Hong
Kong Med J 2019 Jun;25(3):251.e1–3
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Diabetic mastopathy: a breast carcinoma mimic
WK Ng, MB, BS, FRCR1; SK Chan, MB, ChB,
FHKCPath2; KM Kwok, FRCR, FHKAM (Radiology)3; PY
Fung, FRCR, FHKAM (Radiology)3
1 Department of Radiology, Tuen Mun
Hospital, Hong Kong
2 Department of Pathology, Kwong Wah
Hospital, Hong Kong
3 Department of Diagnostic and
Interventional Radiology, Kwong Wah Hospital, Hong Kong
Corresponding author: Dr WK Ng (wingki.ng712@gmail.com)
A 62-year-old woman with a long-standing history of
type 1 diabetes mellitus presented to the breast clinic with a palpable
breast lump. She had incidentally discovered a painless lump in her left
breast that had increased in size over the last 3 months. She
denied nipple discharge or overlying skin changes but reported
a family history of one maternal aunt who had breast cancer diagnosed in
her sixties.
Clinical examination revealed an irregular hard
mass at the upper outer quadrant of the left breast with no evidence of
axillary lymphadenopathy. The right breast was unremarkable.
Mammography showed heterogeneously dense breasts
with asymmetrical density at the left upper breast, but no discrete mass
or spiculations (Fig 1). There were also no suspicious
microcalcifications or architectural distortion. Ultrasonography revealed
an approximately 4-cm irregular hypoechoic lesion with strong posterior
acoustic shadowing at the upper outer quadrant of the left breast and no
increase in vascularity (Fig 2). Overall features were suspicious of
malignancy.
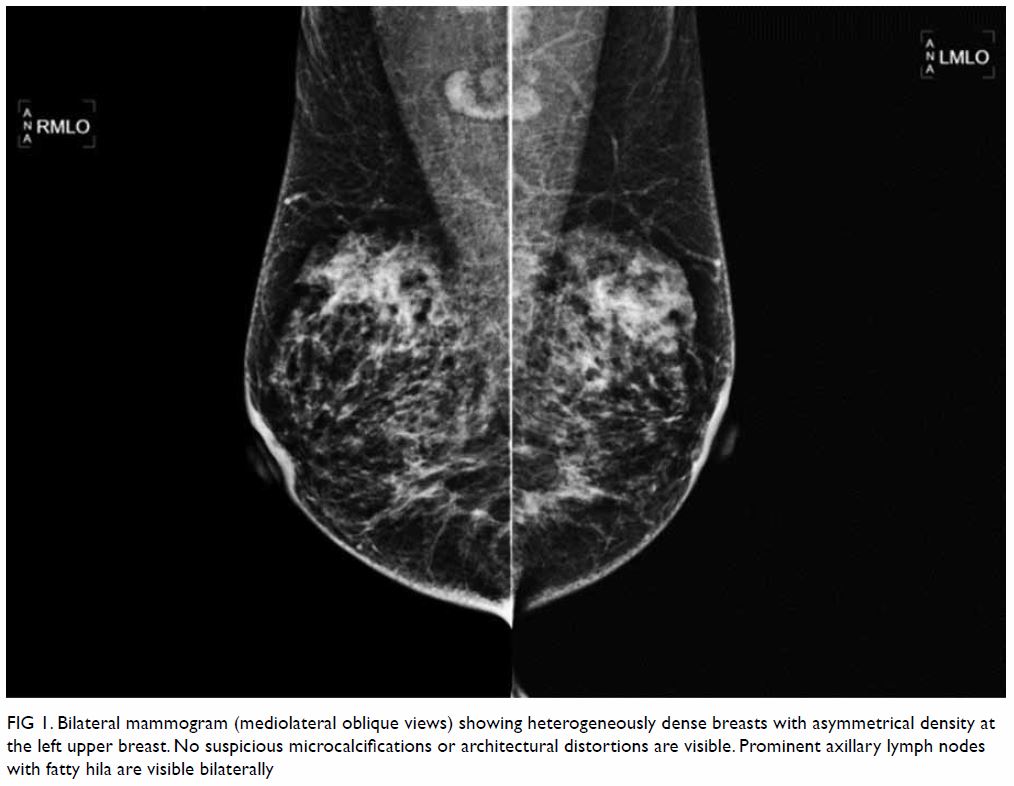
Figure 1. Bilateral mammogram (mediolateral oblique views) showing heterogeneously dense breasts with asymmetrical density at the left upper breast. No suspicious microcalcifications or architectural distortions are visible. Prominent axillary lymph nodes with fatty hila are visible bilaterally
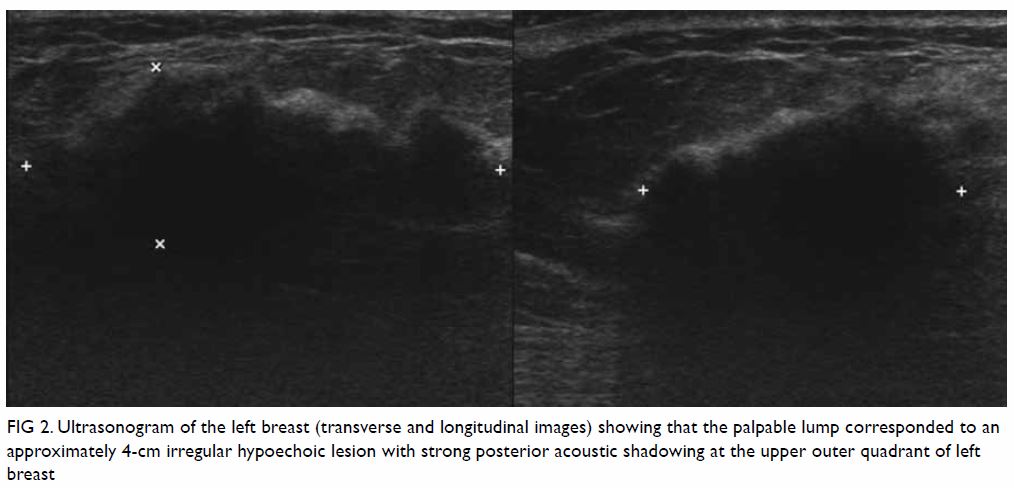
Figure 2. Ultrasonogram of the left breast (transverse and longitudinal images) showing that the palpable lump corresponded to an approximately 4-cm irregular hypoechoic lesion with strong posterior acoustic shadowing at the upper outer quadrant of left breast
Ultrasound-guided core biopsy was performed.
Histological examination showed lymphocytic lobular mastitis associated
with stromal fibrosis of the breast, findings compatible with diabetic
mastopathy (Fig 3).
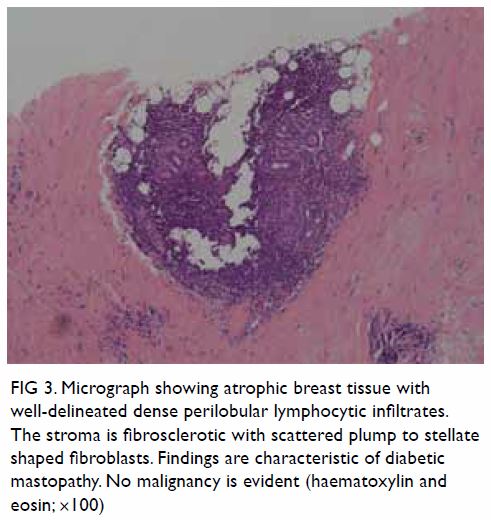
Figure 3. Micrograph showing atrophic breast tissue with well-delineated dense perilobular lymphocytic infiltrates. The stroma is fibrosclerotic with scattered plump to stellate shaped fibroblasts. Findings are characteristic of diabetic mastopathy. No malignancy is evident (haematoxylin and eosin; ×100)
Diabetic mastopathy is a rare fibro-inflammatory
disease of the breast. It is usually seen in association with type 1
diabetes mellitus,1 although rarely
can also been with long-standing type 2 diabetes mellitus. It is typically
found in premenopausal women. Many such patients are known to have other
complications of diabetes mellitus such as retinopathy, nephropathy, and
neuropathy.1 Its exact pathogenesis
is not well understood but likely multifactorial, probably related to an
inflammatory or immunological reaction.
Clinically, diabetic mastopathy often presents as a
hard, painless, irregular breast mass that can also be multiple and
bilateral (60% of the cases). The clinical findings are often suspicious
of breast carcinoma and patients are thus referred for imaging.
On mammogram, diabetic mastopathy may appear as an
ill-defined mass or asymmetric density, without associated calcifications
or spiculations, corresponding to the site of presenting palpable
abnormality, but very often obscured by dense breast tissue.2 On ultrasonogram, diabetic mastopathy appears as an
irregular poorly defined hypoechoic mass of between 2 and 6 cm in size,
with moderate to marked posterior shadowing and absence of vascularity on
colour Doppler imaging.3
Clinical examination and imaging studies cannot
differentiate diabetic mastopathy from breast carcinoma, and ultimately
the diagnosis can only be made on histology from core or excisional
biopsy.
Diabetic mastopathy is a benign entity without
malignant potential4 5 and should therefore be treated conservatively. Surgery
should be avoided as the recurrence rate following surgical excision has
been reported to be rather high at around 32%, and usually within 5 years.6 Recurrences can be single or
multiple, and can occur at the ipsilateral, contralateral, or bilateral
breasts. Clinicians should be aware of this entity if a diabetic patient
presents with a palpable breast lump, after eliminating the possibility of
breast carcinoma. Once this benign condition is diagnosed, the patient
should be advised to perform routine breast self-examination and have
regular clinical breast examinations. If any changes are detected, they
should be referred for imaging and core biopsy performed if necessary.
In summary, diabetic mastopathy is an uncommon but
important benign entity that can mimic breast carcinoma clinically and
radiologically. Ultrasound-guided core needle biopsy of the lesion is
required to establish the diagnosis. Increasing awareness of this
condition and careful correlation of radiological and pathological
findings are essential to avoid unnecessary surgical intervention, reduce
patient anxiety, and ensure optimal patient care.
Author contributions
All authors have made substantial contributions to
the concept or design of the study, acquisition of data, analysis or
interpretation of data, drafting of the manuscript, and critical revision
for important intellectual content. All authors had full access to the
data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to
disclose.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was conducted in accordance with the
principles outlined in the Declaration of Helsinki. The patient provided
verbal informed consent.
References
1. Kudva YC, Reynolds C, O’Brien T, Powell
C, Oberg AL, Crotty TB. “Diabetic mastopathy,” or sclerosing lymphocytic
lobulitis, is strongly associated with type 1 diabetes. Diabetes Care
2002;25:121-6. Crossref
2. Wong KT, Tse GM, Yang WT. Ultrasound and
MR imaging of diabetic mastopathy. Clin Radiol 2002;57:730-5. Crossref
3. Baratelli GM, Riva C. Diabetic fibrous
mastopathy: sonographic-pathologic correlation. J Clin Ultrasound
2005;33:34-7. Crossref
4. Camuto PM, Zetrenne E, Ponn T. Diabetic
mastopathy: a report of 5 cases and a review of the literature. Arch Surg
2000;135:1190-3. Crossref
5. Thorncroft K, Forsyth L, Desmond S,
Audisio RA. The diagnosis and management of diabetic mastopathy. Breast J
2007;13:607-13. Crossref
6. Ely KA, Tse G, Simpson JF, Clarfeld R,
Page DL. Diabetic mastopathy. A clinicopathologic review. Am J Clin Pathol
2000;113:541-5. Crossref

