Hong
Kong Med J 2019 Feb;25(1):38–47 | Epub 31 Jan 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Update on the association between dry eye disease and
meibomian gland dysfunction
Tommy CY Chan, MB, BS, MMedSc1,2,3;
Sharon SW Chow, MB, BS3,4; Kelvin HN Wan, MB, BS1,5;
Hunter KL Yuen, MB, ChB1,6
1 Department of Ophthalmology and Visual
Sciences, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Hong Kong Sanatorium & Hospital,
Happy Valley, Hong Kong
3 Department of Ophthalmology, The
University of Hong Kong, Cyberport, Hong Kong
4 Department of Ophthalmology, Grantham
Hospital, Wong Chuk Hang, Hong Kong
5 Department of Ophthalmology, Tuen Mun
Hospital, Tuen Mun, Hong Kong
6 Hong Kong Eye Hospital, Hong Kong
Corresponding author: Dr Tommy CY Chan (tommychan.me@gmail.com)
Abstract
Dry eye disease is one of the most common
ophthalmic complaints; it results from the activity of various pathways
and is considered a multifactorial disease. An important factor that
contributes to the onset of dry eye disease is meibomian gland
dysfunction. Meibomian gland dysfunction causes a disruption in the tear
film lipid layer which affects the rate of tear evaporation. This
evaporation leads to tear hyperosmolarity, eventually triggering the
onset of dry eye disease. Dry eye disease and meibomian gland
dysfunction are strongly associated with each other, such that many of
their risk factors, signs, and symptoms overlap. This review aimed to
provide an update on the association between dry eye disease and
meibomian gland dysfunction. A stepwise approach for diagnosis and
management is summarised.
Introduction
Dry eye disease (DED) is one of the most common
ocular surface diseases, which can significantly affect the quality of
life of affected patients. The definition of DED has been progressively
established in recent decades. The goal of the Tear Film and Ocular
Surface Society (TFOS) Dry Eye Workshop (DEWS) is to create an
evidence-based definition, a well-defined classification
system, and an appropriate diagnosis and management algorithm for DED.1 In 2007, the TFOS DEWS definition of DED was first
published.2 In 2017, the TFOS DEWS
II amended the definition of DED to be ‘a multifactorial disease of the
ocular surface, characterised by a loss of homeostasis of the tear film,
and accompanied by ocular symptoms, in which tear film instability and
hyperosmolarity, ocular surface inflammation and damage, and neurosensory
abnormalities play etiological roles’.1
The term ‘multifactorial’ indicates that the disease occurs as a result of
multiple influential factors, while the term ‘etiological roles’ suggests
the involvement of various pathways in the onset of DED.1 In 2017, the Asia Dry Eye Society also agreed upon a
new definition of DED, as ‘a multifactorial disease characterised by
unstable tear film causing a variety of symptoms and or visual impairment,
potentially accompanied by ocular surface damage’.3
The two main categories of DED are evaporative dry
eye and aqueous deficient dry eye.2
Evaporative dry eye is related to conditions that affect the eyelids, such
as meibomian gland dysfunction (MGD), poor blinking effort, and lid
disorders, or that affect the ocular surface, such as prolonged contact
lens wear, frequent use of topical drug preservatives, and immune-related
ocular surface disorders (eg, atopic keratoconjunctivitis). Aqueous
deficient dry eye is primarily due to conditions affecting lacrimal gland
function, such as Sjögren’s syndrome, lacrimal gland duct obstruction or
deficiencies, and adverse effects of systemic drugs. Epidemiological
evidence suggests that DED is mainly evaporative in nature,4 and is often associated with MGD.5 6
Meibomian glands are found in the upper and lower
eyelids, where they secrete lipids (meibum) onto the ocular surface,
forming the outermost layer of the tear film. These lipids spread easily,
promoting tear film stability and protecting against evaporation.
Meibomian gland dysfunction is defined as ‘a chronic, diffuse abnormality
of the meibomian glands, commonly characterised by terminal duct
obstruction and/or qualitative/quantitative changes in the glandular
secretion. It may result in alteration of the tear film, symptoms of eye
irritation, clinically apparent inflammation and ocular surface disease’.7
This review aims to provide an update on the
association between MGD and DED, with particular attention to the
diagnosis and management of these conditions. We will discuss the
epidemiology, pathophysiology, risk factors, signs and symptoms,
diagnosis, and ancillary imaging of MGD and DED, along with appropriate
behaviour, medical, and surgical management.
Methods
A comprehensive literature search on PubMed was
performed for studies published between January 2006 and December 2017
with keywords ‘dry eye’, ‘dry eye disease’, ‘tear film’, ‘meibomian
gland’, and ‘meibomian gland dysfunction’. Search results were limited to
clinical studies published in English. Articles reporting DED and MGD were
reviewed. Particular emphasis was placed on papers that investigated the
association between DED and MGD. The reference lists of the retrieved
articles were also examined for relevant studies.
Epidemiology
The reported prevalence of DED ranges from 5% to
50%,4 whereas the reported
prevalence of MGD varies more widely from 3.5% to nearly 70%.8 9 Meibomian
gland dysfunction appears to be more prevalent in Asian populations.5 Meibomian gland dysfunction has been reported to
contribute to 60% of all cases of DED; an additional 20% of cases of DED
are caused by aqueous deficiency.
Pathophysiology of dry eye disease
All forms of DED primarily occur because of water
loss from the tear film, which leads to tear hyperosmolarity due to
evaporative dry eye and/or aqueous deficient dry eye.10 In evaporative dry eye, hyperosmolarity results from
excessive evaporation of tears in the context of normal lacrimal function.
In contrast, in aqueous deficient dry eye, hyperosmolarity occurs due to
an inadequate rate of lacrimal secretion in the context of a normal rate
of evaporation. Environmental factors affect the presence of
hyperosmolarity on the ocular surface, which may trigger the onset of DED,
or cause worsening of the condition.
Pathophysiology of meibomian gland dysfunction
Meibomian gland dysfunction is classified according
to the rate of gland secretion. A low delivery state is characterised by
meibomian gland hyposecretion or obstruction, whereas a high delivery
state is characterised by meibomian gland hypersecretion. Of these two
categories, the most common mechanism is a low delivery state due to duct
obstruction.11 Epithelial
hyperkeratinisation is the most common cause of duct obstruction, leading
to meibum accumulation with chronic inflammation and, eventually, gland
dropout.12 Importantly, this
results in the quantitative and qualitative abnormalities of glandular
secretions. There is a high prevalence of MGD in acne rosacea, which is a
chronic cutaneous inflammatory disorder.
Association between dry eye disease and meibomian gland
dysfunction
The tear film consists of three distinct layers:
the lipid, aqueous, and mucus layers. The lipid layer, a key component of
the tear film, is derived from meibomian glands. The lipid layer prevents
water evaporation from the ocular surface and is thus crucial in the
maintenance of a healthy ocular surface. Dysfunction of the meibomian
glands results in unbalanced lipid secretion, thereby increasing the rate
of ocular surface evaporation and causing tear hyperosmolarity.13 Patients with MGD reportedly exhibit a higher tear
evaporation rate than that of normal subjects.13
This shows that DED is directly correlated with the integrity and quality
of meibum on the ocular surface.
Risk factors
Many risk factors associated with DED also
contribute to MGD. Thus, risk factor modifications can likely improve both
disease states.
Sex
Female sex is a significant risk factor for the
development of both DED and MGD.5 6 This may be due to the effect of
hormonal changes on meibomian secretion, as androgen and oestrogen
receptors are both present within the meibomian glands.14 Importantly, androgens have been reported to
stimulate meibum secretion and suppress inflammation, whereas oestrogens
reduce meibum secretion and increase inflammation.15 Dysfunctional meibomian gland secretion and
concurrent alterations in the lipid layer have been observed in patients
with androgen depletion.16
Additionally, female sex has been identified as a risk factor for the
development of autoimmune diseases that lead to DED, such as Sjögren’s
syndrome.17
Topical medications
Topical medications can cause both DED and MGD;
this may be a result of ocular surface disturbances with various
aetiologies, including allergic reactions, toxic epitheliopathy, and
inflammatory response from chronic chemical irritation. Multiple studies
have revealed a clear relationship between the prevalence of dry eye and
increasing use of eye drops.18 The
primary factor underlying this relationship is the presence of
benzalkonium chloride preservative agent in topical medications.
Benzalkonium chloride has been strongly linked with the onset of DED, as
it dissolves the lipid tear film layer and has been shown to disrupt tear
film osmolarity.19 Similarly, DED
and MGD are commonly reported in glaucoma patients who use topical
glaucoma medications, which contain benzalkonium chloride. Use of these
medications has been associated with changes in meibomian gland structure,
leading to MGD.20
Contact lens wear
Contact lens wear is commonly associated with the
onset of both DED and MGD. An epidemiological study showed that 50% of
contact lens wearers experience dry eye symptoms, whereas only 22% of
non–contact lens wearers experience such symptoms.21 Contact lens wear alters the integrity of the tear
film: a thinner lipid layer has been observed in contact lens wearers,
which causes an increased tear evaporation rate and tear hyperosmolarity.22 Environmental factors, such as
prolonged usage of visual display devices, as well as air pollution and
seasonal changes, further aggravate dry eye symptoms in contact lens
wearers. The occurrence of MGD in contact lens wearers is suspected to be
a result of chronic inflammation,23
as well as clogging of gland orifices due to accumulation of desquamated
epithelial cells.24 Contact lens
wearers demonstrate a high percentage of meibomian gland dropout and
reduction in gland function; these aspects are reportedly directly related
to the duration of contact lens wear.25
Refractive surgery
Worldwide, laser in situ keratomileusis (LASIK) is
the most common corneal refractive surgery currently in use. Dry eye
disease is often associated with a history of LASIK, and can be aggravated
by both preoperative and postoperative factors. Preoperatively, the risk
of DED is significantly increased in patients who are long-term contact
lens wearers, as well as in patients whose eyes exhibit pre-existing tear
film instability.26 Greater
refractive correction magnitude requires deeper ablation, resulting in a
greater extent of sensory nerve damage. This nerve damage results in
reduced corneal sensitivity, leading to neuropathic dry eyes. Notably,
this mechanism is the most common aetiology of post-LASIK dry eyes.26 Corneal refractive surgery has also been shown to
reduce corneal epithelial integrity, conjunctival goblet cell
concentration, and meibomian gland function, resulting in lower ocular
surface disease index and ocular surface staining scores.27
Demodicosis
Two species of mites, Demodex folliculorum
and Demodex brevis, are the only mites that affect human skin;
such infestations are known as demodicosis.28
Reportedly, D folliculorum infests the lash follicles, whereas D
brevis infests the meibomian glands.28
These infestations increase the meibum melting temperature, resulting in a
more viscous lipid layer. A recent study showed that a higher D brevis
count was associated with more severe MGD.29
Furthermore, confocal microscopy analysis revealed lower counts of Demodex
mites in the glands of healthy subjects than in the glands of patients
with MGD-related DED.30
The role of Demodex mites in the pathology
of MGD has not been fully elucidated; however, eradication of Demodex
is particularly helpful in relieving related ocular symptoms. Thus, there
may a pathogenic role for Demodex infestation in MGD.
Symptoms
Many signs and symptoms of DED overlap with those
of MGD. However, most patients with MGD are largely or entirely
asymptomatic; if they are symptomatic, their particular symptoms often do
not directly correlate with the severity of ocular surface disturbance. In
a population-based study in China, 22% of the study population
demonstrated asymptomatic MGD, while 9% showed symptomatic MGD.8 In cases of symptomatic MGD, patients report a variety
of symptoms, including foreign body sensation, dryness, itching, and/or
photosensitivity.7 These
manifestations may be linked to chronic inflammation or mechanical
friction between the ocular surface and meibum that has accumulated in the
gland orifices.
Ocular surface signs and diagnosis
Because DED and MGD are common ophthalmic problems,
a clear diagnosis is crucial for suitable management. Appropriate tests
should be used to diagnose and monitor DED, in accordance with the revised
TFOS DEWS II definition of the disease. For these purposes, the TFOS DEWS
II proposed a battery of diagnostic tests for DED.
The diagnostic tests begin with triaging questions
and risk factor analysis. These are followed by screening for symptoms
using standardised questionnaires, including the five-item dry eye
questionnaire or the ocular surface disease index. Markers of homeostasis
used in diagnostic testing include measures of tear breakup time, staining
of the ocular surface, Schirmer’s test, and tear osmolarity. Tear breakup
time is a non-invasive measurement that is defined as the time required
for the tear film to break up sufficiently that the patient can no longer
refrain from blinking.31 A tear
breakup time of <10 seconds is considered diagnostic for DED (Fig
1). Ocular surface staining is performed by fluorescein staining for
corneal damage and lissamine green staining for conjunctival and lid
margin damage (Fig 2).31
Schirmer’s test consists of the placement of a small strip of filter paper
inside the lower fornix with the eye closed. After 5 minutes, the amount
of moisture is measured as the distance that tear moisture has travelled
on the paper, due to capillary action; a value of <5 mm indicates DED.
Finally, tear osmolarity should be assessed with a calibrated device; a
positive result is defined as ≥308 mOsm/L in the measured eye, or a
difference of >8 mOsm/L between two eyes.32
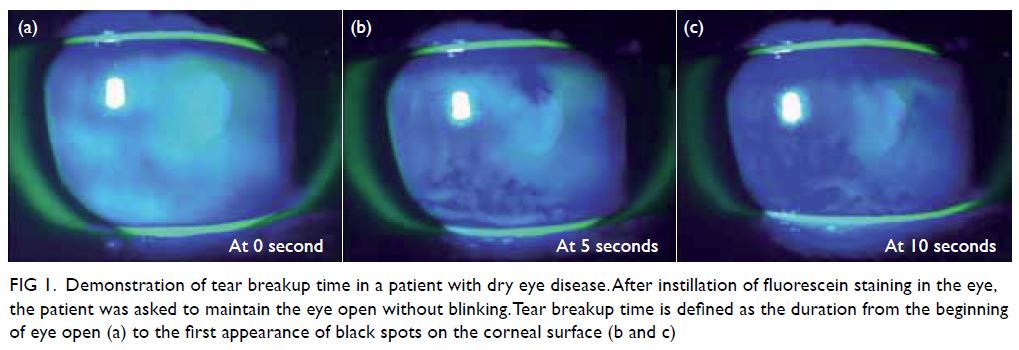
Figure 1. Demonstration of tear breakup time in a patient with dry eye disease. After instillation of fluorescein staining in the eye, the patient was asked to maintain the eye open without blinking. Tear breakup time is defined as the duration from the beginning of eye open (a) to the first appearance of black spots on the corneal surface (b and c)
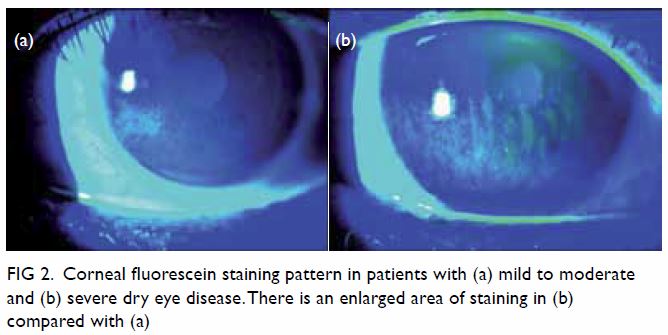
Figure 2. Corneal fluorescein staining pattern in patients with (a) mild to moderate and (b) severe dry eye disease. There is an enlarged area of staining in (b) compared with (a)
The Asia Dry Eye Society recommends diagnosis of
DED by using a combination of symptoms assessed by standardised
questionnaires (ocular surface disease index, McMonnies questionnaire,
women’s health study questionnaire, or five-item dry eye questionnaire),
together with a reduced tear breakup time (with a cut-off value of <5
s).3
Clinical diagnosis of MGD is made based on the
examination of altered anatomical features, such as meibomian gland
dropout, altered meibum excretion, and changes to lid morphology, with
plugging or pouting of the gland orifice. Meibomian glands with normal
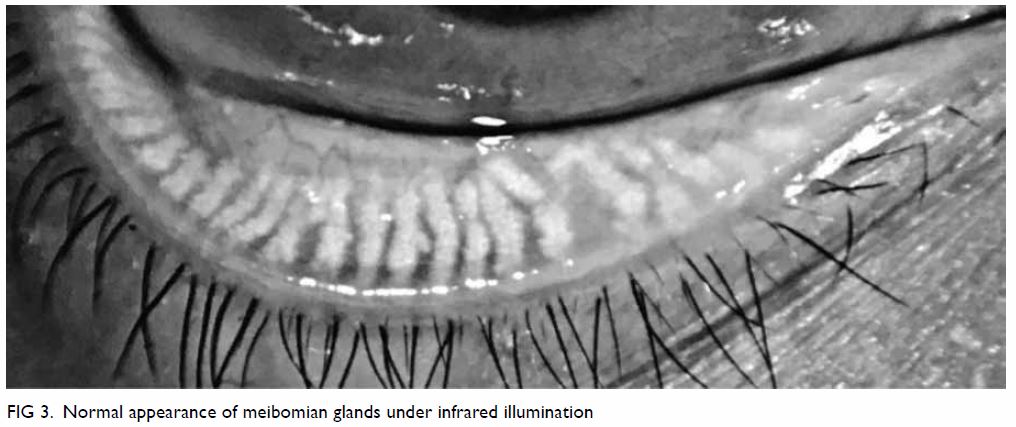
appearance are shown in Figure 3. Gentle gland expression with digital
pressure to the central lower lid can evaluate terminal duct obstruction
and meibum quality (Fig 4). Subtype classification tests, including
identification of MGD features, as well as lipid thickness and tear volume
assessment, are then performed to determine whether the disease
constitutes evaporative dry eye or aqueous deficient dry eye. Lastly, the
severity of disease is evaluated; for this purpose, the International
Workshop on Meibomian Gland Dysfunction has provided a grading system that
can be used to guide management of MGD.33
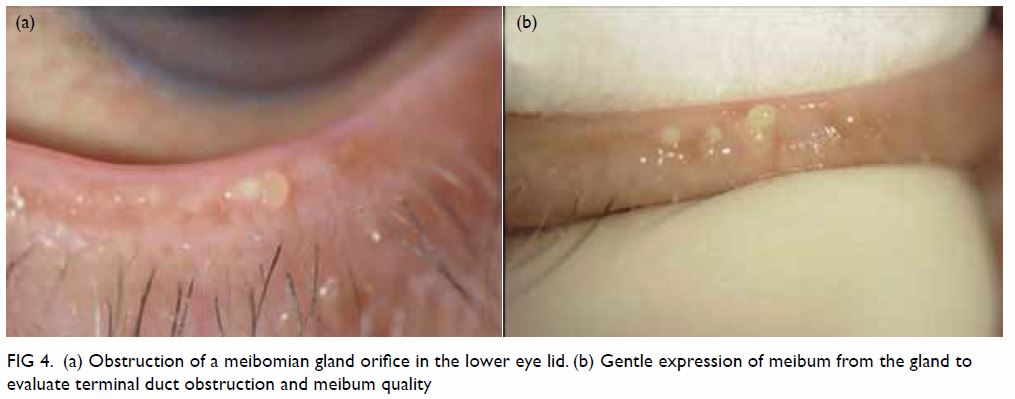
Figure 4. (a) Obstruction of a meibomian gland orifice in the lower eye lid. (b) Gentle expression of meibum from the gland to evaluate terminal duct obstruction and meibum quality
Role of imaging in diagnosis
In recent years, multiple imaging modalities have
been introduced to improve the diagnosis of DED and MGD.34 These modalities aim to facilitate the evaluation of
the structural and dynamic properties of the tear film. In cases of DED
with tear film instability, topographic systems have been used to
determine changes in the edges of the mires of a Placido disc.35 Anterior segment optical coherence tomography aims to
measure the height of the tear meniscus,36
while infrared meibography provides an objective evaluation of gland
structures. Tear film lipid layer thickness can be measured by
interferometry, which allows objective and quantitative measurement of
tear film integrity.37
Both DED and MGD can lower the ocular surface
temperature. In DED, the increased tear film evaporation rate causes heat
loss, lowering ocular surface temperature.38
In MGD, lower tarsal conjunctival temperatures have been observed,
increasing the viscosity of meibum; this change in viscosity leads to
worsening of gland function.39
These advancements in imaging modalities have improved accuracy and
standardised the diagnosis of DED and MGD.
Management
The aim of all treatment in MGD is to increase the
quality and quantity of meibum expression. For this purpose, a stepwise
staged approach is necessary to standardise management of the disease.40 The TFOS DEWS II created an algorithm to implement
various management options, on the basis of disease severity.40 Initially, patients must be educated regarding
environmental and dietary modifications, which include essential fatty
acid supplements. Patients must also be guided to eliminate factors
contributing to the onset of DED, including contact lens wear, as well as
both topical and systemic medications. Several lifestyle modifications,
such as ensuring sufficient sleep or rest, maintaining appropriate
hydration, and discontinuing smoking habits, may help to relieve symptoms.
Ocular lubricants are suggested for mild DED; preferably, these should not
contain benzalkonium chloride preservatives. Some of these modification
approaches are outlined below, along with an overview of the emerging
available treatment devices and options.
Eyelid hygiene
In the presence of MGD, eyelid hygiene is the
cornerstone of MGD management. This treatment modality consists of two
components: eyelid warming and eyelid massage. Meibum in patients with MGD
is more stagnant and viscous and has a higher melting temperature than
that in a healthy individual; thus, warming the eyelid to melt
pathologically altered meibum can improve its secretion.33 Warm compression provides further benefits by melting
abnormal meibum. Secretions from meibomian glands in patients with MGD
exhibit lower levels of lipids, esters, and free sterols.41 Potential involvement of microbes (ie, Staphylococcus
spp, Propionibacterium acnes, Bacillus oleronius,
and the Demodex species described above) contributes to the
pathology of MGD-associated DED by increasing meibum melting temperature
and enhancing inflammation. This illustrates the importance of eyelid
hygiene in MGD management.42 For
patients with MGD who exhibit demodicosis, many treatment options have
been described, including the use of topical 2% metronidazole. Recently,
the use of tea tree oil has also increased in popularity.43 Tea tree oil is a natural essential oil that includes
4-terpineol, which is antimicrobial, anti-inflammatory, and toxic to Demodex.44 Tea tree oil lid scrubs have
shown promising results as management for Demodex-related MGD.45
Effective eyelid hygiene can be achieved by use of
a hot compress (ie, soaking a clean towel in hot water, and applying the
towel over the eyelids), which softens the meibum and allows better flow.
After the application of the hot compress, lipid by-products can be
removed gently by scrubbing both upper and lower lid margins via mild
upward or downward compression of the eyelid, using a moist cotton bud;
this compression begins from the nasal canthus and moves laterally. An
additional therapeutic approach involves the use of mildly diluted baby
shampoo for lid scrubs; this is a widely accepted therapy. Although eyelid
warming and eyelid massage are efficacious for the management of MGD, they
are often time-consuming and labour-intensive; thus, they encounter
patient compliance issues.46 There
are now a wide variety of lid cleansing products, which facilitate
standardisation and simplification of treatment. Additional treatment
options include warming of the lids and expression of meibomian glands,
either manually (similar to above) or with the use of specially designed
devices. One such device, LipiFlow (TearScience; Morrisville [NC], United
States), is designed to transfer heat through the eyelid tissue to
facilitate emptying of gland contents at a therapeutic temperature of
42.5°C.47 LipiFlow treatment has
shown promising results, and may significantly improve symptoms.47
Intense pulsed light was first reported
approximately 10 years ago for the treatment of MGD, and it has
demonstrated an ability to improve tear film quality and quantity, as well
as to promote reduction of dry eye symptoms.48
Intraductal meibomian gland probing provides another approach to remove
abnormal meibum secretions.49 Oral
tetracycline and macrolides are reportedly useful in the treatment of
MGD-related DED.40 These compounds
are used with the assumption that inhibition of lipase production results
in reduction of lipid breakdown, which may contribute to improvement in
MGD. Macrolides, azithromycin in particular, exhibit anti-inflammatory
properties; moreover, these compounds increase cellular accumulation of
cholesterol, which may promote a suitable outcome in patients with
MGDrelated DED.50
Lipid-containing artificial lubricants
The majority of artificial tears are aqueous-based;
however, these offer limited and short-term symptomatic relief, partly due
to the lack of a lipid component. These artificial tears evaporate at a
similar rate to that of natural tears.51
Addition of a lipid component to the artificial lubricant helps to
replenish the lipid layer of the normal tear film.33 These lipid-containing lubricants exhibit long
retention times and can stabilise the tear film lipid layer, reduce tear
evaporation, and improve the signs of MGD.52
Additionally, lipid-containing lubricants have a longer-lasting effect and
cause minimal interference of patient vision. Commercially available
lipid-containing lubricants include mineral oil, high-purity castor oil,
mixtures of light and standard mineral oil, and mixtures of polar
phospholipid surfactant and mineral oil. A systematic review found that
these lipid-containing eye drops are efficacious and safe alternatives to
conventional tear lubricants in their abilities to relieve the signs and
symptoms of DED.52
Anti-inflammatory medications
Because ocular surface inflammation plays an
important role in the development of DED, anti-inflammatory mechanisms
must be considered. For patients with moderate to severe DED, low-dose
topical steroids have been advocated as a treatment choice, likely because
the anti-inflammatory properties of this type of drug can improve ocular
inflammation through suppression of inflammatory cytokines.53 Other anti-inflammatory options include
non-glucocorticoid immunomodulatory drugs, such as topical cyclosporine A,
which is an immunomodulatory drug that can reduce the expression of
inflammatory markers.54 Notably,
topical cyclosporine A has been proven efficacious in the treatment of
DED.55 A randomised trial showed
that cyclosporine A is beneficial in the stabilisation of tear film in
patients with MGD.56 However, its
anti-inflammatory effect is not as remarkable as that observed in DED,
because the main pathophysiology (epithelial gland hyperkeratinisation) is
not clearly resolved.
In severe cases of DED, autologous serum eye drops
can be considered. Autologous serum, which is the fluid component of a
patient’s own blood that remains after centrifugation, exhibits similar
biochemical properties to those of tears.40
Autologous serum reportedly contains specific factors that enhance
epithelial regeneration, and can inhibit the release of inflammatory
cytokines.57 Another treatment
option for patients with severe DED involves scleral contact lenses, which
are rigid gas permeable lenses of large diameter that are supported by the
sclera and serve as a bridge over the corneoscleral junction. A tear
reservoir is maintained between the posterior surface of the scleral
contact lens and the anterior corneal surface, improving tear osmolarity
and relieving dry eye symptoms.58
Omega-3 dietary supplementation
Essential fatty acid supplementation has been
proven beneficial in the treatment of DED and MGD, especially when
administered by intake of foods rich in omega-3 fatty acids, such as
flaxseed and fish oils.59 There is a speculative association between
essential fatty acids and modifications in lipid profile, as well as
reductions in the fatty acid content of meibomian gland secretions. In a
randomised, placebo-controlled, masked trial, omega-3 fatty acid
supplementation resulted in improving ocular surface disease index score,
tear breakup time, and meibum score in patients with MGD.60 Essential fatty acids also enhance the lipid layer,
slow tear evaporation, and reduce apoptosis of lacrimal gland cells.61 Essential fatty acids have been reported to exhibit
anti-inflammatory properties, particularly by promoting the production of
prostaglandin.62 These
modifications improve the tear secretion rate and tear content. Further
research is needed to enhance our understanding of the underlying
mechanism by which fatty acid supplementation supports the management of
MGD.63 64
Surgical and mechanical treatment options
In cases where medical treatment is insufficient,
surgical and mechanical treatment options include tear conservation via
punctual occlusion or moisture chamber goggles. Punctal plugs retain tears
on the ocular surface by blocking lacrimal drainage through the puncta (Fig 5). Permanent surgical closure may be useful
when patients cannot tolerate punctual plugs. Surgical punctual occlusion
blocks tear drainage and improves tear retention, and can be performed by
cauterisation65 or lacrimal
canalicular ligation.66 A
systematic review showed that, when combined with other treatment for DED,
punctual occlusion improves dry eye symptoms.67
A less invasive option, moisture chamber goggles provide a humid
environment and minimise airflow to the ocular surface, thereby slowing
the evaporation of tears.40
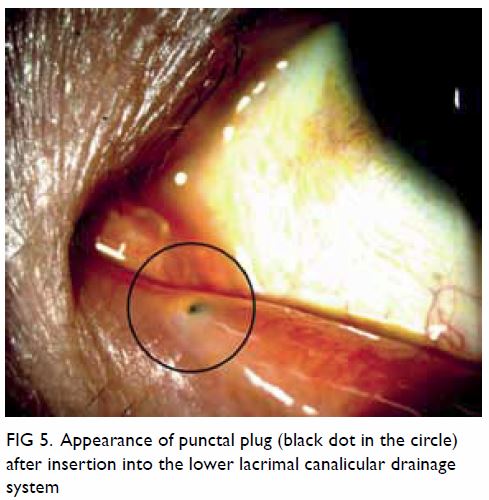
Figure 5. Appearance of punctal plug (black dot in the circle) after insertion into the lower lacrimal canalicular drainage system
Severe DED can lead to corneal erosion, persistent
epithelial defects, corneal ulceration, and eventual corneal scarring.
Amniotic membrane transplant is a reasonable option in such cases.
Amniotic membrane has been shown to contain multiple neurotransmitters and
neurotrophic factors, which are beneficial for the management of severe
DED.68 For patients with severe
DED with persistent epithelial defects that are refractive to medical
treatment, tarsorrhaphy may be useful. Notably, tarsorrhaphy is a
procedure that achieves partial or total closure of the eyelids, either
temporarily or permanently. By reducing ocular surface exposure, the rate
of tear evaporation decreases, such that DED can improve. Due to
unfavourable aesthetic outcomes, this approach is typically one of the
final methods used for management of severe DED.
Suggested treatment guideline for dry eye disease or
meibomian gland dysfunction for non-ophthalmologists
Dry eye disease and MGD are two of the most common
ocular conditions encountered by medical practitioners. To manage these
conditions, risk factors must be identified and modified. Notably, several
environmental and lifestyle modifications can help alleviate these
conditions. Proper lighting, anti-glare filters, ergonomic positioning of
computer monitors, and regular break time from work may help improve the
symptoms.69 A modest increase in
relative humidity, achieved by using a desktop-powered humidifier, has
been shown to increase subjective comfort.70
Reduction or discontinuation of contact lens use, as well as enhancement
of moisture within the surrounding environment, are possible risk factor
modifications. Smoking cessation can also improve the ocular surface
condition and tear function.71
Lubricants can be prescribed to be used as needed for symptomatic relief.
If symptoms do not resolve, benzalkonium chloride–free lubricants should
be considered. Low-dose topical steroids should be implemented with
particular caution, owing to the risk of steroid-related complications
(eg, cataract, glaucoma, and infection). In patients with recalcitrant
disease, referral to an ophthalmologist is necessary to ensure regular
monitoring. In the presence of MGD-related symptoms, lid hygiene and warm
compression are strongly suggested for symptomatic control; careful manual
expression of meibum should also be performed. If the above measures fail,
or if the DED is secondary to other causes of aqueous deficiency (eg,
Sjögren’s syndrome; graft versus host disease; or chronic inflammation in
Stevens-Johnson’s disease, toxic epidermal necrolysis, or ocular
cicatricial pemphigoid), referral to an ophthalmologist is warranted for
further workup (eg, anti-SSA/Ro blood test for Sjögren’s syndrome) and
management.
Conclusion
Dry eye disease is a common ophthalmic problem,
with a cause that is often multifactorial. Meibomian gland dysfunction is
an important contributor to DED, owing to an imbalance in lipid secretion
that affects the rate of tear evaporation. When tears evaporate quickly,
tear osmolarity increases, resulting in DED. There are many risk factors
that contribute to onset of both DED and MGD, many of which may overlap
between these diseases. A clear diagnosis is vital when managing DED.
Various treatment options are available for DED and MGD, and a stepwise
staged approach is often crucial for ensuring appropriate management.
Author contributions
All authors contributed to the concept and design,
acquisition of data, analysis and interpretation of data, drafting of the
manuscript, and critical revision for important intellectual content. All
authors had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
As an epidemiology advisor of the Editorial Board,
HKL Yuen was not involved in the peer review process of the article. All
authors have disclosed no conflicts of interest.
References
1. Craig JP, Nichols KK, Akpek EK, et al.
TFOS DEWS II definition and classification report. Ocul Surf
2017;15:276-83. Crossref
2. The definition and classification of dry
eye disease: report of the Definition and Classification Subcommittee of
the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:75-92. Crossref
3. Tsubota K, Yokoi N, Shimazaki J, et al.
New perspectives on dry eye definition and diagnosis: a consensus report
by the Asia Dry Eye Society. Ocul Surf 2017;15:65-76. Crossref
4. Stapleton F, Alves M, Bunya VY, et al.
TFOS DEWS II epidemiology report. Ocul Surf 2017;15:334-65. Crossref
5. Schaumberg DA, Nichols JJ, Papas EB,
Tong L, Uchino M, Nichols KK. The International Workshop on Meibomian
Gland Dysfunction: report of the subcommittee on the epidemiology of, and
associated risk factors for, MGD. Invest Ophthalmol Vis Sci
2011;52:1994-2005. Crossref
6. The epidemiology of dry eye disease:
report of the Epidemiology Subcommittee of the International Dry Eye
WorkShop (2007). Ocular Surf 2007;5:93-107. Crossref
7. Nichols KK, Foulks GN, Bron AJ, et al.
The International Workshop on Meibomian Gland Dysfunction: executive
summary. Invest Ophthalmol Vis Sci 2011;52:1922-9. Crossref
8. Jie Y, Xu L, Wu YY, Jonas JB. Prevalence
of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond)
2009;23:688-93. Crossref
9. McCarty CA, Bansal AK, Livingston PM,
Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne,
Australia. Ophthalmology 1998;105:1114-9. Crossref
10. Bron AJ, de Paiva CS, Chauhan SK, et
al. TFOS DEWS II pathophysiology report. Ocul Surf 2017;15:438-510. Crossref
11. Chhadva P, Goldhardt R, Galor A.
Meibomian gland disease: the role of gland dysfunction in dry eye disease.
Ophthalmology 2017;124:S20-6. Crossref
12. Gutgesell VJ, Stern GA, Hood CI.
Histopathology of meibomian gland dysfunction. Am J Ophthalmol
1982;94:383-7. Crossref
13. Goto E, Endo K, Suzuki A, Fujikura Y,
Matsumoto Y, Tsubota K. Tear evaporation dynamics in normal subjects and
subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol
Vis Sci 2003;44:533-9. Crossref
14. Sullivan DA, Rocha EM, Aragona P, et
al. TFOS DEWS II sex, gender, and hormones report. Ocul Surf
2017;15:283-333. Crossref
15. Bron AJ, Tiffany JM. The contribution
of meibomian disease to dry eye. Ocul Surf 2004;2:149-65. Crossref
16. Krenzer KL, Dana MR, Ullman MD, et al.
Effect of androgen deficiency on the human meibomian gland and ocular
surface. J Clin Endocrinol Metab 2000;85:4874-82. Crossref
17. Wan KH, Chen LJ, Young AL. Depression
and anxiety in dry eye disease: a systematic review and meta-analysis. Eye
(Lond) 2016;30:1558-67. Crossref
18. Pisella PJ, Pouliquen P, Baudouin C.
Prevalence of ocular symptoms and signs with preserved and preservative
free glaucoma medication. Br J Ophthalmol 2002;86:418-23. Crossref
19. Labbé A, Terry O, Brasnu E, Van Went
C, Baudouin C. Tear film osmolarity in patients treated for glaucoma or
ocular hypertension. Cornea 2012;31:994-9. Crossref
20. Agnifili L, Fasanella V, Costagliola
C, et al. In vivo confocal microscopy of meibomian glands in glaucoma. Br
J Ophthalmol 2013;97:343-9. Crossref
21. Doughty MJ, Fonn D, Richter D, Simpson
T, Caffery B, Gordon K. A patient questionnaire approach to estimating the
prevalence of dry eye symptoms in patients presenting to optometric
practices across Canada. Optom Vis Sci 1997;74:624-31. Crossref
22. Yokoi N, Yamada H, Mizukusa Y, et al.
Rheology of tear film lipid layer spread in normal and aqueous
tear-deficient dry eyes. Invest Ophthalmol Vis Sci 2008;49:5319-24. Crossref
23. Arita R, Itoh K, Inoue K, Kuchiba A,
Yamaguchi T, Amano S. Contact lens wear is associated with decrease of
meibomian glands. Ophthalmology 2009;116:379-84. Crossref
24. Henriquez AS, Korb DR. Meibomian
glands and contact lens wear. Br J Ophthalmol 1981;65:108-11. Crossref
25. Alghamdi WM, Markoulli M, Holden BA,
Papas EB. Impact of duration of contact lens wear on the structure and
function of the meibomian glands. Ophthalmic Physiol Opt 2016;36:120-31. Crossref
26. Nettune GR, Pflugfelder SC. Post-LASIK
tear dysfunction and dysesthesia. Ocul Surf 2010;8:135-45. Crossref
27. Albietz JM, Lenton LM. Management of
the ocular surface and tear film before, during, and after laser in situ
keratomileusis. J Refract Surg 2004;20:62-71.
28. English FP, Nutting WB. Demodicosis of
ophthalmic concern. Am J Ophthalmol 1981;91:362-72. Crossref
29. Liang L, Liu Y, Ding X, Ke H, Chen C,
Tseng SC. Significant correlation between meibomian gland dysfunction and
keratitis in young patients with Demodex brevis infestation. Br J
Ophthalmol 2018;102:1098-102. Crossref
30. Randon M, Liang H, El Hamdaoui M, et
al. In vivo confocal microscopy as a novel and reliable tool for the
diagnosis of Demodex eyelid infestation. Br J Ophthalmol
2015;99:336-41. Crossref
31. Wolffsohn JS, Arita R, Chalmers R, et
al. TFOS DEWS II diagnostic methodology report. Ocul Surf 2017;15:539-74.
Crossref
32. Lemp MA, Bron AJ, Baudouin C, et al.
Tear osmolarity in the diagnosis and management of dry eye disease. Am J
Ophthalmol 2011;151:792-8.e1. Crossref
33. Geerling G, Tauber J, Baudouin C, et
al. The International Workshop on Meibomian Gland Dysfunction: report of
the subcommittee on management and treatment of meibomian gland
dysfunction. Invest Ophthalmol Vis Sci 2011;52:2050-64. Crossref
34. Chan TC, Wan KH, Shih KC, Jhanji V.
Advances in dry eye imaging: the present and beyond. Br J Ophthalmol
2018;102:295-301. Crossref
35. Goto T, Zheng X, Okamoto S, Ohashi Y.
Tear film stability analysis system: introducing a new application for
videokeratography. Cornea 2004;23:S65-70. Crossref
36. Ibrahim OM, Dogru M, Takano Y, et al.
Application of visante optical coherence tomography tear meniscus height
measurement in the diagnosis of dry eye disease. Ophthalmology
2010;117:1923-9. Crossref
37. Finis D, Pischel N, Schrader S,
Geerling G. Evaluation of lipid layer thickness measurement of the tear
film as a diagnostic tool for meibomian gland dysfunction. Cornea
2013;32:1549-53. Crossref
38. Purslow C, Wolffsohn J. The relation
between physical properties of the anterior eye and ocular surface
temperature. Optom Vis Sci 2007;84:197-201. Crossref
39. Arita R, Shirakawa R, Maeda S,
Yamaguchi M, Ohashi Y, Amano S. Decreased surface temperature of tarsal
conjunctiva in patients with meibomian gland dysfunction. JAMA Ophthalmol
2013;131:818-9. Crossref
40. Jones L, Downie LE, Korb D, et al.
TFOS DEWS II management and therapy report. Ocul Surf 2017;15:575-628. Crossref
41. Shine WE, McCulley JP. Polar lipids in
human meibomian gland secretions. Curr Eye Res 2003;26:89-94. Crossref
42. Knop E, Knop N, Millar T, Obata H,
Sullivan DA. The International Workshop on Meibomian Gland Dysfunction:
report of the subcommittee on anatomy, physiology, and pathophysiology of
the meibomian gland. Invest Ophthalmol Vis Sci 2011;52:1938-78. Crossref
43. Gao YY, Di Pascuale MA, Elizondo A,
Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea
tree oil. Cornea 2007;26:136-43. Crossref
44. Tighe S, Gao YY, Tseng SC.
Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex
mites. Transl Vis Sci Technol 2013;2:2. Crossref
45. Gao YY, Xu DL, Huang IJ, Wang R, Tseng
SC. Treatment of ocular itching associated with ocular demodicosis by 5%
tea tree oil ointment. Cornea 2012;31:14-7. Crossref
46. Korb DR, Blackie CA. Meibomian gland
therapeutic expression: quantifying the applied pressure and the
limitation of resulting pain. Eye Contact Lens 2011;37:298-301. Crossref
47. Lane SS, DuBiner HB, Epstein RJ, et
al. A new system, the LipiFlow, for the treatment of meibomian gland
dysfunction. Cornea 2012;31:396-404. Crossref
48. Craig JP, Chen YH, Turnbull PR.
Prospective trial of intense pulsed light for the treatment of meibomian
gland dysfunction. Invest Ophthalmol Vis Sci 2015;56:1965-70. Crossref
49. Sik Sarman Z, Cucen B, Yuksel N,
Cengiz A, Caglar Y. Effectiveness of intraductal meibomian gland probing
for obstructive meibomian gland dysfunction. Cornea 2016;35:721-4. Crossref
50. Liu Y, Kam WR, Ding J, Sullivan DA.
Can tetracycline antibiotics duplicate the ability of azithromycin to
stimulate human meibomian gland epithelial cell differentiation? Cornea
2015;34:342-6. Crossref
51. Trees GR, Tomlinson A. Effect of
artificial tear solutions and saline on tear film evaporation. Optom Vis
Sci 1990;67:886-90. Crossref
52. Lee SY, Tong L. Lipid-containing
lubricants for dry eye: a systematic review. Optom Vis Sci
2012;89:1654-61. Crossref
53. Djalilian AR, Nagineni CN, Mahesh SP,
Smith JA, Nussenblatt RB, Hooks JJ. Inhibition of inflammatory cytokine
production in human corneal cells by dexamethasone, but not cyclosporin.
Cornea 2006;25:709-14. Crossref
54. Gao J, Sana R, Calder V, et al.
Mitochondrial permeability transition pore in inflammatory apoptosis of
human conjunctival epithelial cells and T cells: effect of cyclosporin A.
Invest Ophthalmol Vis Sci 2013;54:4717-33. Crossref
55. Wan KH, Chen LJ, Young AL. Efficacy
and safety of topical 0.05% cyclosporine eye drops in the treatment of dry
eye syndrome: a systematic review and meta-analysis. Ocul Surf
2015;13:213-25. Crossref
56. Prabhasawat P, Tesavibul N, Mahawong
W. A randomized double-masked study of 0.05% cyclosporine ophthalmic
emulsion in the treatment of meibomian gland dysfunction. Cornea
2012;31:1386-93. Crossref
57. López-García JS, García-Lozano I,
Rivas L, Giménez C, Acera A, Suárez-Cortés T. Effects of autologous serum
eye drops on conjunctival expression of MUC5AC in patients with ocular
surface disorders. Cornea 2016;35:336-41. Crossref
58. La Porta Weber S, Becco de Souza R,
Gomes JÁ, Hofling-Lima AL. The use of the Esclera scleral contact lens in
the treatment of moderate to severe dry eye disease. Am J Ophthalmol
2016;163:167-73.e1. Crossref
59. Liu Y, Kam WR, Sullivan DA. Influence
of omega 3 and 6 fatty acids on human meibomian gland epithelial cells.
Cornea 2016;35:1122-6. Crossref
60. Macsai MS. The role of omega-3 dietary
supplementation in blepharitis and meibomian gland dysfunction (an AOS
thesis). Trans Am Ophthalmol Soc 2008;106:336-56.
61. Rosenberg ES, Asbell PA. Essential
fatty acids in the treatment of dry eye. Ocul Surf 2010;8:18-28. Crossref
62. Das UN. Essential fatty acids—a
review. Curr Pharm Biotechnol 2006;7:467-82. Crossref
63. Barabino S, Rolando M, Camicione P, et
al. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome
with an inflammatory component. Cornea 2003;22:97-101. Crossref
64. Aragona P, Bucolo C, Spinella R,
Giuffrida S, Ferreri G. Systemic omega-6 essential fatty acid treatment
and pge1 tear content in Sjögren’s syndrome patients. Invest Ophthalmol
Vis Sci 2005;46:4474-9. Crossref
65. Ohba E, Dogru M, Hosaka E, et al.
Surgical punctal occlusion with a high heat-energy releasing cautery
device for severe dry eye with recurrent punctal plug extrusion. Am J
Ophthalmol 2011;151:483-7.e1. Crossref
66. DeMartelaere SL, Blaydon SM,
Tovilla-Canales JL, Shore JW. A permanent and reversible procedure to
block tear drainage for the treatment of dry eye. Ophthalmic Plast
Reconstr Surg 2006;22:352-5. Crossref
67. Ervin AM, Law A, Pucker AD. Punctal
occlusion for dry eye syndrome. Cochrane Database Syst Rev
2017;(6):CD006775. Crossref
68. Sakuragawa N, Elwan MA, Uchida S,
Fujii T, Kawashima K. Non-neuronal neurotransmitters and neurotrophic
factors in amniotic epithelial cells: expression and function in humans
and monkey. Jpn J Pharmacol 2001;85:20-3. Crossref
69. Blehm C, Vishnu S, Khattak A, Mitra S,
Yee RW. Computer vision syndrome: a review. Surv Ophthalmol
2005;50:253-62. Crossref
70. Wang MT, Chan E, Ea L, et al.
Randomized trial of desktop humidifier for dry eye relief in computer
users. Optom Vis Sci 2017;94:1052-7. Crossref
71. Aktaş S, Tetikoğlu M, Koçak A, et al.
Impact of smoking on the ocular surface, tear function, and tear
osmolarity. Curr Eye Res 2017;42:1585-9. Crossref