Hong
Kong Med J 2019 Apr;25(2):102–12 | Epub 10 Apr 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Poisoning by toxic plants in Hong Kong: a 15-year
review
WY Ng, MB, ChB, PhD1; LY Hung, MB, BS1;
YH Lam, MPhil1; SS Chan, MSc1; KS Pang, MSc2;
YK Chong, FHKAM (Pathology)1; CK Ching, FRCPA, FHKAM
(Pathology)1; Tony WL Mak, FRCPath, FHKAM (Pathology)1
1 Hospital Authority Toxicology
Reference Laboratory, Department of Pathology, Princess Margaret Hospital,
Laichikok, Hong Kong
2 Hong Kong Herbarium, Agriculture,
Fisheries and Conservation Department, Hong Kong
Corresponding author: Dr Tony WL Mak (makwl@ha.org.hk)
Abstract
Introduction: Hong Kong has a
great diversity of plants, many of which are toxic to humans. The aim of
this study was to identify the plant species most commonly involved in
cases of plant poisoning in Hong Kong and to provide clinicians with a
reference tool for the diagnosis and management of plant poisoning.
Methods: We retrospectively
reviewed all plant poisoning cases referred to the Hospital Authority
Toxicology Reference Laboratory from 1 January 2003 to 31 December 2017.
Demographics, clinical presentation, laboratory findings, treatment and
outcomes of patients, as well as morphological
identification and analytical testing of the plant specimens, were
investigated.
Results: A total of 62 cases
involving 26 poisonous plant species were identified, among which Alocasia
macrorrhizos (Giant Alocasia), Gelsemium elegans (Graceful
Jessamine), and Rhododendron (Azalea) species were the three most
commonly encountered. Gastrointestinal toxicity (n=30, 48%),
neurological toxicity (n=22, 35%), and hepatotoxicity (n=6, 10%) were
the three most common clinical problems. Forty-nine (79%) and eight
(13%) patients had mild and moderate toxicity, respectively; they all
recovered shortly with supportive treatment. The remaining five (8%)
patients experienced severe toxicity requiring intensive care support.
Most patients (n=61, 98%) used the plants intentionally: as a medicinal
herb (n=31), as food (n=29), and for attempting suicide (n=1). Reasons
for using the poisonous plants included misidentification (n=34, 55%),
unawareness of the toxicity (n=20, 32%), and contamination (n=6, 10%).
Conclusions: Although most plant
exposure resulted in a self-limiting disease, severe poisonings were
encountered. Epidemiology of plant poisonings is geographically
specific. Clinicians should be aware of local poisonous plants and their
toxicities.
New knowledge added by this study
- The three most common plants causing poisoning in Hong Kong were Alocasia macrorrhizos, Gelsemium elegans, and Rhododendron species.
- Plants causing severe and potentially fatal poisonings in Hong Kong were Abrus precatorius, Gelsemium elegans, Rhododendron species, and Emilia sonchifolia.
- Plant poisoning is uncommon but can be severe.
- Raising public awareness minimises unintentional poisoning.
- This study provided useful reference for the local clinicians to diagnose and manage plant poisonings.
Introduction
Many plants are poisonous to humans. Surveys of
various toxicology centres in Australia,1
Germany,2 3 Morocco,4 New
Zealand,5 Sweden,6 Thailand,7 the
United Kingdom,8 and the United
States9 10
showed that plant exposure was responsible for 1.8% to 8% of all
inquiries. Most plant exposures did not result in significant toxicity,
but severe and life-threatening poisonings have been reported. As reported
by the Moroccan Poison Control Centre from 1980 to 2011, plants were the
cause of approximately 5% of all fatal cases of intoxication encountered.4 Consumption of wild plants as
“medicinal herbs” or food is not an uncommon practice in Hong Kong, and
severe plant poisoning cases have been reported.11
12 13
14 15
16 17
18 19
However, local epidemiology data on plant poisoning are sparse and
limited.
Owing to the variable clinical features and rare
occurrence of plant poisonings, diagnosing and treating these patients
remain a challenge to our frontline clinicians. Although history of plant
exposure is important, it is often insufficient to pinpoint the
incriminating toxic plant. Misidentification of plant is a common cause of
poisoning in the first place. Morphological identification of a poisonous
plant and biochemical confirmation are generally not available to guide
immediate clinical management. Therefore, clinical features are critical
for initiating supportive treatment, which is a common strategy for
treating poisonings of an uncertain nature.
Classifications of clinically significant plant
toxins have been proposed in order to aid rapid recognition and management
of these patients.20 21 Plant toxins can be broadly classified into four
groups: (1) cardiotoxic toxins, such as cardiac glycosides; (2) neurotoxic
toxins, such as gelsemium alkaloids, grayanotoxins, solanaceous tropane
(anticholinergic) alkaloids, strychnine, and brucine; (3) cytotoxic
toxins, such as colchicine, mimosine, plumbagin, toxalbumins; vinblastine,
and vincristine; and (4) gastrointestinal-hepatotoxic toxins, such as
amaryllidaceous alkaloids, calcium oxalate raphide, cycasin, pentacyclic
triterpenoids, phorbol esters, phytolaccatoxins, plumericin, pyrrolizidine
alkaloids, saponins, steroidal alkaloids, tetrahydropalmatine, and
teucvin.
The Hospital Authority Toxicology Reference
Laboratory (HATRL), the only tertiary clinical toxicology laboratory in
Hong Kong, provides support to all local hospitals in managing patients
with complex poisoning problem, including cases of plant poisonings.
Besides plant identification, HATRL provides specialised toxicology
testing for certain plant toxins, especially for those with significant
clinical toxicity and management impact.16
22 The aim of the present study
was to identify the plant species most commonly involved in cases of plant
poisoning in Hong Kong, in order to promote awareness among local
clinicians and to provide a reference for diagnosing and managing plant
poisonings.
Methods
All cases of suspected plant-related poisoning
referred to the HATRL from January 2003 to December 2017 were
retrospectively reviewed. Clinical data were collected by reviewing the
laboratory database and patient medical records. Demographic
characteristics, clinical presentation, drug history, laboratory and
toxicological findings, progress, and outcomes of these patients were
reviewed. The causal relationships between the clinical presentation and
plant use were evaluated based on known adverse effects of the specified
plant or toxins, the temporal sequence, exclusion of other underlying or
concurrent diseases which could otherwise account for the clinical
presentation, and progress after discontinuation of plant use. Severity of
the poisoning cases was graded according to an established poisoning
severity score as follows23: mild,
transient, and spontaneously resolving symptoms (mild); pronounced or
prolonged symptoms (moderate); severe or life-threatening symptoms
(severe); and fatal poisoning (fatal). Descriptive statistics were used to
present the results.
In collaboration with the Hong Kong Herbarium,
Agriculture, Fisheries and Conservation Department, available plant
specimens were sent for morphological identification. Mass
spectrometry–based and microscopy-based tests for specific plant toxin(s)
were developed, and performed on selected cases, guided by clinical
presentation of the patients and types of the toxic plant(s) exposed. Mass
spectrometry–based analytical platforms can identify the majority of the
clinically significant plant toxins affecting the cardiovascular,
neurological, gastrointestinal, hepatological, and renal systems in plant
and biological specimens. The poisonous plants commonly encountered in
Hong Kong and the corresponding plant toxins are summarised in Table
1.24
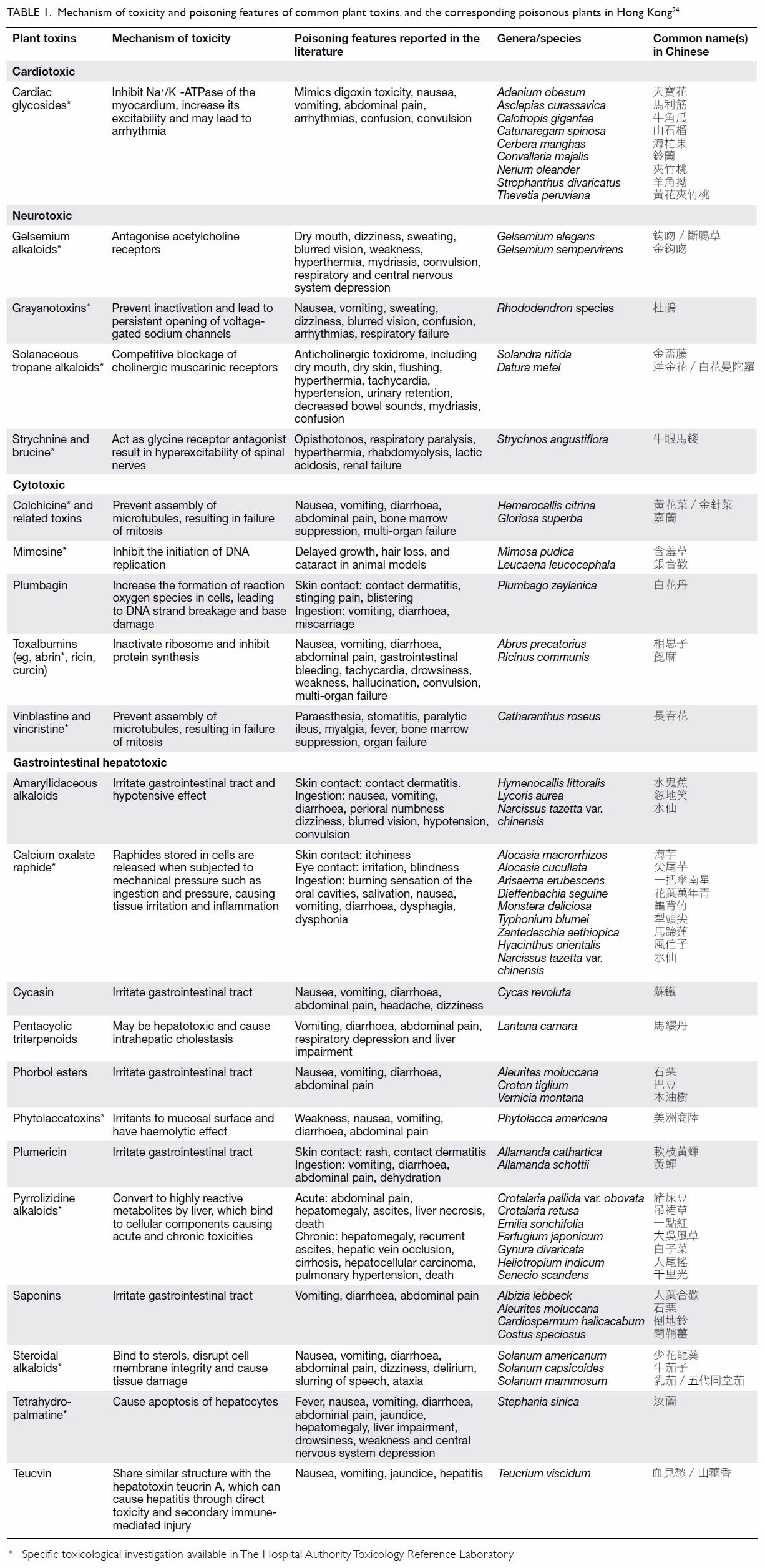
Table 1. Mechanism of toxicity and poisoning features of common plant toxins, and the corresponding poisonous plants in Hong Kong24
The ethics committee exempted the study group from
obtaining patient consent because the presented data were anonymised, and
the risk of identification was low. The STROBE guidelines were used to
ensure the reporting of this study.25
Results
A total of 62 cases of confirmed plant poisoning,
involving 26 plant species, were identified within the study period. The
patients were referred from 14 local hospitals administered by the
Hospital Authority. Almost all patients (n=60, 97%) were Chinese, 29 (47%)
were male, and 33 (53%) were female. The median age was 50 years (range, 1
month to 83 years), including three children (range, 1-23 months) and four
adolescents (range, 15-18 years). The route of exposure was oral in 60
(97%) patients and topical in two (3%) patients. The majority of the
patients (n=55, 89%) developed acute toxic symptoms after a single use of
the plants, while the remaining patients (n=7, 11%) reported a history of
prolonged exposure. Details of the plant poisoning cases, including the
involved plant species and toxins, clinical presentation and outcome of
these patients are summarised in Table 2.
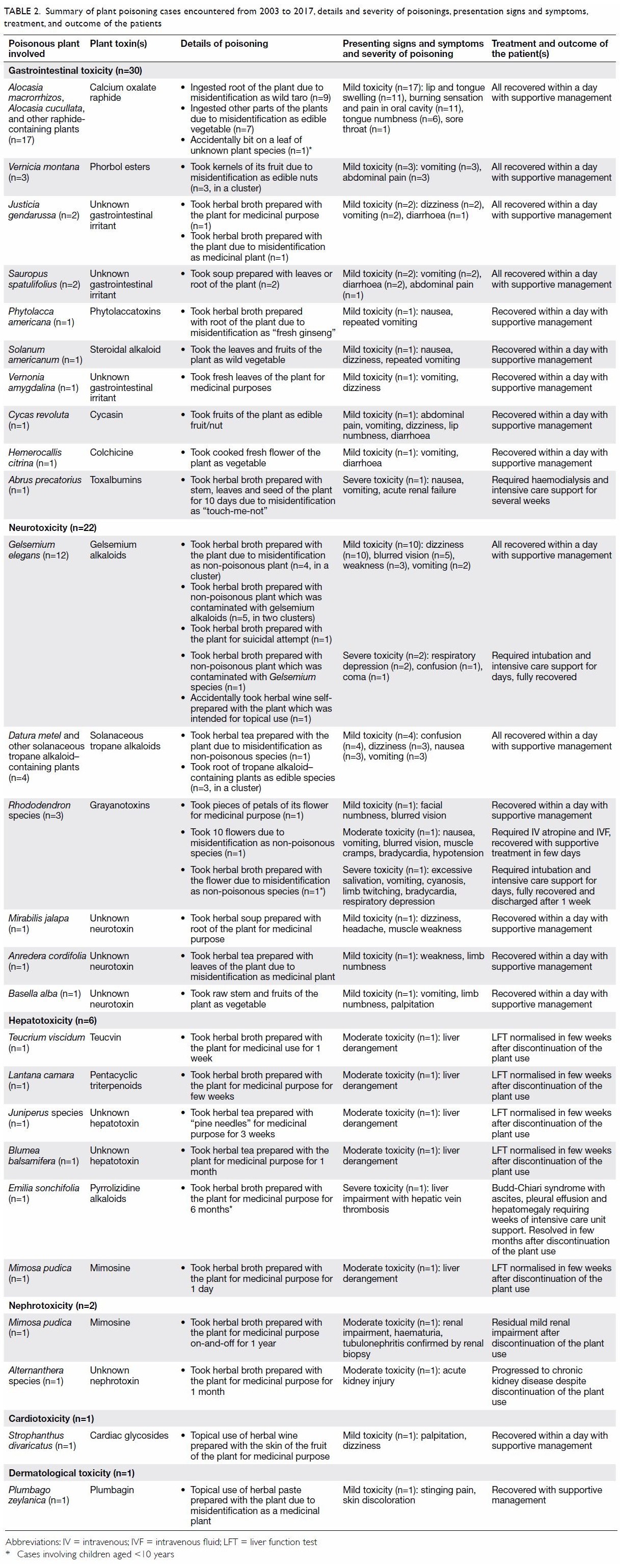
Table 2. Summary of plant poisoning cases encountered from 2003 to 2017, details and severity of poisonings, presentation signs and symptoms, treatment, and outcome of the patients
Gastrointestinal toxicity was the most common
clinical presentation (n=30, 48%). Of these 30 patients, 17 developed
oromucosal irritation after ingestion of calcium oxalate
raphide–containing plants. The other 13 patients presented with
gastroenteritis-like symptoms, such as nausea, vomiting, abdominal pain
and diarrhoea, after ingestion of plant containing gastrointestinal
irritants (n=11) or cytotoxic toxins (n=2). Neurological toxicity was the
second most common presenting symptoms (n=22, 35%), mainly caused by
gelsemium alkaloids (n=12), Solanaceous tropane alkaloids (n=4), and
grayanotoxins (n=3). Hepatotoxicity was encountered in six (10%) patients.
Five patients had abnormal liver function test results owing to the use of
teucvin (n=1), pyrrolizidine alkaloids (n=1), and other unknown
hepatotoxin(s) (n=3); one patient developed cholestasis after exposure to
pentacyclic triterpenoids. Cases of nephrotoxicity (n=2, 3%),
cardiotoxicity (n=1, 2%), and dermatological toxicity (n=1, 2%) were also
recorded.
Most of the patients experienced mild (n=49, 79%)
or moderate toxicity (n=8, 13%). Of them, 55 patients recovered within
days with supportive treatment. The other two patients with nephrotoxicity
had residual renal impairment after discontinuation of plant use; in one
of them a renal biopsy study showed tubulonephritic changes. The remaining
five patients (8%) experienced severe toxicity after the use of Abrus
precatorius (n=1), Gelsemium elegans (n=2), Rhododendron
species (n=1), and Emilia sonchifolia (n=1), and all five required
intensive care support.
The morphological identification and biochemical
analysis process followed to identify the plants involved in the 62 cases
of intoxication is shown in Figure 2. Most patients provided fresh plant
specimens (n=53, 85%) or photograph of the plant (n=3, 5%) they had
consumed. Among these specimens received by our laboratory, 43 could be
identified morphologically with the aid of the Hong Kong Herbarium,
Agriculture, Fisheries and Conservation Department. Biochemical analysis
for specific plant toxin(s) were attempted in the plant or the biological
specimens in 45 (73%) cases, with 41 yielding diagnostic information,
including six cases with no plant specimens for identification and 13
cases with unsuccessful identification. There were 22 (35%) cases with
both informative morphological identification and biochemical results.
Among these 22 cases, the morphological identification and biochemical
results were coherent in 20. For the remaining two cases with clinical
gelsemium poisoning, gelsemium alkaloids were detected in both the urine
and plant specimens. However, the plant specimen was morphologically
identified as Cassytha filiformis. Cassytha filiformis is
a non-toxic plant that has been shown to parasitise Gelsemium elegans
and absorb gelsemium alkaloids, leading to poisoning.18 Patient accuracy in identification of plants was low.
Although 56 (90%) patients had given common names of the plants they
consumed, only 19 of them were correct.
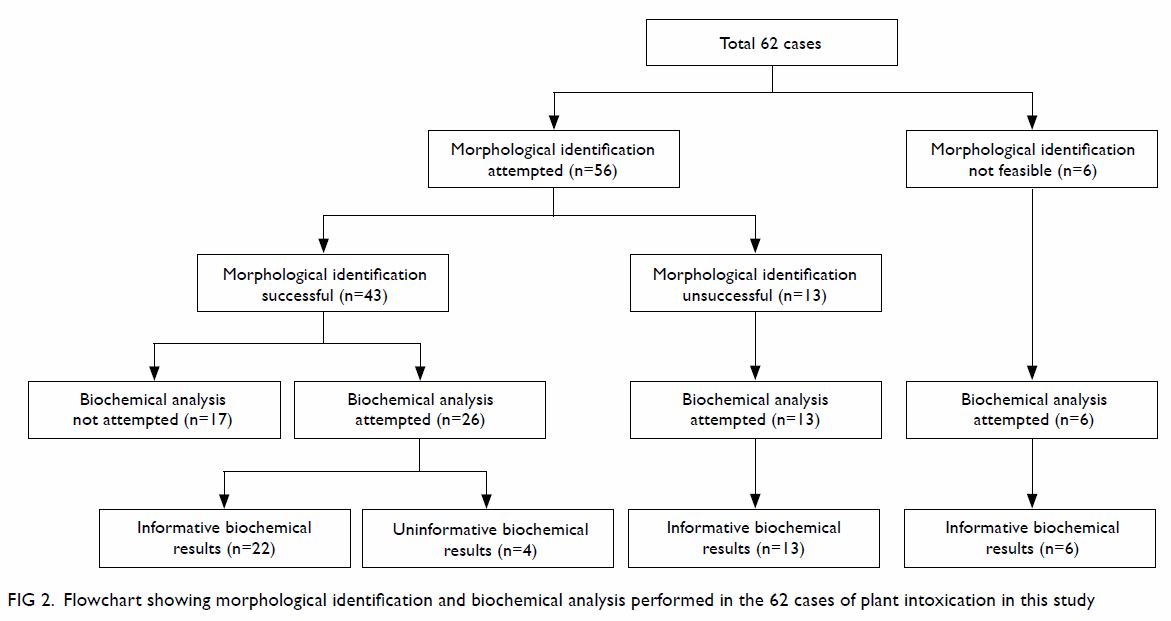
Figure 2. Flowchart showing morphological identification and biochemical analysis performed in the 62 cases of plant intoxication in this study
Patients consumed the plants as medicinal herbs
(n=31, 50%), as food (n=29, 47%), in an attempted suicide (n=1, 2%), or
accidentally, in one paediatric patient (n=1, 2%). Causes of poisoning in
patients using the plants as medicinal herbs or food included plant
misidentification (n=34), unawareness/underestimation of the potential
toxicity (n=20), or contamination of non-toxic plants with poisonous ones
(n=6). The sources of the poisonous plants used included self-collecting
from parks or the countryside (n=37, 60%), obtaining from friends or
relatives (n=20, 32%), growing at home (n=3, 5%), and buying from wet
markets (n=2, 3%). The plants were obtained in Hong Kong (n=49, 79%) or
mainland China (n=12, 19%), with one (2%) case collected from the
Philippines.
Discussion
The 62 cases reported herein represent the largest
series of plant poisoning cases in Hong Kong confirmed by either
morphological identification or biochemical confirmation. Comparing our
results with those of studies from other regions, the pattern of plant
poisoning is considerably different. Plant poisonings reported in Western
countries are predominantly accidental exposure in children,3 7 10 whereas in the present study, most patients were
adults poisoned after intentional use of wild plants. The highly urbanised
and industrialised nature of Hong Kong explains the low incidence of
paediatric accidental poisoning. The relatively high rate of adult
poisoning may be related to the long-standing tradition and Chinese
culture of using wild plant as “medicinal herbs” and “vegetables”.
Recreational abuse of toxic plants, such as Datura species,26 27 which are
responsible for a significant number of poisoning cases reported in other
regions, was not observed locally.
The most common type of poisoning in the current
study was the use of raphide-containing plants, such as Alocasia
macrorrhizos, accounting for more than 25% of cases. The roots of
these plants are frequently mistaken as edible taro.28 Although all cases in this study were mild, severe
outcomes including oropharyngeal oedema, upper airway obstruction, or
systemic toxicity are possible.29
30
Similar to previous reports of plant poisoning,3 5 7 most patients in the present study developed mild
transient symptoms and recovered with supportive management and
discontinuation of exposure. However, there were five cases of severe
poisoning requiring intensive care support, involving the use of Gelsemium
elegans, Rhododendron species, Abrus precatorius,
and Emilia sonchifolia (Fig 1).

Figure 1. The four types of poisonous plants causing severe toxicity in our patients (reproduced from reference 24 with permission)
Gelsemium elegans is one of the most
notorious poisonous plants in Hong Kong and South-East Asian countries and
has been used for homicidal and suicidal purposes.31 32 Severe
gelsemium toxicity can result in respiratory depression and even death. In
our case series, two clusters of “hidden” gelsemium poisoning were
identified where the patients consumed non-toxic parasitic Cassytha
filiformis which absorbed gelsemium alkaloids from Gelsemium
elegans on which it grew.18
Similar cases of hidden gelsemium poisoning from contaminated dried herbs
Ficus hirta have been reported in Hong Kong.16
Rhododendron species and other plants in the
Ericaceae family contain grayanotoxins.33
Severe grayanotoxin poisoning may result in respiratory depression and
arrhythmias.34 “Mad honey”
containing nectar of Ericaceae plants is known to be a source of
exposure in Hong Kong,35 but
poisonings due to direct consumption of Rhododendron flowers are
not uncommon.12
The seed of Abrus precatorius contains the
protein abrin, an extremely poisonous toxin similar to ricin that can
result in multi-organ failure.36 37 These seeds are sometimes used
in beaded jewellery, in addition to being collected, providing another
potential source of exposure.37
Cases of abrin poisoning due to suicidal attempt or accidental ingestion
have been reported worldwide,38 39 40
but such poisoning is rare in Hong Kong.
The toxic constituents in Emilia sonchifolia
are pyrrolizidine alkaloids.41
Massive acute ingestion can lead to hepatotoxicity and coagulopathy, while
chronic low-dose exposure can result in liver cirrhosis and hepatic
veno-occlusive disease.42 43 44 Certain
types of traditional Chinese medicine, such as Flos farfarae and Herba
senecionis scandentis, are potential sources of pyrrolizidine
alkaloids exposure in Hong Kong,45
46 but toxicity due to wild plant
consumption is relatively rare.14
Timely diagnosis of plant poisonings is very
difficult. Local epidemiology data are scarce, and reports published in
other parts of the world are of limited use because plant species are
geographically specific. Patients might volunteer a history of plant
exposure, but the information they provide may be uninformative,
misleading, or incorrect. In our case series, among those who provided a
common name of the plant, only 34% were correct.
Our results demonstrate the importance of adopting
a complementary approach, incorporating clinical toxidrome, morphological
identification of plant specimens, and biochemical analysis of plant
toxins, in order to achieve maximal diagnostic efficacy. Clinical history
may not be particularly informative. The presenting toxidrome is more
objective and useful for identifying the plant responsible for the
poisoning, and this diagnostic approach is well supported in the
literature.20 21
Table 1 summarises and categorises commonly
encountered local poisonous plants by toxidromes. This may serve as quick
reference for clinicians to allow rapid identification of the plant and
prompt management of the poisoning. However, clinicians should also be
aware that the toxicity of some plants may not be well characterised, and
that some plants may give rise to non-specific toxidrome, rendering this
approach less useful in certain cases.
Specialised toxicological investigation may provide
additional evidence to confirm or refute the provisional diagnosis. In
most situations, morphological identification of the plant is sufficient
to achieve a diagnosis, if a well-preserved plant specimen is provided.
However, a plant specimen is not always available; even if available, it
may not be representative of the plants consumed by the patient, or may
have been deformed due to prior processing. An equally important tool is
target testing for specific plant toxin(s) in the plant or biological
specimens based on clinical suspicion. This is a powerful tool for
confirming the diagnosis, especially when plant specimen is not available;
and for identifying cases of contamination with an unclear or hidden
source.18 However, these
investigations cannot aid immediate management in the emergency room.
Diagnosis of plant poisoning requires a proactive
rather than a reactive approach. A knowledge database comprising the
clinical toxicity, morphology, and biochemical analysis of local toxic
plants is an important tool to prepare clinicians and healthcare
professionals, such as that provided by the Hospital Authority.24 This public resource can also be used to educate the
general public on the dangers of wild plants.
As a retrospective study, this study has several
limitations. This study is subjected to selection bias as the data
collection is a passive procedure depending on test or consultation
requests by clinicians, and there might be incomplete or missing data.
Moreover, for those with history of exposure or mild clinical symptoms,
toxicology testing or consultation may not be requested, our data might
therefore include only cases with more severe outcome in the overall
continuum of plant poisoning. Inevitably, some plant exposures were not
reported to our laboratory, and thus our findings may underestimate the
actual number of cases.
Conclusions
Diagnosing plant poisoning is challenging and the
epidemiology of plant poisonings is geographically specific. Clinicians
should consider a complementary approach with consideration of the
clinical toxidromes, local epidemiological data, botanical and
toxicological findings to help recognition of the plants involved in cases
of intoxication. Plant specimens and biological specimens should be saved
whenever possible for toxicological analysis. Clinicians should be aware
of local poisonous plants and their toxicities. Although most plant
exposure resulted in a self-limiting disease, severe poisonings were
encountered. The public should be educated about the potential hazards of
consuming plants obtained from the wild and discouraged from engaging in
this practice.
Author contributions
All authors had full access to the data,
contributed to the study (including concept or design, acquisition of
data, analysis or interpretation of data, drafting of the manuscript, and
critical revision for important intellectual content), approved the final
version for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Declaration
Some of the cases have been published by the
authors in electronic form24 and
in book form (Mak WL, Lam YH, Ching CK, Chan SS, Chong YK, Ng WY, editors.
Atlas of Poisonous Plants in Hong Kong—A Clinical Toxicology Perspective.
Hong Kong: Hospital Authority Toxicology Reference Laboratory; 2016), and
presented at the Hospital Authority Toxicology Services Scientific
Conference 2016 (“Poisonous plants in Hong Kong—a clinical perspective”).
Some of the cases have been previously published as case reports by the
authors and other units.12 13 14 15 16 17 18 19
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Hong Kong Hospital
Authority Kowloon West Cluster Research Ethics Committee (Ref. KW/EX-16-036[96-18(TCM)]).
References
1. Children’s Health Queensland Hospital
and Health Service, Queensland Government. Queensland Poisons Information
Centre Annual report 2014. Available from:
https://www.childrens.health.qld.gov.au/wp-content/uploads/PDF/poisons/annual-reports/2014.pdf.
Accessed 30 May 2018.
2. Müller D, Desel H. Common causes of
poisoning: etiology, diagnosis and treatment. Dtsch Arztebl Int
2013;110:690-9.
3. Plenert B, Prasa D, Hentschel H, Deters
M. Plant exposures reported to the Poisons Information Centre Erfurt from
2001-2010. Planta Med 2012;78:401-8. Crossref
4. Rhalem N, Chaoui H, Lahcen O, Soulaymani
A, Bencheikh RS. Toxic deaths: data from the Poison Control Centre of
Morocco (CAPM). XXXV International Congress of the European Association of
Poisons Centres and Clinical Toxicologists (EAPCCT) 26-29 May 2015, St
Julian’s, Malta. Clin Toxicol 2015;53:265.
5. Slaughter RJ, Beasley DM, Lambie BS,
Wilkins GT, Schep LJ. Poisonous plants in New Zealand: a review of those
that are most commonly enquired about to the National Poisons Centre. N Z
Med J 2012;125:87-118.
6. Swedish Poisons Information Centre.
Swedish Poisons Information Centre. Annual Report 2015. Available from:
https://giftinformation.se/globalassets/publikationer/annual-report-2015.pdf.
Accessed 18 May 2018.
7. Sriapha C, Tongpoo A, Wongvisavakorn S,
et al. Plant poisoning in Thailand: a 10-year analysis from Ramathibodi
Poison Center. Southeast Asian J Trop Med Public Health 2015;46:1063-76.
8. Public Health England. National Poisons
Information Service Report 2016/17. Available from:
http://www.edinburghclinicaltoxicology.org/s/NPIS-2016-17-report.pdf.
Accessed 30 May 2018.
9. Gummin DD, Mowry JB, Spyker DA, Brooks
DE, Fraser MO, Banner W. 2016 annual report of the American Association of
Poison Control Centers’ National Poison Data System (NPDS): 34th annual
report. Clinical Toxicol (Phila) 2017;55:1072-252. Crossref
10. Enfield B, Brooks DE, Welch S, et al.
Human plant exposures reported to a regional (Southwestern) Poison Control
Center over 8 years. J Med Toxicol 2018;14:74-8. Crossref
11. Chan TY, Chan LY, Tam LS, Critchley
JA. Neurotoxicity following the ingestion of a Chinese medicinal plant, Alocasia
macrorrhiza. Hum Exp Toxicol 1995;14:727-8. Crossref
12. Poon WT, Ho CH, Yip KL, et al.
Grayanotoxin poisoning from Rhododendron simsii in an infant. Hong
Kong Med J 2008;14:405-7.
13. Poon WT, Chau TL, Lai CK, et al.
Hepatitis induced by Teucrium viscidum. Clin Toxicol (Phila)
2008;46:819-22. Crossref
14. Wu JS, Poon WT, Ma CK, et al.
Budd-Chiari syndrome secondary to toxic pyrrolizidine alkaloid exposure.
Hong Kong Med J 2013;19:553-5. Crossref
15. Pang CT, Ng HW, Lau FL. Oral mucosal
irritating plant ingestion in Hong Kong: epidemiology and its clinical
presentation. Hong Kong J Emerg Med 2010;17:477-81. Crossref
16. Lai CK, Chan YW. Confirmation of
gelsemium poisoning by targeted analysis of toxic gelsemium alkaloids in
urine. J Anal Toxicol 2009;33:56-61. Crossref
17. Fung HT, Lam KK, Lam SK, Wong OF, Kam
CW. Two cases of Gelsemium elegans Benth. poisoning. Hong Kong J
Emerg Med 2007;14:221-4. Crossref
18. Cheung WL, Law CY, Lee HC, et al.
Gelsemium poisoning mediated by the non-toxic plant Cassytha
filiformis parasitizing Gelsemium elegans. Toxicon
2018;154:42-9. Crossref
19. Cheng KL, Chan YC, Mak TW, Tse ML, Lau
FL. Chinese herbal medicine-induced anticholinergic poisoning in Hong
Kong. Hong Kong Med J 2013;19:38-41.
20. Diaz JH. Poisoning by herbs or plants:
rapid toxidromic classification and diagnosis. Wilderness Environ Med
2016;27:136-52. Crossref
21. Lin TJ, Nelson LS, Tsai JL, et al.
Common toxidromes of plant poisonings in Taiwan. Clin Toxicol (Phila)
2009;47:161-8. Crossref
22. Ng SW, Ching CK, Chan AY, Mak TW.
Simultaneous detection of 22 toxic plant alkaloids (aconitum alkaloids,
solanaceous tropane alkaloids, sophora alkaloids, strychnos alkaloids and
colchicine) in human urine and herbal samples using liquid
chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol
Biomed Life Sci 2013;942-943:63-9. Crossref
23. Persson HE, Sjöberg GK, Haines JA,
Pronczuk de Garbino J. Poisoning severity score. Grading of acute
poisoning. J Toxicol Clin Toxicol 1998;36:205-13. Crossref
24. Hospital Authority Toxicology
Reference Laboratory. Atlas of poisonous plants in Hong Kong—a clinical
toxicology perspective. 2016. Available from:
http://www3.ha.org.hk/toxicplant. Accessed 30 May 2018.
25. von Elm E, Altman DG, Egger M, Pocock
SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening
the Reporting of Observational Studies in Epidemiology (STROBE) statement:
guidelines for reporting observational studies. J Clin Epidemiol
2008;61:344-9. Crossref
26. Centers for Disease Control and
Prevention (CDC). Suspected moonflower intoxication-Ohio, 2002. MMWR Morb
Mortal Wkly Rep 2003;52:788-91.
27. Boumba VA, Mitselou A, Vougiouklakis
T. Fatal poisoning from ingestion of Datura stramonium seeds. Vet
Hum Toxicol 2004;46:81-2.
28. Lin TJ, Hung DZ, Hu WH, Yang DY, Wu
TC, Deng JF. Calcium oxalate is the main toxic component in clinical
presentations of Alocasis macrorrhiza (L) Schott and Endl
poisonings. Vet Hum Toxicol 1998;40:93-5.
29. Farré M, Xirgu J, Salgado A, Peracaula
R, Reig R, Sanz P. Fatal oxalic acid poisoning from sorrel soup. Lancet
1989;2:1524. Crossref
30. Kalliala H, Kauste O. Ingestion of
rhubarb leaves as cause of oxalic acid poisoning. Ann Paediatr Fenn
1964;10:228-31.
31. Xiang H, Zhou YJ, Huang PL, et al.
Lethal poisoning with Gelsemium elegans in Guizhou, China. Public
Health 2016;136:185-7. Crossref
32. Zhou Z, Wu L, Zhong Y, et al. Gelsemium
elegans poisoning: a case with 8 months of follow-up and review of
the literature. Front Neurol 2017;8:204. Crossref
33. Lampe KF. Rhododendrons,
mountain laurel, and mad honey. JAMA 1988;259:2009. Crossref
34. Lennerz C, Jilek C, Semmler V,
Deisenhofer I, Kolb C. Sinus arrest from mad honey disease. Ann Intern Med
2012;157:755-6. Crossref
35. Chen SP, Lam YH, Ng VC, et al. Mad
honey poisoning mimicking acute myocardial infarction. Hong Kong Med J
2013;19:354-6. Crossref
36. Dickers KJ, Bradberry SM, Rice P,
Griffiths GD, Vale JA. Abrin poisoning. Toxicol Rev 2003;22:137-42. Crossref
37. Fernando C. Poisoning due to Abrus
precatorius (jequirty bean). Anaesthesia 2001;56:1178-80. Crossref
38. Wooten JV, Pittman CT, Blake TA, et
al. A case of abrin toxin poisoning, confirmed via quantitation of
L-abrine (N-methyl-L-tryptophan) biomarker. J Med Toxicol 2014;10:392-4. Crossref
39. Jang DH, Hoffman RS, Nelson LS.
Attempted suicide, by mail order: Abrus precatorius. J Med Toxicol
2010;6:427-30. Crossref
40. Subrahmanyan D, Mathew J, Raj M. An
unusual manifestation of Abrus precatorius poisoning: a report of
two cases. Clin Toxicol (Phila) 2008;46:173-5. Crossref
41. Cheng D, Röder E. Pyrrolizidine
alkaloids from Emilia sonchifolia [in German]. Planta Med
1986;6:484-6. Crossref
42. Wiedenfeld H. Plants containing
pyrrolizidine alkaloids: toxicity and problems. Food Addit Contam Part A
Chem Anal Control Expo Risk Assess 2011;28:282-92. Crossref
43. Chen Z, Huo JR. Hepatic veno-occlusive
disease associated with toxicity of pyrrolizidine alkaloids in herbal
preparations. Neth J Med 2010;68:252-60.
44. Kumana CR, Ng M, Lin HJ, Ko W, Wu PC,
Todd D. Herbal tea induced hepatic veno-occlusive disease: quantification
of toxic alkaloid exposure in adults. Gut 1985;26:101-4. Crossref
45. Centre for Food Safety, Food and
Environmental Hygiene Department, Hong Kong SAR Government. Risk
assessment studies report no. 56. Chemical hazard evaluation.
Pyrrolizidine alkaloids in food. 2017. Available from:
https://www.cfs.gov.hk/english/programme/programme_rafs/files/Pyrrolizidine_Alkaloids_in_Food_e.pdf.
Accessed 10 Nov 2018.
46. Gao H, Ruan JQ, Chen J, et al. Blood
pyrrole-protein adducts as a diagnostic and prognostic index in
pyrrolizidine alkaloid-hepatic sinusoidal obstruction syndrome. Drug Des
Devel Ther 2015;9:4861-8. Crossref

