14 15 16 18 19 20 21 22 23 24 25 26 27 28 29 30 33 39 40 41
Age
Korean data suggested that a 10-year-old female
child was the first paediatric case, and the youngest
paediatric case of COVID-19 as of 2 March 2020
was a 45-day-old male baby.42 To date, the youngest reported paediatric case was a 36-hour-old baby in
China.13
The median age of the paediatric patients with
COVID-19 from Madrid, Spain was 1 year (range,
0-15 years). The largest dataset of 2143 paediatric
patients from China reported the median age at
diagnosis as 7 years.14 However, in the largest-to-date
American dataset of 2572 COVID-19 cases
in children aged <18 years, the median age was
reported as 11 years.10
Comparing the two large studies,10 14 we believe
that there is variation in the median age between
different regions of the world. However, paediatric
data from other parts of the world to confirm this are
still lacking. We deduce that children of all ages can
be infected, including neonates, infants, and young
children.
Sex
An observational cohort study of 36 patients
identified that 64% of the patients infected with
SARS-CoV-2 were male.15 This is consistent with
findings from other small studies.16 17 However,
a large study by Dong et al14 did not find any
statistically significant trend in the sex of paediatric
patients, with boys comprising 1213 cases (56.6%).
This is in line with another large study from the US
describing 2490 paediatric cases: 57% of the patients
were male.10
Incubation period
The incubation period of COVID-19 ranged
from less than 1 day to as long as 16 days.19 The
median incubation period in children reported by
Han et al18 and Shen et al19 was 5 days and 7.5 days,
respectively.
Symptoms
Infected children were either asymptomatic or
reported symptoms like fever and dry cough as the
most common symptoms.13 15 19 20 21 22 23 24 25 26 Data reported by
the US Centers for Disease Control and Prevention
resonate with these findings: fever and cough were
reported in 56% and 54% of the paediatric patients,
respectively, whereas in another study, 68% of the
paediatric cases had no obvious symptoms.10 A
case series comprising of six patients reported fever and cough in all of them and vomiting in four of
them.27 These results were confirmed by Xia et al,16
who found cough almost equally frequently as fever
(65% and 60%, respectively). However, polypnoea
has been reported as the most common symptom
in severely affected patients, followed by fever and
cough.28 A high proportion of patients presented
initially with gastrointestinal symptoms such as
nausea, vomiting, abdominal pain, constipation,
and diarrhoea.10 15 16 18 19 21 22 27 28 29 Other reported
symptoms were fatigue, myalgia, headache,10 16 18 22 28
and upper respiratory tract symptoms such as
sore throat, nasal discharge, tachypnoea, and
expectoration.10 13 15 16 17 19 21 22 23 24 25 28 29
Presentation of COVID-19 in paediatric
patients is much milder than that in adults.
Therefore, children may be a hidden source of
infection.22 Fever, cough, and shortness of breath
were more commonly reported among adult patients
(93%) than paediatric patients (73%).10 Another
study showed a statistically significant difference
between symptomatic presentation in children and
adults (P<0.0001): fever (36% and 86%, respectively),
cough (19% and 62%), pneumonia (53% and 95%),
and severe disease type (0% and 23%).15 In contrast,
gastrointestinal symptoms were more common
in children.10 18 Another difference was observed
between the median duration of fever in children
(1 day; range, 0-3 days) and adults (4 days; range,
1-10 days).18
Dong et al14 reported that only 34.1% of cases
were laboratory-confirmed, whereas the remainder
had clinically suspected disease on the basis of
their symptoms. However, this does not rule out
their chance of other respiratory infections, so we
cannot fully rely on clinical suspicion. Children
with SARS-CoV, SARS-CoV-2, and H1N1 influenza
presented with somewhat similar symptoms, with
the most common being fever. However, cough
and pharyngeal congestion are not as common in
SARS-CoV-2 (7% and 3%, respectively) as in
SARS-CoV (64% and 14%) and H1N1 influenza (83%
and 95%). These findings indicate that SARS-CoV-2
has little effect on the upper respiratory tract of
children.15 24 25
There are many plausible explanations for
why the disease’s manifestations are milder in the
paediatric population as compared with adults. First,
it is very rare for children to have co-morbidities like
diabetes, cardiovascular disease, and hypertension.
Adults have a higher prevalence of C-reactive
protein and longer duration of fever, suggesting a
stronger immunological response compared with
that in children.15 18 Further, children tend to remain
at home and have fewer opportunities for exposure to pathogens or patients. Angiotensin-converting
enzyme II is the receptor speculated to be affected
by SARS-CoV-2.43 44 Children are less sensitive
to COVID-19 because of their lower maturity
and binding ability to a different distribution of
angiotensin-converting enzyme II receptors.45
Furthermore, children experience respiratory
infections in winter more often than adults do, which
may result in higher levels of antibodies against
other respiratory viruses, providing cross-protection
against SARS-CoV-2. Additionally, children are
mostly infected by the second or third generation of
viral infections; hence, the viruses with which they
are infected have weak virulence. Further, children’s
immune response is still under development and
may show different responses to pathogens than that
of adults.23 24 A different yet possible explanation is
that like SARS and MERS-CoV infection in children,
SARS-CoV-2 infection may follow a much milder
and shorter course.21
Radiological findings
Among the studies that focused on radiographic
findings in paediatric patients (n=224), 147 (65.6%)
patients showed positive findings suggestive
of pneumonia. Most of the patients presented
with abnormalities, such as multiple bilateral,
peripheral ground-glass opacities (GGO), and
consolidation.15 18 19 20 23 24 27 29 30 In comparison with
adults, pulmonary inflammatory changes have been
reported to be milder,15 23 24 and nodular changes
(75%), revealed as halo sign and air bronchogram sign
on computed tomography (CT), are more common
in paediatric patients.16 22 23 Therefore, these should
be considered as typical signs in paediatric patients.
Chest CT images demonstrated bilateral lung
involvement in about 70% of children aged <3 years,
and unilateral lesions and normal lungs were reported
more frequently in children aged ≥6 years.21 Lesions
were mainly distributed in the middle and outer
bands of the lungs near the pleura. However, 53% had
no obvious abnormalities.22 When another group of
24 asymptomatic carriers was reviewed, 29.2% had
normal CT images.30 Feng et al24 examined nine of the
15 confirmed paediatric patients, of whom 54% had
no clear symptoms on admission. However, their CT
results were typical of SARS-CoV-2 infection. Chest
CT scans revealed improvement in children after
3 to 5 days of treatment.17 20 24 29 However, lesions
were sometimes still visible on chest CT despite two
consecutive negative nucleic acid tests.16
The severity of the lesions was limited in
the early stage, but they increased in density as
the disease advanced, involving multiple lobes
bilaterally.16 22 Earlier in the course of disease, CT
showed consolidation with surrounding halo sign,
GGO, fine mesh shadow, and tiny nodules in 50%,
60%, 20%, and 15% of cases, respectively.16 In severely ill patients, bilateral multiple patch-like shadows,
GGO, ‘white lung’ change accompanied by air
bronchogram sign, and pleural thickening was the
characteristic picture on CT.16 21 28 In neonates, chest
X-ray commonly showed GGO and blurred lung
margins followed by bilateral pneumothorax and
signs of neonatal respiratory distress syndrome.17
Computed tomography features could play
an important role in screening suspected cases
radiographically while awaiting confirmation
by real-time reverse transcription–polymerase
chain reaction (PCR), the results of which could
be used to decide the subsequent plan of action.
Computed tomography can also be useful in cases
that yield multiple false-negative real-time reverse
transcription–PCR tests despite being clinically
symptomatic.
Laboratory findings
Analysis of laboratory tests has revealed different
laboratory parameters in children compared with
adults. In children, the peripheral white blood cell
count and absolute lymphocyte count are usually
normal or slightly reduced.11 15 19 20 22 24 27 28 29 31 In
contrast, laboratory analyses of adults have shown
low leukocyte counts and significant reductions
in absolute peripheral blood lymphocyte counts.
One study revealed leukopenia as a common
finding among adults (20%), whereas leukocytosis
was more frequent in children (28.6%; P=0.014).18
One study found an elevated lymphocyte count on
the initial routine blood test in 66% of paediatric
subjects.23 Although the association is not clear,
this altered immune response and lack of significant
lymphopenia might help to cause the milder
presentation in children. Some features differed
significantly according to disease severity. Sun et al28
observed normal or mildly raised levels of leukocytes,
neutrophils, and lymphocytes in severely ill patients,
whereas low counts were observed in critically ill
patients with serious complications. Abnormalities
in the cytokine spectrum, characterised by increased
plasma concentrations of inflammatory cytokines,
were seen more frequently in critically ill than severe
patients.28
Inflammatory markers like C-reactive protein
and erythrocyte sedimentation rate were normal
or transiently elevated.19 22 23 38 Raised levels of
procalcitonin (PCT) were linked with severe disease
in children.11 16 22 The erythrocyte sedimentation rate
was raised significantly in adults as compared with
children (P=0.047), whereas PCT was elevated in
42.9% of children but no adult patients (P=0.007).18
Elevated PCT in children may indicate bacterial
co-infection, and timely administration of antibiotics
might prove beneficial.
Another characteristic feature of COVID-19
is that it affects vital organs like the lungs, liver, and heart, indicated by increased levels of myocardial
enzymes, aspartate aminotransferase, alanine
aminotransferase, and D-dimer. Myocardial
zymography revealed a higher frequency of
elevated levels of isoenzyme in children than in
adults.11 16 18 20 28 The level of creatine kinase, an
indicator of myocardial injury, is significantly higher
in severely ill patients.28 Moreover, brain natriuretic
peptide has been found in a few paediatric patients.28
The presence of creatine kinase and brain natriuretic
peptide indicates that SARS-CoV-2 has the potential
to cause heart injury. Therefore, attention should be
paid to those laboratory results.
In summary, the laboratory findings reported
in children with SARS-CoV-2 are inconsistent with
those observed in adult cases. Disease progression
is characterised by amplified inflammatory response
or cytokine storm. Monitoring of laboratory
parameters is suggested to identify patients who
might show improvement with anti-inflammatory
treatments.
Treatment
The principles of early identification, early isolation,
early diagnosis, and early treatment should be
stressed. Our review did not identify any treatment
protocols or trials specific to the paediatric
population. Although most children with mild
disease may not have indications for hospitalisation,
supervision must be ensured to contain and prevent
transmission. As no vaccine is currently available,
management plans include bed rest and supportive
treatments like maintenance of water electrolyte
balance and homeostasis, administration of
antipyretics, and administration of broad-spectrum
antibiotics because of the probability of co-infecti
on.10 16 19 20 21 22 27 29 30 31 32 33 34 38
Given by spray or nebulisation in the early
phase of disease, interferons, alone or in combination
with other antivirals, have been shown to improve
symptoms.10 11 20 22 23 29 30 33 38 Oral lopinavir/ritonavir or
ribavirin have been used; however, their efficacy and
safety remain to be determined.10 11 19 22 23 27 29 30 33 34 38
Corticosteroids and intravenous immunoglobulin
have been used in severe cases only.10 18 19 22 27 33 38
Use of steroids for treatment of SARS-CoV and
MERS-CoV resulted in increased rates of secondary
bacterial and fungal infections and longer duration
of hospital stay. Thus, in addition to suppressing
the inflammatory response, steroids also delay viral
clearance.46 Because of the lack of evidence regarding
efficacy, the World Health Organization’s interim
guidance advised against the use of steroids for
treatment of novel coronavirus, unless indicated.47
For such cases, it is recommended to use steroids
only in the short term, and only as a part of a clinical
trial, to efficiently weigh their harms and benefits.48
Patients with COVID-19 should be closely monitored for signs of clinical deterioration, such
as rapidly progressive respiratory failure, central
cyanosis, coma, convulsion, and sepsis. If respiratory
distress develops despite the use of a nasal catheter
or mask oxygenation, a heated humidified high-flow
nasal cannula and non-invasive ventilation should be
used to target SpO2 ≥94%.9 11 17 18 19 27 32 42 Mechanical
ventilation with endotracheal intubation should
be adopted when no improvement is seen.10 11 38
Increased levels of pro-inflammatory factors have
been seen in children.11 16 19 20 22 23 24 27 28 29 31 38 Thus,
targeted anti-inflammatory therapies that might
help with early control of disease progression
are warranted in the future. Monitoring patients’
conditions closely and the application of timely
and effective therapeutic protocols through
multidisciplinary approaches could serve as the
cornerstones of COVID-19 treatment.
Co-morbidities
Compared with adults, children rarely had
co-morbidities.15 18 Zheng et al21 reported two
patients with congenital heart disease, one of whom
also had malnutrition and metabolic diseases.
Among 345 paediatric cases with information
on underlying conditions, 23% had at least one
underlying condition, with chronic lung disease
being the most common, followed by cardiovascular
and immunosuppressive diseases.10 Tagarro et al11
reported that 27% of patients had underlying disease.
Furthermore, Xia et al16 reported that 35% patients
had a history of underlying diseases, which may
indicate that such patients are more susceptible to
SARS-CoV-2.
Outcomes
Although paediatric patients are susceptible to
COVID-19, the case fatality rate of severe paediatric
patients is much lower than that of adults (49.0%),15
which indicates that they have favourable outcomes
compared with adults.21 Paediatric patients
mostly recover in 1 to 3 weeks and are generally
discharged after consecutive negative nucleic acid
tests.15 16 18 19 20 21 22 24 27 29 30 31 33 Tagarro et al11 reported that
60% of paediatric patients were hospitalised, with
only 10% admitted to paediatric intensive unit
care. Sun et al28 also reported that most severely ill
patients recovered and were discharged. Another
two children who received paediatric intensive
unit care recovered without any adverse outcomes
reported.21
Disease duration is relative to the severity of the
disease: the duration is over 10 days across all patients
and over 20 days in critically ill patients.28 Qiu et al15
concluded that patients with the moderate clinical
type spent more days in hospital compared with
those with mild clinical type (P=0.017). The length
of hospital stay has varied between different studies: the minimum and maximum averages reported have
been 8.327 and 15.3 days,19 respectively. Because
this parameter is multifactorial and influenced by
isolation policy and the availability of health facilities
and laboratory tests in different hospitals, the length
of hospital stay does not necessarily predict the
prognosis, and detailed analysis is expected on its
significance.
Overall, the prognosis in neonates is also good.
Most of them have been discharged after consecutive
negative nucleic acid test results.13 A few have been
kept under observation despite stable condition and
negative clinical and radiological findings because
of positive COVID-19 pharyngeal swab nucleic acid
test results.13 16 17
Complications
Almost all studies have reported recovery without any
complications. In critically ill patients, septic shock
and multiple organ dysfunction syndrome were the
most common complications, and intussusception,
toxic encephalopathy, status epilepticus,
disseminated intravascular coagulation (DIC),
hydronephrosis, cardiac insufficiency, coagulopathy,
hypoglobulinaemia, and gastroenteritis were also
reported.28 One study reported two critical cases
with abnormal renal function and coagulapathy.21
Deaths
In China, the deaths of a 14-year-old boy14 and
a 10-month-old baby with intussusception who
developed multi-organ failure 4 weeks post-admission
were reported.39 Three paediatric deaths were reported
in the US.10 None had been reported in Italy or Spain
as of 15 March and 8 April 2020, respectively.9 11
Window of stool polymerase chain reaction
detection
The ability of SARS-CoV-2 to infect the
gastrointestinal tract is supported by detection of
its nucleic acids in stool samples from adults and
children.15 20 26 29 32 In spite of negative nucleic acid
results from throat swab specimens, children’s stools
were still nucleic acid–positive after 10 days of
recovery.29 Other studies have reported re-admission
of discharged children with positive stool specimens
but negative respiratory specimens. Although their
prognosis is better than that of adults; the period of
PCR positivity is longer in children.15 20 26 Poorer hand
hygiene practices causing faecal-oral transmission
might be a reason for the delayed clearance of viral
RNA in children’s stools. Although positive results
cannot confirm that live virus is present in the stool,
this still increases the infection risk to the public,
so follow-up of specimen collection should be
considered. The isolation period for children should
be reviewed because of the transmission risk.
Transmission patterns
The virus is mainly transmitted through respiratory
droplets or contact.28 Transmission among the
paediatric population mostly occurs by close contact
with family members,14 18 19 20 21 22 23 29 a history of exposure
to the epidemic area, or both.15 21 According to
Wang et al,22 90% of cases were clustered in families.
Another study reported that 62.5% cases were
associated with familial clustering.28 Neonates and
infants are more likely than adults to be infected
via close contact with COVID-19-positive family
members.16 25
Potential of intrauterine vertical
transmission
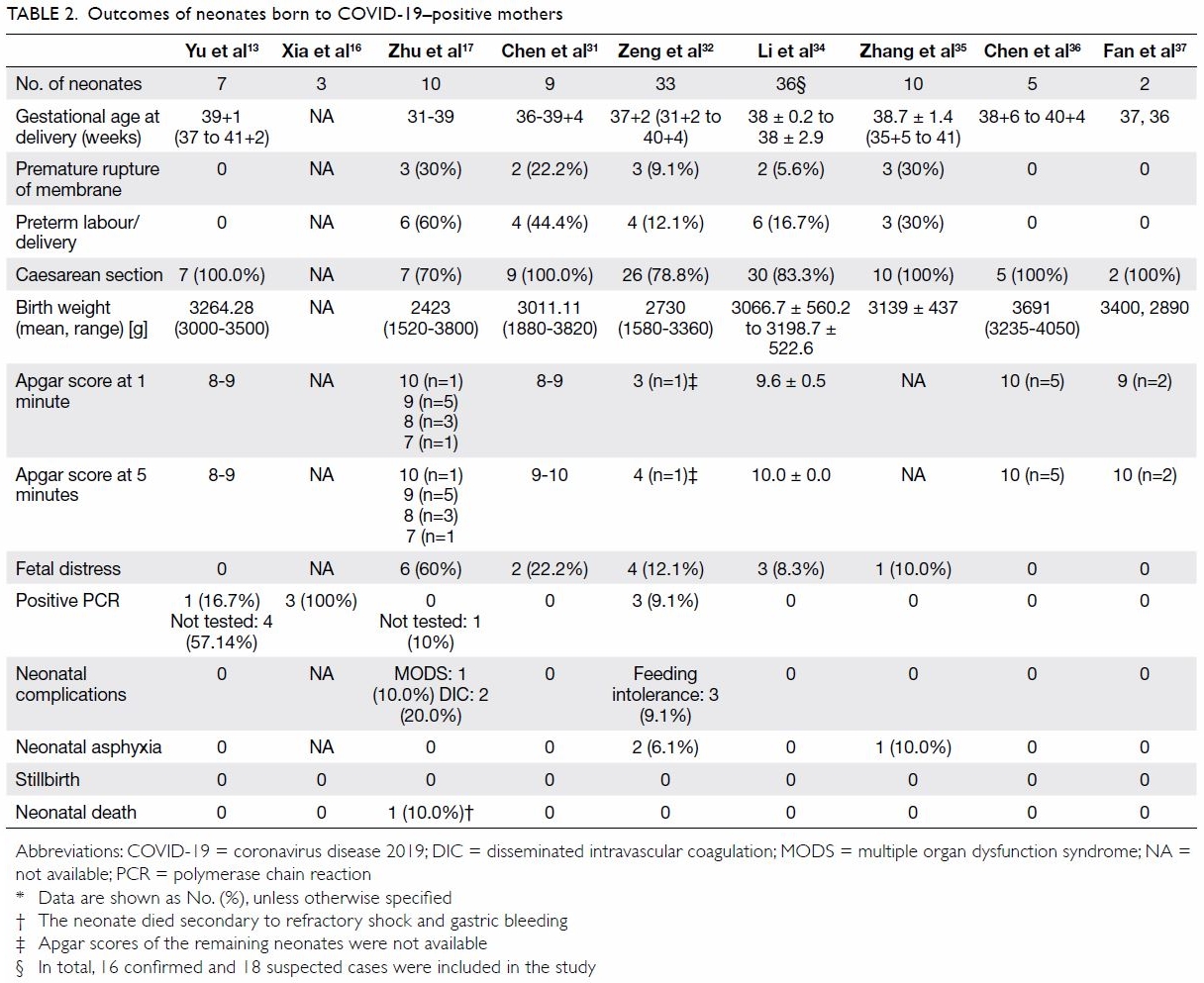
To date, nine studies have reported on neonates born
to COVID-19 positive mothers (n=115); only seven
of those neonates were SARS-CoV-2-positive.13 16 32
Xia et al,16 Yu et al,13 and Zeng et al32 reported that 3/20 (15%), 1/7 (14%), and 3/33 (9%) neonates
were positive, respectively. Despite the placenta
and cord blood being negative for SARS-CoV-2,
one neonate was diagnosed as positive 36 hours
after birth.13 Other studies have also found that
the placenta, cord blood, and breast milk were
negative for SARS-CoV-2.17 31 34 37 Because of the
limited evidence regarding vertical transmission,
we speculate that close contact could explain the
positive results. We are still not confident about the
probability of vertical maternal-fetal transmission,
and further studies need to be performed on this
subject. Neonates born to infected mothers should
be separated immediately after birth and undergo an
isolation period.
Impact of COVID-19 on fetal outcomes
Although the majority of the studies showed that
neonates were negative for SARS-COV-2 and that most neonates had excellent outcomes,36 a few
studies revealed that neonates born to COVID-19-positive mothers could develop other complications
that could lead to poor neonatal outcomes. The
rate of premature birth among newborns born to
mothers with confirmed COVID-19 pneumonia
(23.5%) was significantly higher than the 2020 and
2019 control rates (5.8% and 5.0%, respectively).
Low birth weight was also more frequent in infants
of infected mothers (17.6%) than in the control
group (2.5%).34 Some incidence of premature birth,
fetal distress, premature rupture of membranes,
small size for gestational age, and large size
for gestational age was observed in neonates
born to COVID-19-positive mothers.17 35 Two
COVID-19-positive neonates developed DIC, of
whom one died on the 9th day secondary to refractory
shock, multiple organ dysfunction syndrome, and
DIC.17 The neonatal outcomes are summarised in
Table 2.13 16 17 31 32 34 35 36 37
Conclusion
Paediatric patients make up a small fraction of
COVID-19 cases, and they have a better prognosis
than adult patients have. The differences in
the mechanisms behind COVID-19’s clinical
manifestations between children and adults need to
be verified by large, well-designed studies. Children
are likely to become a hidden source of infection,
which may delay the diagnosis of COVID-19, leading
to unfavourable outcomes and causing community
transmission. The probability of intrauterine vertical
transmission in neonates is low, and close contact
is the only plausible explanation for the observed
positive results in neonates.
Author contributions
M Jahangir designed the study. All authors contributed to the
acquisition and analysis of data, and wrote the manuscript.
M Jahangir had critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
The authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. World Health Organization. Coronavirus disease
(COVID-19)–events as they happen. Rolling updates
on coronavirus disease (COVID-19). Available from:
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed 30 Apr 2020.
2. World Health Organization. Coronavirus disease 2019
(COVID-19) situation report–51. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10. Accessed 30 Apr 2020.
3. World Health Organization. Coronavirus disease 2019
(COVID-19) situation report–101. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200430-sitrep-101-covid-19.pdf?sfvrsn=2ba4e093_2. Accessed 30 Apr 2020.
4. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19–3 March 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---3-march-2020. Accessed 30 Apr 2020.
5. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R.
Features, evaluation and treatment coronavirus (COVID-19).
StatPearls Publishing; 2020 Jan. Available from: https://www.statpearls.com/as/infectious/52171/. Accessed 30
Apr 2020.
6. Mahase E. Coronavirus: COVID-19 has killed more people
than SARS and MERS combined, despite lower case fatality
rate. BMJ 2020;368:m641. Crossref
7. Worldometer. Age, sex, existing conditions of COVID-19
cases and deaths. Available from: https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/.
Accessed 30 Apr 2020.
8. Wu Z, McGoogan JM. Characteristics of and important
lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314
cases from the Chinese Center for Disease Control and
Prevention. JAMA 2020;323:1239-42. Crossref
9. Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA 2020;323:1335. Crossref
10. CDC COVID-19 Response Team. Coronavirus disease 2019 in children–United States, February 12–April 2, 2020.
MMWR Morb Mortal Wkly Rep 2020;69:422-6. Crossref
11. Tagarro A, Epalza C, Santos M, et al. Screening and severity
of coronavirus disease 2019 (COVID-19) in children in
Madrid, Spain. JAMA Pediatr 2020;Apr 8. Epub ahead of
print. Crossref
12. Health Advisory Platform by Ministry of National Health
Services Regulations and Coordination, Government of
Pakistan. COVID-19. Available from: http://covid.gov.pk/stats/pakistan. Accessed 5 May 2020.
13. Yu N, Li W, Kang Q, et al. Clinical features and obstetric and
neonatal outcomes of pregnant patients with COVID-19 in
Wuhan, China: a retrospective, single-centre, descriptive
study. Lancet Infect Dis 2020;20:559-64. Crossref
14. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19
among children in China. Pediatrics 2020;145:e20200702. Crossref
15. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and
epidemiological features of 36 children with coronavirus
disease 2019 (COVID-19) in Zhejiang, China: an
observational cohort study. Lancet Infect Dis 2020;20:689-
96. Crossref
16. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and
CT features in pediatric patients with COVID-19
infection: different points from adults. Pediatr Pulmonol
2020;55:1169-74. Crossref
17. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia.
Transl Pediatr 2020;9:51-60. Crossref
18. Han YN, Feng ZW, Sun LN, et al. A comparative-descriptive
analysis of clinical characteristics in 2019-coronavirusinfected
children and adults. J Med Virol 2020;Apr 6. Epub
ahead of print. Crossref
19. Shen Q, Guo W, Guo T, et al. Novel coronavirus infection
in children outside of Wuhan, China. Pediatr Pulmonol
2020;55:1424-9. Crossref
20. Su L, Ma X, Yu H, et al. The different clinical characteristics
of corona virus disease cases between children and
their families in China—the character of children with
COVID-19. Emerg Microbes Infect 2020;9:707-13. Crossref
21. Zheng F, Liao C, Fan QH, et al. Clinical characteristics of
children with coronavirus disease 2019 in Hubei, China.
Curr Med Sci 2020;40:275-80. Crossref
22. Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases
of 2019 novel coronavirus infection in children from six
provinces (autonomous region) of northern China [in
Chinese]. Zhonghua Er Ke Za Zhi 2020;58:269-74.
23. Zhou Y, Yang GD, Feng K, et al. Clinical features and chest
CT findings of coronavirus disease 2019 in infants and
young children [in Chinese]. Zhongguo Dang Dai Er Ke Za
Zhi 2020;22:215-20.
24. Feng K, Yun YX, Wang XF, et al. Analysis of CT features
of 15 children with 2019 novel coronavirus infection [in
Chinese]. Zhonghua Er Ke Za Zhi 2020;58:E007.
25. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel
coronavirus infection in hospitalized infants under 1 year
of age in China. JAMA 2020;323:1313-4. Crossref
26. Ma X, Su L, Zhang Y, Zhang X, Gai Z, Zhang Z. Do
children need a longer time to shed SARS-CoV-2 in stool
than adults? J Microbiol Immunol Infect 2020;53:373-6. Crossref
27. Liu W, Zhang Q, Chen J, et al. Detection of COVID-19
in children in early January 2020 Wuhan, China. N Engl J
Med 2020;382:1370-1. Crossref
28. Sun D, Li H, Lu X, et al. Clinical features of severe pediatric
patients with coronavirus disease 2019 in Wuhan: a single
center’s observational study. World J Pediatr 2020;16:251-9. Crossref
29. Zhang T, Cui X, Zhao X, et al. Detectable SARS-CoV-2
viral RNA in feces of three children during recovery period
of COVID-19 pneumonia. J Med Virol 2020;92:909-14. Crossref
30. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24
asymptomatic infections with COVID-19 screened among
close contacts in Nanjing, China. Sci China Life Sci
2020;63:706-11. Crossref
31. Chen H, Guo J, Wang C, et al. Clinical characteristics and
intrauterine vertical transmission potential of COVID-19
infection in nine pregnant women: a retrospective review
of medical records. Lancet 2020;395:809-15. Crossref
32. Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection
with SARS-CoV-2 in 33 neonates born to mothers with
COVID-19 in Wuhan, China. JAMA Pediatr 2020;174:722-5. Crossref
33. Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr
Radiol 2020;50:796-9. Crossref
34. Li N, Han L, Peng M, et al. Maternal and neonatal outcomes
of pregnant women with COVID-19 pneumonia: a case-control
study. Clin Infect Dis 2020;Mar 30. Epub ahead of
print. Crossref
35. Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy
outcomes in pregnant women with COVID-19 in Hubei
Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi
2020;55:166-71.
36. Chen S, Liao E, Cao D, Gao Y, Sun G, Shao Y. Clinical
analysis of pregnant women with 2019 novel coronavirus
pneumonia. J Med Virol 2020;Mar 28. Epub ahead of print. Crossref
37. Fan C, Lei D, Fang C, et al. Perinatal transmission of
COVID-19 associated SARS-CoV-2: Should we worry?
Clin Infect Dis 2020;Mar 17. Epub ahead of print.
38. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA
Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA Statement. PLoS Med
2009;6:e1000097. Crossref
39. Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in
children. N Engl J Med 2020;382:1663-5. Crossref
40. Recommendations for the diagnosis, prevention and
control of the 2019 novel coronavirus infection in children
(first interim edition) [in Chinese]. Zhonghua Er Ke Za Zhi
2020;58(0):E004.
41. National Health Commission of the People’s Republic
of China. Diagnosis and treatment scheme for new
coronavirus infected pneumonia (fifth interim edition,
revised). Available from: http://www.gov.cn/zhengce/zhengceku/2020-02/09/5476407/files/765d1e65b7d1443081053c29ad37fb07.pdf. Accessed 30 Apr 2020.
42. Park JY, Han MS, Park KU, Kim JY, Choi EH. First pediatric
case of coronavirus disease 2019 in Korea. J Korean Med
Sci 2020;35:e124. Crossref
43. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak
associated with a new coronavirus of probable bat origin.
Nature 2020;579:270-3. Crossref
44. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure
of the 2019-nCoV spike in the prefusion conformation.
Science 2020;367:1260-3. Crossref
45. Fang F, Luo XP. Facing the pandemic of 2019 novel
coronavirus infections: the pediatric perspectives [in
Chinese]. Zhonghua Er Ke Za Zhi 2020;58:81-5.
46. Russell CD, Millar JE, Baillie JK. Clinical evidence does
not support corticosteroid treatment for 2019-nCoV lung
injury. Lancet 2020;395:473-5. Crossref
47. World Health Organization. Clinical management of
severe acute respiratory infection (SARI) when COVID-19
disease is suspected: interim guidance. Available from:
https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed 29 May 2020.
48. Shen K, Yang Y, Wang T, et al. Diagnosis, treatment, and
prevention of 2019 novel coronavirus infection in children:
experts’ consensus statement. World J Pediatr 2020;16:223-31. Crossref