Hong Kong Med J 2020 Oct;26(5):390–6 | Epub 10 Sep 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Molecular detection of Mycoplasma genitalium in endocervical swabs and associated rates of macrolide and fluoroquinolone resistance in Hong Kong
Kevin KM Ng, MB, ChB, FRCPath; Patricia KL Leung, MPhil; Terence KM Cheung, MPhil
Public Health Laboratory Services Branch, Centre for Health Protection, Department of Health, Hong Kong SAR Government, Hong Kong
Corresponding author: Dr Kevin KM Ng (kevinkmng@yahoo.com.hk)
Abstract
Introduction: There is a global trend of increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium (MG), such that international guidelines recommend molecular detection of resistance if a patient has MG-positive test results. Tests for MG are not routinely performed in Hong Kong. This study examined the detection of MG in endocervical swabs and the associated macrolide and fluoroquinolone resistance rates.
Methods: Endocervical swabs received from two sexual health clinics in Hong Kong for routine assessments of Chlamydia trachomatis and Neisseria gonorrhoeae were also subjected to detection of MG. All MG-positive samples were tested for resistance-mediating mutations in 23S rRNA, parC, and gyrA genes. Laboratory records and past results for each patient were analysed.
Results: In total, endocervical swabs from 285 patients were included in this study. Mycoplasma genitalium was detected in swabs from 21 patients (7.4%) by real-time polymerase chain reaction with a commercial kit. Among MG-positive samples which were successfully analysed further, macrolide resistance-mediating mutations in 23S rRNA were found in 42.1% (8/19); fluoroquinolone resistance–related mutations in parC and gyrA were found in 65% (13/20) and 0% (0/20), respectively. All macrolide-resistant MG strains were also fluoroquinolone-resistant (42.1%, 8/19). No assessed factors were associated with the detection of MG or resistance-related mutations.
Conclusion: In Hong Kong, MG was detected in endocervical swabs from 7.4% of patients in sexual health clinics, with high rates of macrolide and fluoroquinolone resistance. These findings warrant careful review of testing, clinical correlation, and treatment strategies for MG in the context of increasing antibiotic resistance.
New knowledge added by this study
- Mycoplasma genitalium (MG) was detected in endocervical swabs from 7.4% of patients in two sexual health clinics in Hong Kong.
- High rates of macrolide and fluoroquinolone resistance–associated mutations (42.1% and 65%, respectively) were detected in MG-positive specimens.
- All macrolide-resistant MG strains were also fluoroquinolone-resistant.
- No clinical or demographic factors were significantly associated with the detection of MG or resistance-related mutations.
- Our findings support the existing recommendation that testing should be reserved only for patients with increased risks or for whom treatment has failed, as well as their contacts.
- The empirical uses of macrolide and fluoroquinolone regimens utilised in Hong Kong might explain the high rates of resistance found in this study.
- Careful review is required with respect to testing, clinical correlation, and treatment strategies for MG in the context of increasing antibiotic resistance.
Introduction
Mycoplasma genitalium (MG) is capable of causing
urogenital infection, especially urethritis, in men.
There is increasing evidence to support its ability to cause cervicitis and pelvic inflammatory disease in
women.1 Data from a recent meta-analysis showed
a prevalence of 1.3% in the general populations
of developed countries, with similar rates among men and women; furthermore, the prevalence
ranged from 0.6% to 12.6% in clinic-based
studies.2 In terms of therapy, macrolides such as
azithromycin are considered first-line treatment,
while fluoroquinolones (FQ) such as moxifloxacin
are considered second-line treatment. However,
antibiotic resistance is an emerging problem, with
cited rates of macrolide and FQ resistance both
reaching approximately 70% in the Asia-Pacific
region.3 Consequently, in the context of increasing
reports of treatment failure,4 5 multiple international
guidelines indicate that all MG-positive specimens
should be subjected to testing for macrolide
resistance-mediating mutations and test-of-cure
purposes.6 7 8
In Hong Kong, testing for MG is not routinely
undertaken in the public sector. Notably, a
cross-sectional study performed in 2008 in Hong
Kong showed respective prevalences of 10% and 2%
in symptomatic and asymptomatic men who sought
sexual health services.9 Empirical treatment for
non-gonococcal urethritis or non-specific genital
tract infection encompassing MG infection generally
comprises either a single dose of azithromycin or
a 1-week course of doxycycline. Data regarding
resistance profiles in Hong Kong are not available.
This laboratory-based study aimed to determine
the prevalences of MG in both symptomatic and
asymptomatic women attending sexually transmitted
infection (STI) clinics, as well as to determine the rates of macrolide and FQ resistance in MG-positive
endocervical swabs, by using molecular methods.
Methods
Specimen and data collection
From March to May 2019, endocervical swabs of
female patients sent from two STI clinics in Hong
Kong for routine molecular detection of Chlamydia
trachomatis (CT) and Neisseria gonorrhoeae (NG)
were also subjected to detection of MG. Information
regarding the presence or absence of genitourinary
symptoms was provided by attending physicians.
All available laboratory information for each tested
patient was reviewed to determine demographic
data, history of STI, human immunodeficiency virus
status, and test results from the current visit.
Detection of Mycoplasma genitalium
The cobas TV/MG assay (Roche Diagnostics,
Rotkreuz, Switzerland) performed on the cobas
6800 System (Roche Diagnostics) was used for
detection of MG. The cobas TV/MG assay is a
CE-marked, Food and Drug Administration–cleared, commercial qualitative nucleic acid test that
utilises the real-time polymerase chain reaction for
dual detection of Trichomonas vaginalis (TV) and
MG. Its reported overall sensitivity and specificity
rates were 83.1% and 98.4%, respectively, for MG
detection in endocervical swabs from female
patients.10 Tests were performed in accordance with
the manufacturer’s instructions.
Detection of other sexually transmitted
pathogens
In brief, CT and NG were detected by the cobas
CT/NG assay (Roche Diagnostics), while herpes
simplex virus (HSV) 1 and HSV 2 were detected by
the cobas HSV 1 and 2 assay (Roche Diagnostics),
both performed on the cobas 4800 System (Roche
Diagnostics). In addition, NG was detected by the
culture method on modified Thayer-Martin agars.
Either molecular- or culture-based test indicating
the presence of NG was considered a positive result.
Trichomonas vaginalis was detected by the cobas
TV/MG assay (Roche Diagnostics) and wet mount
microscopy after enrichment in Feinberg medium.
Similar to NG, either test indicating the presence
of TV was considered a positive result. Syphilis was
detected by serological tests of serum specimens,
including enzyme immunoassay (DiaSorin, Saluggia,
Italy), Venereal Disease Research Laboratory
test (Becton, Dickinson and Company, Franklin
Lakes [NJ], US), fluorescent treponemal antibody
absorption test (ImmunoDiagnostics Limited, Hong
Kong, China), or Treponema pallidum passive
particle agglutination test (Fujirebio Diagnostics AB,
Göteborg, Sweden).
Detection of macrolide and fluoroquinolone
resistance
Mutations at nucleotide positions 2071 and 2072
(2058 and 2059, respectively, by Escherichia coli
numbering) of the 23S rRNA gene have been
associated with macrolide resistance in MG and
subsequent treatment failure11 12; mutations in the
quinolone resistance-determining region of the
parC gene, and possibly the gyrA gene, have been
associated with FQ resistance.5 Polymerase chain
reaction analysis of these genes was performed
as described previously.13 14 Sequencing was
performed using a 3730xl DNA Analyzer (Applied
Biosystems, Foster City [CA], US), in accordance
with the manufacturer’s instructions. Primers used
for sequencing were the same as those used for the
polymerase chain reaction. Resulting sequences
were compared to the sequence of wild-type strain
MG G37 by using BLAST (https://blast.ncbi.nlm.
nih.gov/Blast.cgi).
Statistical analysis
Calculation of odds ratios, and the Chi squared
test or Fisher’s exact test, were performed as
univariate analysis to identify associations between
assessed factors (ie, age, previous STI clinic visit,
history of STI, symptoms, and sexually transmitted
co-infections) and outcomes (ie, detection of MG
and detection of resistance mutations), as well as
between STI and symptoms. Because of the low
outcome frequency, Firth logistic regression was
employed to analyse associations between factors
with P<0.25 in univariate analysis and the detection
of resistance mutations. IBM SPSS Statistics
Subscription (Windows version, IBM Corp, Armonk
[NY], US) was used for data analysis.
The STROBE statement reporting guidelines
were followed in this study.
Results
In total, 285 non-duplicated specimens from
285 patients were included in this study. The mean
patient age was 35.5 years (range, 16-76 years); 23.9%
of the patients (n=68) were in the younger age-group
(≤25 years). Of the 285 patients, 59.6% (n=170) were
new patients without a previous relevant testing
record. In all, 18.9% of the patients (n=54) had a
documented history of STI. None were known
human immunodeficiency virus carriers; however,
human immunodeficiency virus status was not
available for seven patients. Regarding the current
clinic visit, 60.7% of the patients (n=173) were
symptomatic, and 7.4% (n=21) had MG-positive
test results (14 specimens exhibited MG alone;
7 specimens exhibited MG and another pathogen).
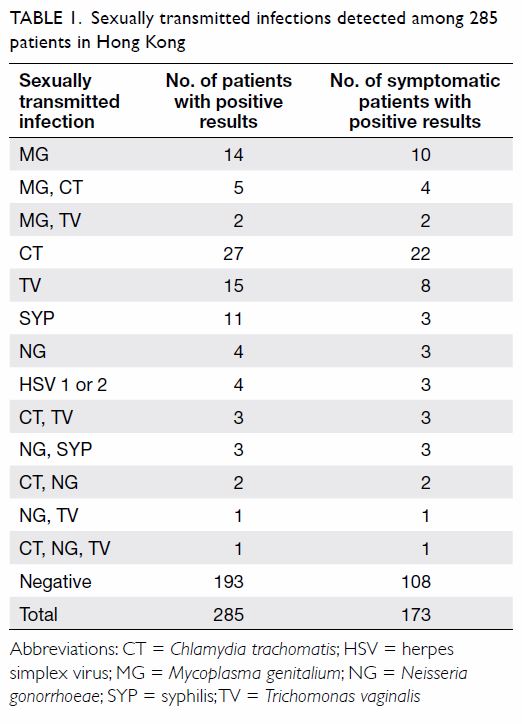
Table 1 shows the numbers of STIs detected among
these 285 patients.
Younger age, previous STI clinic visit, history
of STI, symptoms, and sexually transmitted
co-infections were not associated with the detection
of MG (Table 2). Symptoms were only significantly
associated with the detection of CT (Table 3).
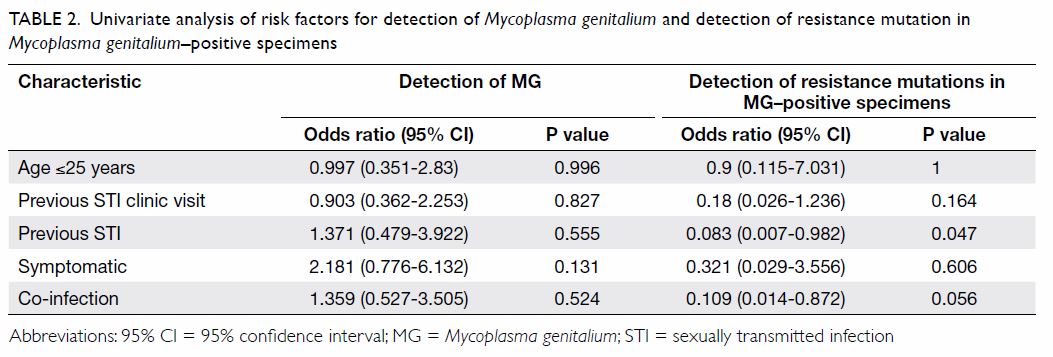
Table 2. Univariate analysis of risk factors for detection of Mycoplasma genitalium and detection of resistance mutation in Mycoplasma genitalium–positive specimens
Among the 21 MG-positive endocervical
swabs, sequencing results for 23S rRNA were
available for 19 specimens. In total, 42.1% of the
specimens (n=8) harboured the macrolide resistance-mediating
mutations A2071G or A2072G. Regarding
parC and gyrA, sequencing results were available
for 20 specimens. Overall, 65% of the specimens
(n=13) harboured the FQ resistance–related
mutations G248T (Ser83Ile), G259T (Asp87Tyr),
or G259A (Asp87Asn) within parC; no mutations
were detected in gyrA. Among the 19 specimens
with sequencing results available regarding both
macrolide and FQ resistance, dual resistance was
detected in 42.1% of the specimens (n=8); thus, all
macrolide-resistant strains were also resistant to FQ.
Furthermore, C184T (Pro62Ser) in parC, for which
clinical significance is unknown, was detected in one
specimen without any other mutation and in one
specimen with dual resistance.
In subgroup analysis of patients with
MG-positive specimens, younger age, previous STI
clinic visit, symptoms, and sexually transmitted
co-infections were not associated with the detection
of resistance mutations, similar to the findings
among all specimens; however, a history of STI was negatively associated with the detection of mutations
(Table 2). This association did not remain after
multivariable logistic regression (odds ratio=0.151,
95% confidence interval=0.004-2.983, P=0.221).
Discussion
In this study, 7.4% of endocervical swabs from
women attending STI clinics exhibited MG-positive
results, although no assessed factors were obviously
associated with the detection of MG. Mycoplasma
genitalium–positive rates did not significantly differ
(P=0.131) among patients who were symptomatic
(9.2%) and those who were asymptomatic (4.5%).
These rates were comparable with the findings of the
aforementioned 2008 cross-sectional study in Hong
Kong involving male patients with STI,9 as well as
with the findings of a multicentre clinical study in the
US.15 Reported rates of MG detection have exhibited
considerable variability. A 2018 systematic review2
revealed that higher rates were prevalent among
at-risk groups (eg, commercial sex workers and men
who have sex with men), in clinic-based settings, and
in countries with lower economic development. In Australia, the prevalence of MG was found to range
from 2.1% to 13%, depending on the population
tested16; while a higher prevalence of approximately
15% has been reported in Japan.17 Nevertheless,
the prevalence in the general population and
asymptomatic patients remained low (1.3%),2
which did not support universal screening. In the
present study, symptoms were not associated with
the detection of MG. Considering the organism’s
uncertain clinical significance and natural history,
it is necessary to balance the need to test and the
risks of unnecessary treatment, including potential
aggravation of antibiotic resistance. We agree with
the existing recommendation that testing should be
carefully selected, reserved only for patients with
increased risks or for whom treatment has failed, as
well as their contacts.18
Importantly, the choice of specimen might
affect the detection of MG. A recent prospective,
multicentre study showed that self or clinician-collected
vaginal swabs exhibited the best sensitivities
(92%-98.9%), while urinary and endocervical swabs
were less sensitive (81.5% and 77.8%, respectively).15
These findings were consistent with the results of
prior studies19 20 21; notably, some studies found that
endocervical swabs were more sensitive than urinary
specimens,20 21 presumably because of the lower
bacterial load in urine.22 Endocervical swabs are
the routine specimens sent to our laboratory from
STI clinics for molecular detection of CT and NG;
we perform assays for detection of MG and CT, as
recommended by European guidelines.6 Of note, the
relatively low sensitivity of the test with respect to
endocervical swabs might also have underestimated
the prevalence of MG in our study.
Resistance-related mutations in 23S rRNA
and parC genes were detected in 42.1% and 65% of
MG-positive samples, respectively, among which
none harboured mutations in gyrA. All macrolide-resistant
strains were also FQ-resistant (42.1%).
Although the populations have differed among
studies, similarly high rates of macrolide resistance have been reported, while rates of FQ and dual
resistance have varied among regions (Table 4).3 23 24 25 26 27 28
Several studies also demonstrated consistent
increases in resistance rates over time.3 28 29
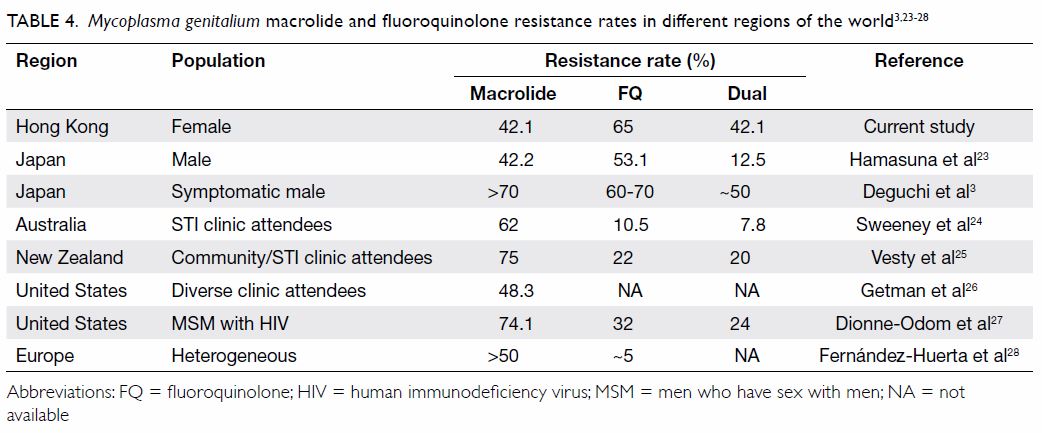
Table 4. Mycoplasma genitalium macrolide and fluoroquinolone resistance rates in different regions of the world3 23 24 25 26 27 28
All mutations detected in this study have been
described previously; while C184T (Pro62Ser) in
parC is of unknown significance, others are known
to confer antibiotic resistance leading to higher
minimal inhibitory concentrations and treatment
failure.5 11 12 23 In particular, the extent of FQ resistance
is reportedly related to the presence of concurrent
parC and gyrA mutations. Although MG strains with
lone parC mutations had reduced susceptibilities to
FQ, they were able to be eradicated by sitafloxacin
and (possibly) moxifloxacin. However, concurrent
gyrA mutations have been shown to further increase
the FQ minimal inhibitory concentrations, leading
to treatment failure.23 30
No assessed factors were significantly
associated with the detection of MG in this study,
possibly due to the limited number of positive
samples. The authors of other studies have suggested
that a syndromic approach (ie, management of a
patient whereby a syndrome is used as a basis for
the treatment of the causative organisms) and the
use of a single dose of azithromycin for treatment
of NG (as part of dual therapy), non-gonococcal
urethritis/non-specific genital tract infection, or
known MG infection contribute to the emergence
of macrolide resistance in MG, because this regimen
is suboptimal and might exert selective pressure
on resistant strains.28 29 31 A similar phenomenon
has been observed with respect to FQ, especially
in Japan, where frequent use of the second-line
antibiotic sitafloxacin caused selection of resistant
strains, leading to high rates of FQ resistance in
MG.3 In public clinics in Hong Kong, a single dose
of azithromycin or a 1-week course of doxycycline
is used as empirical treatment for non-gonococcal urethritis or non-specific genital tract infection.
If no culprit pathogen is identified and the patient
complains of persistent symptoms during follow-up,
a 1-week course of moxifloxacin for possible MG
is considered, following exclusion of other causes
(eg, non-compliance). These empirical uses of
macrolide and FQ regimens might explain the
high rates of resistance found in this study. For
other regions with lower rates of FQ resistance, the
relationship between FQ use and emergence of its
resistance in MG requires further investigation.
In the context of increasing drug resistance,
international guidelines have suggested follow-up
molecular testing for resistance determinants in
MG-positive specimens.6 7 8 In particular, the most
recent British and Australian guidelines include
revised treatment regimens, which suggest 1 week
of doxycycline followed by 3 days of azithromycin as
treatment for macrolide-sensitive (or susceptibility
unknown) MG, or followed by 7 to 10 days of
moxifloxacin as treatment for macrolide-resistant
MG.7 8 Alternative antibiotics that might be effective
(eg, pristinamycin) require further evaluation.32 33
When the detection of MG and its drug
resistance profile is considered after patient selection
and careful review of clinical indications, testing
at a private laboratory may be sought, because this
service is not readily available in the public sector
in Hong Kong. Our findings of high macrolide and
FQ resistance rates in MG implied that the use of
azithromycin and moxifloxacin as empirical first- and
second-line therapies, respectively, might be
ineffective; furthermore, this approach could induce
greater drug resistance. These findings should be
taken into consideration in future assessments of
treatment guidelines for Hong Kong. The acquisition
of updated treatment strategies from international
guidelines may also be useful.
Because our laboratory is a reference laboratory that serves all public STI clinics in Hong Kong, our
database is comprehensive in terms of laboratory
testing records. Specimens in this study were unique
and not duplicated for any patient. In addition, we
have considerable capacity to perform arrays of
confirmatory tests for various sexually transmitted
pathogens. However, this study was limited by the
absence of other clinical information such as sexual
practices, antimicrobials prescribed, and treatment
outcomes, because these data were not available
to the authors. The small number of MG-positive
samples also limited our ability to assess correlations
with factors considered in this study.
Conclusion
Mycoplasma genitalium was detected in 7.4%
of 285 endocervical swabs collected from both
symptomatic and asymptomatic women attending
STI clinics in Hong Kong. Among the MG-positive
samples, macrolide resistance-mediating mutations
and fluoroquinolone resistance–related mutations
were detected in 42.1% and 65%, respectively.
Dual resistance was also detected in all macrolide-resistant
strains (42.1%). These findings suggest that
both testing and treatment strategies require careful
review to avoid further enhancing the prevalence of
antibiotic resistance.
Author contributions
Concept or design: KKM Ng, PKL Leung.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: KKM Ng.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: KKM Ng.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors acknowledge the excellent work and contributions
by staff at the Special Investigation Laboratory of Public Health
Laboratory Services Branch, Centre for Health Protection,
Department of Health, Hong Kong SAR Government.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study obtained ethics approval (Ref LM 424/2019) from
the Ethics Committee of the Department of Health, Hong
Kong SAR Government. Patients consented to testing for
sexually transmitted pathogens.
References
1. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma
genitalium infection and female reproductive tract disease:
a meta-analysis. Clin Infect Dis 2015;61:418-26. Crossref
2. Baumann L, Cina M, Egli-Gany D, et al. Prevalence of
Mycoplasma genitalium in different population groups:
systematic review and meta-analysis. Sex Transm Infect
2018;94:255-62. Crossref
3. Deguchi T, Ito S, Yasuda M, et al. Surveillance of the
prevalence of macrolide and/or fluoroquinolone resistance-associated
mutations in Mycoplasma genitalium in Japan. J
Infect Chemother 2018;24:861-7. Crossref
4. Lau A, Bradshaw CS, Lewis D, et al. The efficacy of
azithromycin for the treatment of genital Mycoplasma
genitalium: a systematic review and meta-analysis. Clin
Infect Dis 2015;61:1389-99. Crossref
5. Murray GL, Bradshaw CS, Bissessor M, et al. Increasing
macrolide and fluoroquinolone resistance in Mycoplasma
genitalium. Emerg Infect Dis 2017;23:809-12. Crossref
6. Jensen JS, Cusini M, Gomberg M, Moi H. 2016 European
guideline on Mycoplasma genitalium infections. J Eur
Acad Dermatol Venereol 2016;30:1650-6. Crossref
7. Australasian Sexual Health Alliance. Australian STI
management guidelines for use in primary care. Available
from: http://www.sti.guidelines.org.au/sexually-transmissible-infections/mycoplasma-genitalium. Accessed 15 Mar 2020.
8. Soni S, Horner P, Rayment M, et al. British Association
for Sexual Health and HIV national guideline for the
management of infection with Mycoplasma genitalium
(2018). Int J STD AIDS 2019;30:938-50. Crossref
9. Yu JT, Tang WY, Lau KH, et al. Role of Mycoplasma
genitalium and Ureaplasma urealyticum in non-gonococcal
urethritis in Hong Kong. Hong Kong Med J
2008;14:125-9.
10. US Food and Drug Administration. cobas TV/MG
Premarket Notification 510(k). Table 34. Available from:
https://www.accessdata.fda.gov/cdrh_docs/pdf19/K190433.pdf. Accessed 15 Mar 2020.
11. Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R.
Azithromycin treatment failure in Mycoplasma
genitalium–positive patients with nongonococcal
urethritis is associated with induced macrolide resistance.
Clin Infect Dis 2008;47:1546-53. Crossref
12. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance
and azithromycin failure in a Mycoplasma genitalium–
infected cohort and response of azithromycin failures
to alternative antibiotic regimens. Clin Infect Dis
2015;60:1228-36. Crossref
13. Peuchant O, Ménard A, Renaudin H, et al. Increased
macrolide resistance of Mycoplasma pneumoniae in
France directly detected in clinical specimens by real-time
PCR and melting curve analysis. J Antimicrob Chemother
2009;64:52-8. Crossref
14. Shimada Y, Deguchi T, Nakane K, et al. Emergence of
clinical strains of Mycoplasma genitalium harbouring
alterations in ParC associated with fluoroquinolone
resistance. Int J Antimicrob Agents 2010;36:255-8. Crossref
15. Gaydos CA, Manhart LE, Taylor SN, et al. Molecular
testing for Mycoplasma genitalium in the United States:
results from the AMES Prospective Multicenter Clinical
Study. J Clin Microbiol 2019;57:e01125-19. Crossref
16. Trevis T, Gossé M, Santarossa N, Tabrizi S, Russell D, McBride WJ. Mycoplasma genitalium in the Far North
Queensland backpacker population: an observational
study of prevalence and azithromycin resistance. PLoS
One 2018;13:e0202428. Crossref
17. Hamasuna R. Mycoplasma genitalium in male urethritis: diagnosis and treatment in Japan. Int J Urol 2013;20:676-
84. Crossref
18. Stewart JD, Webb BQ, Francis M, Graham M, Korman TM.
Should we routinely test for Mycoplasma genitalium when
testing for other sexually transmitted infection? Med J Aust
2020;212:30-1. Crossref
19. Jensen JS, Björnelius E, Dohn B, Lidbrink P. Comparison of
first void urine and urogenital swab specimens for detection
of Mycoplasma genitalium and Chlamydia trachomatis by
polymerase chain reaction in patients attending a sexually
transmitted disease clinic. Sex Transm Dis 2004;31:499-507. Crossref
20. Wroblewski JK, Manhart LE, Dickey KA, Hudspeth MK,
Totten PA. Comparison of transcription-mediated
amplification and PCR assay results for various genital
specimen types for detection of Mycoplasma genitalium. J
Clin Microbiol 2006;44:3306-12. Crossref
21. Lillis RA, Nsuami MJ, Myers L, Martin DH. Utility of
urine, vaginal, cervical, and rectal specimens for detection
of Mycoplasma genitalium in women. J Clin Microbiol
2011;49:1990-2. Crossref
22. Murray GL, Danielewski J, Bodiyabadu K, et al. Analysis
of infection loads in Mycoplasma genitalium clinical
specimens by use of a commercial diagnostic test. J Clin
Microbiol 2019;57:e00344-19. Crossref
23. Hamasuna R, Le PT, Kutsuna S, et al. Mutations
in ParC and GyrA of moxifloxacin-resistant and
susceptible Mycoplasma genitalium strains. PLoS One
2018;13:e0198355. Crossref
24. Sweeney EL, Trembizki E, Bletchly C, et al. Levels of
Mycoplasma genitalium antimicrobial resistance differ
by both region and gender in the state of Queensland,
Australia: implications for treatment guidelines. J Clin Microbiol 2019;57:e01555-18.Crossref
25. Vesty A, McAuliffe G, Roberts S, Henderson G, Basu I.
Mycoplasma genitalium antimicrobial resistance in
community and sexual health clinic patients, Auckland,
New Zealand. Emerg Infect Dis 2020;26:332-5. Crossref
26. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma
genitalium prevalence, coinfection, and macrolide
antibiotic resistance frequency in a multicenter clinical
study cohort in the United States. J Clin Microbiol
2016;54:2278-83. Crossref
27. Dionne-Odom J, Geisler WM, Aaron KJ, et al. High
prevalence of multidrug-resistant Mycoplasma genitalium
in human immunodeficiency virus–infected men who have
sex with men in Alabama. Clin Infect Dis 2018;66:796-
8. Crossref
28. Fernández-Huerta M, Barberá MJ, Serra-Pladevall J, et al.
Mycoplasma genitalium and antimicrobial resistance
in Europe: a comprehensive review. Int J STD AIDS
2020;31:190-7. Crossref
29. Martens L, Kuster S, de Vos W, Kersten M, Berkhout H,
Hagen F. Macrolide-resistant Mycoplasma genitalium in
southeastern region of the Netherlands, 2014-2017. Emerg
Infect Dis 2019;25:1297-303. Crossref
30. Murray GL, Bodiyabadu K, Danielewski J, et al.
Moxifloxacin and sitafloxacin treatment failure in
Mycoplasma genitalium infection: association with parC
mutation G248T (S83I) and concurrent gyrA mutations. J
Infect Dis 2020;221:1017-24.
31. Horner P, Ingle SM, Garrett F, et al. Which azithromycin
regimen should be used for treating Mycoplasma
genitalium? A meta-analysis. Sex Transm Infect
2018;94:14-20. Crossref
32. Bradshaw CS, Jensen JS, Waites KB. New horizons
in Mycoplasma genitalium treatment. J Infect Dis
2017;216:S412-9. Crossref
33. Read TR, Jensen JS, Fairley CK, et al. Use of pristinamycin
for macrolide-resistant Mycoplasma genitalium infection.
Emerg Infect Dis 2018;24:328-35. Crossref