© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
COMMENTARY
Rechallenge of ponatinib in chronic myeloid
leukaemia after hepatotoxicity
YL Boo, MD, MRCP (UK)1; Christopher CK
Liam, MD, MRCP (UK)2; SG Toh, MRCP (UK)1; SM Lim,
MRCP (UK)1
1 Department of Haematology, Hospital
Sultanah Aminah, Johor Bahru, Malaysia
2 Department of Haematology, Hospital
Ampang, Selangor, Malaysia
Corresponding author: Dr YL Boo (coolrontin@gmail.com)
Commentary
Chronic myeloid leukaemia (CML) is a
myeloproliferative neoplasm associated with an oncogenic fusion gene
breakpoint cluster region-Abelson (BCR-ABL) encoding a protein with
tyrosine kinase activity. Tyrosine kinase inhibitors (TKIs) have
revolutionised the treatment and improved overall survival in patients
with CML. Nonetheless, first- and second-generation TKIs are ineffective
against BCR-ABL T315I mutation. This has led to the development of a
third-generation inhibitor, ponatinib. Ponatinib can cause hepatotoxicity
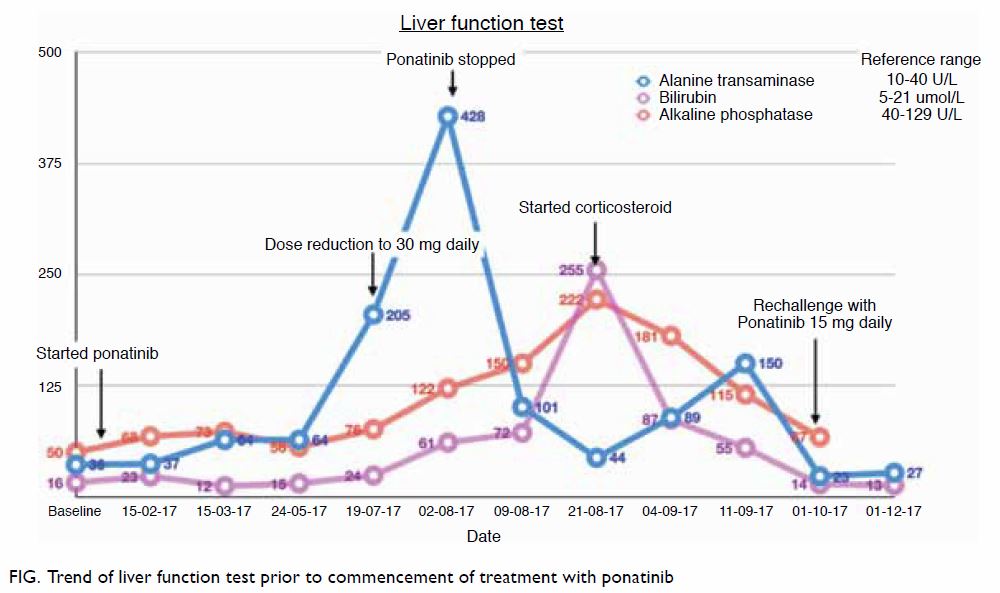
including fulminant hepatic failure and even deaths.1 We recently encountered a case of successful
rechallenge with ponatinib following severe ponatinib-induced
hepatotoxicity that resolved with corticosteroid. A 30-year-old man
diagnosed with CML was started on ponatinib 45 mg once daily after the
discovery of BCR-ABL T315I mutation. Despite initially responding well,
the patient developed hepatotoxicity after 6 months of treatment. The
patient’s alanine aminotransferase level was 5 times the upper normal
limit, and his total bilirubin and alkaline phosphatase levels were
normal. There were no other significant identifiable causes for the
underlying liver impairment. The ponatinib dose was reduced, but the
patient’s alanine aminotransferase level increased further, and he
developed conjugated hyperbilirubinaemia within 2 weeks. Ponatinib was
subsequently withheld, but the patient developed intense pruritus and deep
jaundice. Ultrasound scan of the hepatobiliary system and endoscopic
ultrasonography was normal, autoimmune and viral hepatitis screening tests
were negative, and liver biopsy was consistent with mild steatohepatitis.
Oral prednisolone was started at 40 mg daily, and the patient’s liver
function showed marked improvement after 2 weeks of treatment and
normalised within 1 month of tapering steroids (Fig). The patient was rechallenged with ponatinib at
a lower dose; this was tolerated well and liver function was normal during
subsequent review. Ponatinib was gradually escalated during 6 months of
follow-up without any adverse events. Ponatinib, a third-generation TKI,
has been shown to be effective against T315I mutation. A phase II clinical
trial evaluated ponatinib in patients resistant or intolerant to nilotinib
or dasatinib, or who had T315I mutation produced a major cytogenetic
response in 60% of patients with chronic-phase CML. It also achieved major
haematological response in 52% of patients with accelerated-phase CML, 31%
of patients with blast-phase CML, and 41% of Philadelphia
chromosome–positive patients with acute lymphoblastic leukaemia.2 However, the therapeutic efficacy of ponatinib needs to
be weighed carefully against the risks before commencement of treatment.
Cardiovascular safety profile, hepatotoxicity, pancreatitis, and
cytopenias are among the serious adverse events that may lead to
significant morbidity and mortality.3
The incidence of serum alanine aminotransferase and
aspartate aminotransferase elevations has been reported as 56% for all
grades, and 8% for Grades 3 or 4.1
These adverse events were graded based on National Cancer Institute Common
Terminology Criteria for Adverse Events, version 4.0.4 The presence of serum aminotransferase levels above 3
times the normal upper limit should lead to dose reduction or temporary
cessation of treatment, with resumption at a lower dose once levels
normalise. In patients with clinically apparent liver injury and jaundice,
treatment should be discontinued.1 In a phase I trial of ponatinib,
observed adverse events, which included hepatotoxicity, were
dose-dependent and reported as self-limiting in most of the events.4 Subsequent rechallenge of ponatinib at lower dose was
generally successful; however, Grade 4 adverse events in some patients
warranted cessation of treatment for these patients.4 Successful reversion of imatinib-induced hepatotoxicity
in CML patients has been previously reported.5
We observed rapid improvement in our patient’s liver function following
corticosteroid therapy, and thus managed to avoid permanent
discontinuation of ponatinib in the treatment of T315I mutation CML. The
commencement of ponatinib at lower dose is recommended, with gradual
escalation of dosage as tolerated by the patient. In preclinical studies,
at doses of >30 mg, trough blood concentrations were reported to
completely suppress the emergence of BCR-ABL mutations.4
The pathogenic mechanisms of ponatinib-induced
hepatotoxicity are not fully understood. However, the histopathology
findings of steatohepatitis and its response to corticosteroid suggested
an inflammatory response; this has been previously described in
imatinib-induced hepatotoxicity.6
The clinical finding of severe hepatic impairment was disproportionate to
the liver biopsy findings in our patient, possibly partly contributed to
by the commencement of corticosteroid therapy before biopsy was carried
out. As the patient had no significant past medical illness to suggest
non-alcoholic steatohepatitis, we were unable to conclude its relationship
with the development of hepatic impairment in our patient.
Corticosteroid therapy for severe hepatotoxicity
and steatohepatitis induced by ponatinib has not, to our knowledge, been
previously reported. Corticosteroid therapy and rechallenge of ponatinib
have potential for successfully treating patients with severe
ponatinib-induced hepatotoxicity and T315I mutation CML.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: All authors.
Acquisition of data: YL Boo, SG Toh.
Analysis or interpretation of data: YL Boo, CCK Liam.
Drafting of the manuscript: YL Boo, CCK Liam.
Critical revision for important intellectual content: All authors.
Acquisition of data: YL Boo, SG Toh.
Analysis or interpretation of data: YL Boo, CCK Liam.
Drafting of the manuscript: YL Boo, CCK Liam.
Critical revision for important intellectual content: All authors.
Acknowledgement
The authors would like to thank the Director
General of Health for permission to publish this article.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Patient consent
This study was conducted in accordance with the
Declaration of Helsinki. The patient provided written informed consent for
publication.
References
1. Iclusig [package insert]. Cambridge, MA:
Ariad Pharmaceuticals; 2012.
2. Kantarjian HM, Pinilla-Ibarz J, Le
Coutre PD, et al. Five-year results of the ponatinib phase II PACE trial
in heavily pretreated CP-CML patients (pts). J Clin Oncol 2017;35(Suppl
15):7012. Crossref
3. Price KE, Saleem N, Lee G, Steinberg M.
Potential of ponatinib to treat chronic myeloid leukemia and acute
lymphoblastic leukemia. Onco Targets Ther 2013;6:1111-8.
4. Cortes JE, Kantarjian H, Shah NP, et al.
Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl
J Med 2012;367:2075-88. Crossref
5. Ferrero D, Pogliani EM, Rege-Cambrin G,
et al. Complete reversion of imatinib-induced hepatotoxicity in chronic
myeloid leukemia patients by low-intermediate dose corticosteroid. Blood
2005;106:4856.
6. Ferrero D, Pogliani EM, Rege-Cambrin G,
et al. Corticosteroids can reverse severe imatinib-induced hepatotoxicity.
Haematologica 2006;91(6 Suppl):ECR27.