© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Kaposi’s sarcoma presenting with multiple
cervical lymphadenopathies in a renal transplant recipient: a case report
TL Leung, MRCP (UK), LY Wong, FHKAM (Medicine), A Cheuk, FHKAM (Medicine)
Department of Medicine and Geriatrics, Princess Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr TL Leung (aaronleungtl@hotmail.com)
Case report
A 57-year-old man underwent cadaveric renal
transplantation in January 2018 and was prescribed
mycophenolate mofetil, tacrolimus, and prednisolone
as post-transplant immunosuppressive therapy. He
developed multiple cervical lymphadenopathies at 6
months after transplantation. Fine needle aspiration
cytology of the left submandibular lymph node,
performed in the private sector, revealed only a
hypocellular lesion. Considering the possibility of a
post-transplantation lymphoproliferative disorder,
excisional biopsy was arranged in our unit, but the
patient defaulted from his appointment.
The patient attended the emergency
department 9 months after transplantation
complaining of shortness of breath. Physical
examination on admission revealed generalised
lymphadenopathy. Chest radiograph showed left
lower, left middle, and right lower zone infiltrates.
Despite the use of empirical piperacillin/tazobactam
and withholding of mycophenolate mofetil, his
condition deteriorated with worsening type one
respiratory failure and increasing bilateral lung
infiltrates on serial chest radiographs. Tacrolimus was
discontinued. However, serial procalcitonin levels
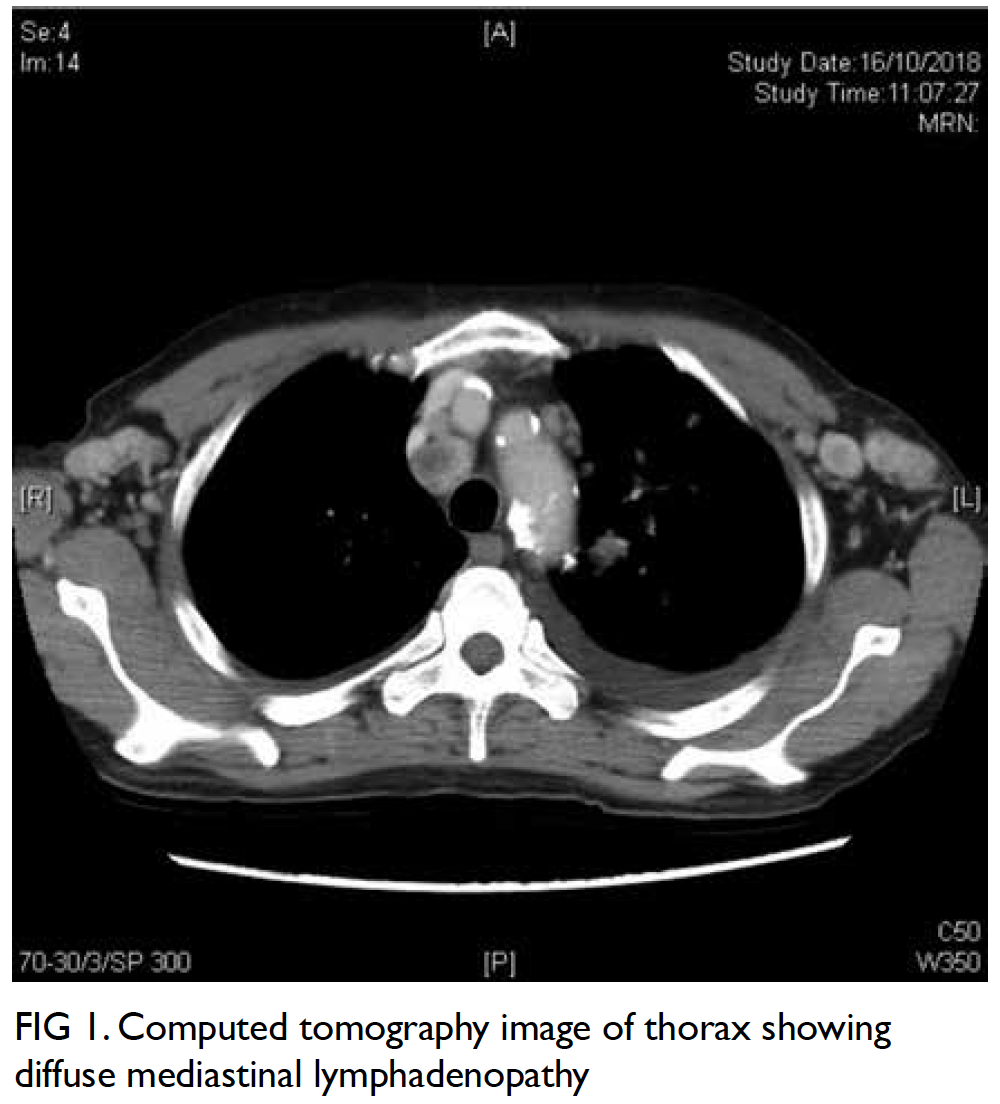
were undetectable. Computed tomography scan of
the thorax suggested focal consolidative changes
with diffuse cervical, axillary, mediastinal, hilar and
abdominal lymphadenopathy (Fig 1). Biopsy of the
groin and cervical lymph node showed spindle cell
proliferation associated with surrounding vascular
channels, red cell extravasation between spindle cells
(Fig 2a), and positive human herpesvirus 8 (HHV-8)
staining (Fig 2b). This confirmed the diagnosis of
Kaposi’s sarcoma (KS). There was no plasmablastic
histopathology to suggest the presence of multi-centric
Castleman disease. Despite maximum
supportive therapy with mechanical ventilation,
empirical antimicrobials and antifungal treatment,
his condition further deteriorated and he succumbed
due to respiratory failure.
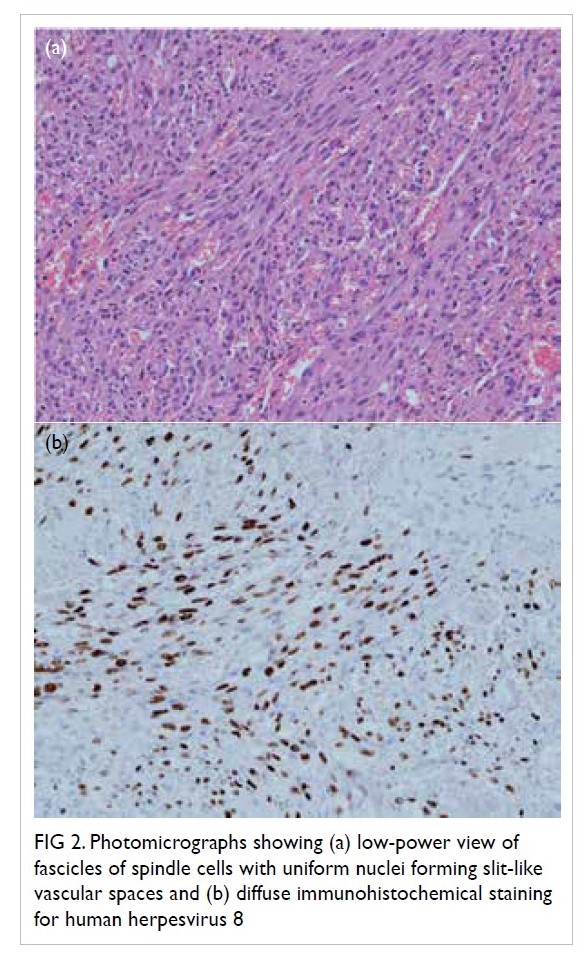
Figure 2. Photomicrographs showing (a) low-power view of fascicles of spindle cells with uniform nuclei forming slit-like vascular spaces and (b) diffuse immunohistochemical staining for human herpesvirus 8
Discussion
Immunosuppressive therapy is known to increase
the risk of infection and malignancy. In a study of incidence of malignancy among a cohort of Hong
Kong kidney transplant recipients from 1972 to
2011, the most prevalent malignancies were non-Hodgkin’s lymphoma followed by colorectal cancer,
lung cancer, kidney cancer, and non-melanoma skin
cancer. Only five cases of KS were reported.1 The low
incidence of KS may be due to the low prevalence
of HHV-8 seroprevalence in Asia.2 There were only
68 reports of KS in Hong Kong between 1983 and
2016 according to the Hong Kong Cancer Registry.
In this report, we describe a case of KS in a renal
transplant recipient who presented with multiple
cervical lymphadenopathies.
In the clinical context of multiple cervical
lymphadenopathies in a post-transplant recipient,
post-transplant lymphoproliferative disorder, and
multi-centric Castleman disease are our initial
top differential diagnoses. However, the histology
of the lymph node of our patient did not suggest
these diagnoses but KS. The clinical presentation
of post-transplant KS can be divided into exclusive dermatological lesions or mucocutaneous lesions,
with or without visceral involvement. Most patients
present with single or multiple pigmented skin lesions
with or without lower limb skin lymphoedema.3 4 5 6 7
Non-cutaneous KS is uncommon and was reported
to account for only 5.4% of KS in a large AIDSassociated
KS cohort.8 The gastrointestinal tract
and the lungs are the most common sites for
visceral involvement.4 7 Lymph node involvement is
commonly associated with diffuse dermatological
lesions or visceral involvement.7 9 Concomitant
lymphoma is a possible but uncommon diagnosis
for multiple cervical lymphadenopathies in a patient
with KS.5 Overall, post-transplant KS that presents
with multiple cervical lymphadenopathies without
skin lesions is rare.
The first case of KS after renal transplantation
was reported in 1969. Its prevalence has been
reported as 0.4% to 5.3%, depending on the
geographical prevalence of HHV-8 seropositivity.10 11
The average time to diagnosis has been reported as 12 to 39 months after transplantation.3 4 10 There is a
male preponderance with a male-to-female ratio of
3:1.4 The 5-year survival rate is around 70%, although
those with visceral involvement generally carry a
poorer prognosis.12
There are no established guidelines for
treatment of post-transplant KS so treatment often
depends on clinical presentation. Tapering or
withdrawal of immunosuppressants is the mainstay
of therapy. Intralesional chemotherapy may be
used for a single dermatological lesion. Systemic
chemotherapy, such as liposomal anthracycline or
taxanes, may be considered for widespread disease.
Complete remission with immunosuppressant
reduction or withdrawal has been reported as 50%
to 60% and graft loss as 20% to 30% in the pre-mammalian
target of rapamycin (mTOR) inhibitor
era.7 10 Treatment with mTOR inhibitor, such as
sirolimus, has gained recognition with its anti-angiogenic
and anti-neoplastic activity, particularly
among patients with an exclusive dermatological
presentation. Three previous case series of 25
patients reported a 100% remission rate after
switching from a calcineurin inhibitor to sirolimus,
while two patients had graft loss due to causes
other than rejection.3 5 6 Other case series have
reported treatment failure with sirolimus. Visceral
involvement and delay in switching from calcineurin
inhibitor to mTOR inhibitor after diagnosis of KS
may contribute to treatment failure.9 Further study
is essential to determine the optimal treatment for
post-transplant KS, especially for those with visceral
involvement.
Conclusion
Post-transplant KS presenting with multiple
cervical lymphadenopathies is rare and may
signify an aggressive subtype. Withdrawing
immunosuppressive therapy alone failed to salvage
our patient. Further study is required to evaluate the
potential of switching mycophenolate mofetil and
calcineurin inhibitor to mTOR inhibitor to improve
the prognosis for this subgroup of patients.
Author contributions
All authors contributed to the concept of the study, acquisition and analysis of the data, drafting of the manuscript, and critical revision of the manuscript for important intellectual
content. All authors had full access to the data, contributed to
the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Acknowledgement
We thank Dr Matthew KL Tong, Dr Hilda WH Chan, Dr Hon-lok Tang, and Dr Samuel KS Fung for their valuable
opinions and supervision on writing the manuscript. We
thank Dr Yuen-fun Mak for her expert guidance on pathology
review.
Declaration
Part of the content about this case was presented in the Nephrology interhospital meeting in September 2019.
Conflict of interest
All authors have disclosed no conflicts of interest.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
The patient was treated in accordance with the Declaration of Helsinki. The patient provided informed consent for all
procedures.
References
1. Cheung CY, Lam MF, Chu KH, et al. Malignancies after kidney transplantation: Hong Kong renal registry. Am J
Transplant 2012;12:3039-46. Crossref
2. Zhang T, Shao X, Chen Y, et al. Human herpesvirus 8 seroprevalence, China. Emerg Infect Dis 2012;18:150-2. Crossref
3. Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J
Med 2005;352:1317-23. Crossref
4. Penn I. Sarcomas in organ allograft recipients. Transplantation 1995;60:1485-91. Crossref
5. Yaich S, Charfeddine K, Zaghdane S, et al. Sirolimus for the treatment of Kaposi Sarcoma after renal transplantation: a series of 10 cases. Transplant Proc 2012;44:2824-6. Crossref
6. Gutiérrez-Dalmau A, Sánchez-Fructuoso A, Sanz-Guajardo A, et al. Efficacy of conversion to sirolimus in
posttransplantation Kaposi’s sarcoma. Transplant Proc
2005;37:3836-8. Crossref
7. Barete S, Calvez V, Mouquet C, et al. Clinical features and contribution of virological findings to the management
of Kaposi sarcoma in organ-allograft recipients. Arch
Dermatol 2000;136:1452-8. Crossref
8. Stebbing J, Mazhar D, Lewis R, et al. The presentation and survival of patients with non-cutaneous AIDS-associated
Kaposi’s sarcoma. Ann Oncol 2006;17:503-6. Crossref
9. Lebbé C, Euvrard S, Barrou B, et al. Sirolimus conversion for patients with posttransplant Kaposi’s sarcoma. Am J
Transplant 2006;6:2164-8. Crossref
10. Montagnino G, Bencini PL, Tarantino A, Caputo R,
Ponticelli C. Clinical features and course of Kaposi’s
sarcoma in kidney transplant patients: report of 13 cases.
Am J Nephrol 1994;14:121-6. Crossref
11. Qunibi W, Akhtar M, Sheth K, et al. Kaposi’s sarcoma: the most common tumor after renal transplantation in Saudi
Arabia. Am J Med 1988;84:225-32. Crossref
12. Woodle ES, Hanaway M, Buell J, et al. Kaposi sarcoma: an analysis of the US and international experiences from
the Israel Penn International Transplant Tumor Registry.
Transplant Proc 2001;33:3660-1. Crossref