Hong
Kong Med J 2018 Oct;24(5):473–83 | Epub 28 Sep 2018
DOI: 10.12809/hkmj187303
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Risk factors associated with 1-year mortality among
patients with HIV-associated tuberculosis in areas with intermediate
tuberculosis burden and low HIV prevalence
CK Chan, FHKCP, FHKAM (Medicine)1; KH
Wong, FHKCP, FHKAM (Medicine)2; MP Lee, FHKCP, FHKAM (Medicine)3;
Kenny CW Chan, FHKCP, FHKAM (Medicine)4; CC Leung, FHKCP, FHKAM
(Medicine)1; Eric CC Leung, FHKCP, FHKAM (Medicine)1;
WK Chan, BNurs (Hons), MNurs4; Ida KY Mak, BSc, MPhil1
1 Tuberculosis and Chest Service, Centre
for Health Protection, Department of Health, Hong Kong
2 Head Office, Centre for Health
Protection, Department of Health, Hong Kong
3 Department of Medicine, Queen
Elizabeth Hospital, Hospital Authority, Hong Kong
4 Integrated Treatment Centre, Special
Preventive Programme, Centre for Health Protection, Department of Health,
Hong Kong
Corresponding author: Dr CK Chan (chikuen_chan@dh.gov.hk)
Abstract
Introduction: Data are limited
regarding risk factors for mortality among patients with human
immunodeficiency virus (HIV)-associated tuberculosis (TB) in areas with
low HIV prevalence and intermediate TB burden, such as the Western
Pacific region. This study aimed to assess such risk factors in Hong
Kong, which has an intermediate TB burden and low HIV prevalence.
Methods: We conducted a
retrospective cohort analysis of adult patients reported to the Hong
Kong TB-HIV Registry between 2006 and 2015. Baseline characteristics
were compared with Kaplan-Meier estimates. Cox proportional hazards
regression modelling was used to identify factors associated with
mortality.
Results: Of 299 patients
studied, 21 (7.0%) died within 12 months of anti-TB treatment (median
[interquartile range], 7.5 [3.8-10] months). The median age of death was
54 (interquartile range, 40.5-75.0) years. The cause of death was TB in
five and unrelated to TB in the remaining 16. Cox proportional hazards
regression showed that older age (adjusted hazard ratio=4.5; 95%
confidence interval [CI]=1.4-14.9), history of drug addiction (4.6; 95%
CI=1.6-13.0), and low baseline CD4 cell count of <50/μL (2.9; 95%
CI=1.1-7.7) were independent risk factors for death within 12 months.
Conclusion: This study
complements previous studies by providing information regarding risk
factors associated with mortality among patients with HIV-associated TB
in areas with intermediate TB burden and low HIV prevalence. The results
from our study may guide targeted measures to improve survival in other
areas with intermediate TB burden and low HIV prevalence, such as the
Western Pacific region.
New knowledge added by this study
- Previous studies on risk factors associated with mortality among patients with human immunodeficiency virus (HIV)–associated tuberculosis (TB) have mainly examined patients from developing countries with high TB burden and high HIV prevalence, especially from Africa.
- The present study showed that older age, a history of drug addiction, and a low baseline CD4 cell count of <50/μL were independent risk factors for death within 12 months in an area with intermediate TB burden, low HIV prevalence, and good infrastructure.
- This study might facilitate formulation of innovative strategies to improve health outcomes among patients with HIV-associated TB in areas with intermediate TB burden and low HIV prevalence.
Introduction
In 2016, there were an estimated 10.4 million new
tuberculosis (TB) cases worldwide, of which 1.03 million (10%) were among
people living with human immunodeficiency virus (HIV).1 Of the estimated 1.7 million deaths resulting from TB
disease, 0.4 million were among people living with HIV.1 The success rate of TB treatment is generally worse for
patients with HIV than for those without. Globally, the proportion of
patients with TB who died during treatment has been reported as
approximately three-fold higher among patients with HIV than those without
(11% vs 4%); this difference is greatest in the Western Pacific region
(13% vs 2%).1
Identification of risk factors associated with
mortality and innovative strategies to manage disease in high-risk groups
may contribute to reduction in mortality among patients coinfected with TB
and HIV. Lack of drug susceptibility testing, suboptimal initial anti-TB
treatment, disseminated TB, and a low CD4 cell count have been reported as
prognostic factors for increased TB-related mortality in Eastern Europe.2 3
In some areas of Africa with high HIV prevalence, increased odds of
mortality are associated with the following factors: advanced age,
pretreatment sputum smear status, history of TB, and lack of
antiretroviral therapy (ART).4 5 6
However, data regarding risk factors for mortality in the Western Pacific
region are scarce.7 8 In particular, there have been few assessments in areas
with intermediate burden of TB in the Western Pacific region.
Hong Kong has been classified by the World Health
Organization as a place with intermediate TB burden and good
infrastructure. The TB notification rate in Hong Kong was 60.5 per 100 000
population in 2015, of which 40.2% were patients aged >65 years.9 The overall TB mortality rate was 2.3 per 100 000
population in the same year. The prevalence of HIV infection in the
general population in Hong Kong is low (<0.1%).10 Notably, patients with HIV-associated TB constituted
approximately 1% of all TB notifications in Hong Kong.9 Treatment outcomes of a cohort of patients reported to
the territory-wide TB-HIV Registry of the Hong Kong Department of Health,
as of 31 December 2009, have been reported previously.11 The aim of the present study was to assess the 1-year
mortality rate among patients with HIV-associated TB and to identify risk
factors associated with mortality. Therefore, we retrospectively reviewed
the data of patients reported to the TB-HIV Registry between 1 January
2006 and 31 December 2015. The results of this study are expected to guide
targeted measures to improve survival and to inform TB control policies in
areas with intermediate TB burden, such in Hong Kong and throughout the
Western Pacific region.
Methods
Data regarding sex, age, ethnicity, case category,
history of drug addiction, site of TB infection, sputum smear and culture
results, CD4 cell count at TB diagnosis, and ART usage of consecutive
patients reported to the TB-HIV Registry between 1 January 2006 and 31
December 2015 were retrieved and retrospectively reviewed. Tuberculosis
treatment outcomes at 12 months after initiation of treatment, and
mortality data such as the date and cause(s) of death, were obtained from
the database. Further mortality data were obtained by cross-matching with
the statutory death registry by using patients’ identity card
numbers/passport numbers as unique identifiers. For patients who died
within 12 months during anti-TB treatment, relevant clinical records from
chest clinics and hospitals were traced and reviewed.
All the data collected were imported into Epi-Info
software, and exported into SPSS (Windows version 16.0; SPSS Inc, Chicago
[IL], US) for analysis. For the identification of variables potentially
associated with mortality, comparisons of survival status were made by
using Kaplan-Meier estimates. A two-tailed P value <0.05 was considered
statistically significant. An evaluation of the effects of covariates on
mortality was performed by using Cox proportional hazards regression
modelling. Variables that were significant in the univariate analysis or
were of clinical significance were included in the model. Patients who had
been transferred out of Hong Kong before completion of treatment were
censored at the date they were last known to be alive, based on the date
of their most recent contact. For patients who were lost to follow-up, but
were not deceased according to the statutory death registry, the censor
date was 12 months. Kaplan-Meier survival curves were generated to
demonstrate differences in mortality, stratified for a variety of
covariates.
Results
Of 337 patients reported to the TB-HIV Registry
between 2006 and 2015, 17 were excluded either because of duplicate
reporting or revision of diagnosis. Twenty-one patients were tourists or
illegal immigrants with a very short stay in Hong Kong; these were also
excluded, resulting in 299 patients in the analysis. Baseline demographic
and clinical characteristics of these 299 patients, stratified by survival
status at 12 months from the date of start of anti-TB treatment, are shown
in Table 1. Overall, extrapulmonary TB was common,
present in approximately 65% of patients. Sputum smear positivity was
present in more than one-third of patients. Of note, the multidrug
resistance rate among the bacteriologically proven cases was low (2.2%
overall). The overall median CD4 cell count at the time of TB diagnosis
was 100/μL (interquartile range [IQR], 36-204/μL). Of 284 patients who
were alive and had not defaulted or transferred out at 8 weeks from the
initiation of TB treatment, 73 (25.7%) were undergoing ART. The proportion
undergoing ART at 8 weeks was higher among patients reported to the TB-HIV
Registry between 2011 and 2015 than among those reported between 2006 and
2010 (37.3% vs 18.4%; P=0.0005).
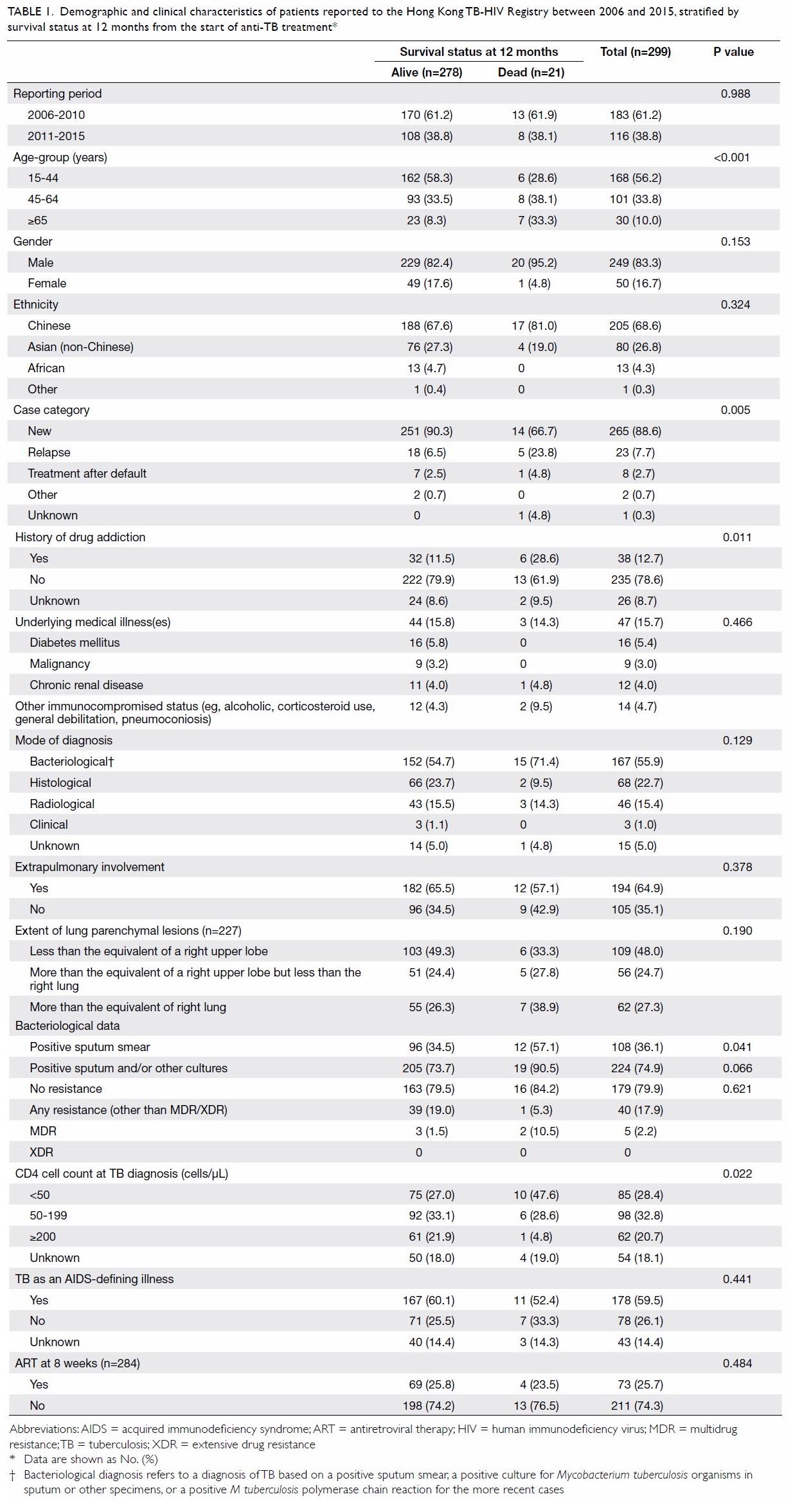
Table 1. Demographic and clinical characteristics of patients reported to the Hong Kong TB-HIV Registry between 2006 and 2015, stratified by survival status at 12 months from the start of anti-TB treatment
Overall, 135 (45.2%) were cured or had completed
treatment at 12 months, whereas 96 (32.1%) remained on an anti-TB
treatment regimen at 12 months due to extensive disease, use of a
non-standard treatment regimen, and/or drug resistance. Twenty-three
(7.7%) patients defaulted treatment for more than 2 months, while 24
(8.0%) patients were transferred out; 21 (7.0%) patients died within 12
months of the initiation of anti-TB treatment. The proportions of patients
who died within 12 months among patients reported to the TB-HIV Registry
between 2011 and 2015 and those reported between 2006 and 2010 were
similar (7.1% vs 6.9%). The median time interval between the initiation of
anti-TB treatment and death was 7.5 months (IQR, 3.8-10 months); the
median age of death was 54 (IQR, 40.5-75.0) years. The cause of death was
due to TB in five patients and unrelated to TB in the remaining 16 (Table
2). A similar proportion of patients with early death within 2
months died from TB (25.0%), compared with those who died after 2 months
(23.5%; P=0.72).
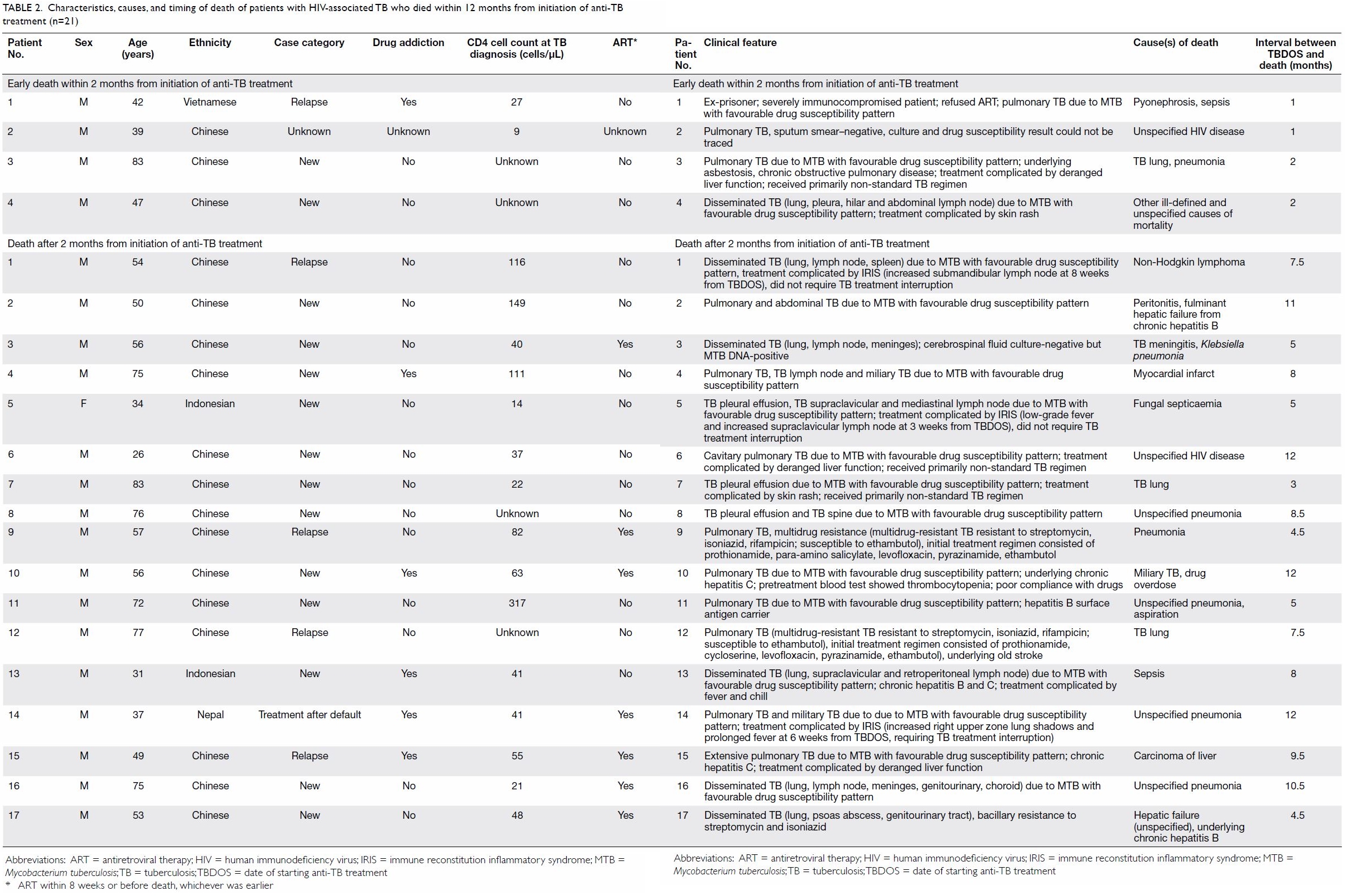
Table 2. Characteristics, causes, and timing of death of patients with HIV-associated TB who died within 12 months from initiation of anti-TB treatment (n=21)
Table 3 summarises the results of univariate (using
Kaplan-Meier estimates) and Cox proportional hazards regression analysis
with respect to 1-year mortality of the 299 cases. In the univariate
analysis, older age, case categories other than new cases, a positive
sputum smear, a history of drug addiction, and a low CD4 cell count
(<50/μL) at the time of TB diagnosis were associated with mortality.
Further analysis with Cox proportional hazards regression showed that
older age (adjusted hazard ratio=4.5; 95% confidence interval
[CI]=1.4-14.9; P=0.012), a history of drug addiction (adjusted hazard
ratio=4.6; 95% CI=1.6-13.0; P=0.005) and a low CD4 cell count (<50/μL)
at the time of TB diagnosis (adjusted hazard ratio=2.9; 95% CI=1.1-7.7;
P=0.03) remained the sole independent risk factors for death within 12
months. Similar findings were observed when ART at 8 weeks was included in
the Cox proportional hazards regression model (results not shown).
Unadjusted Kaplan-Meier survival curves stratified by covariates are shown
in the Figure.

Table 3. Survival status at 12 months of 299 patients with HIV-associated TB and comparison by univariate and Cox proportional hazards regression modelling
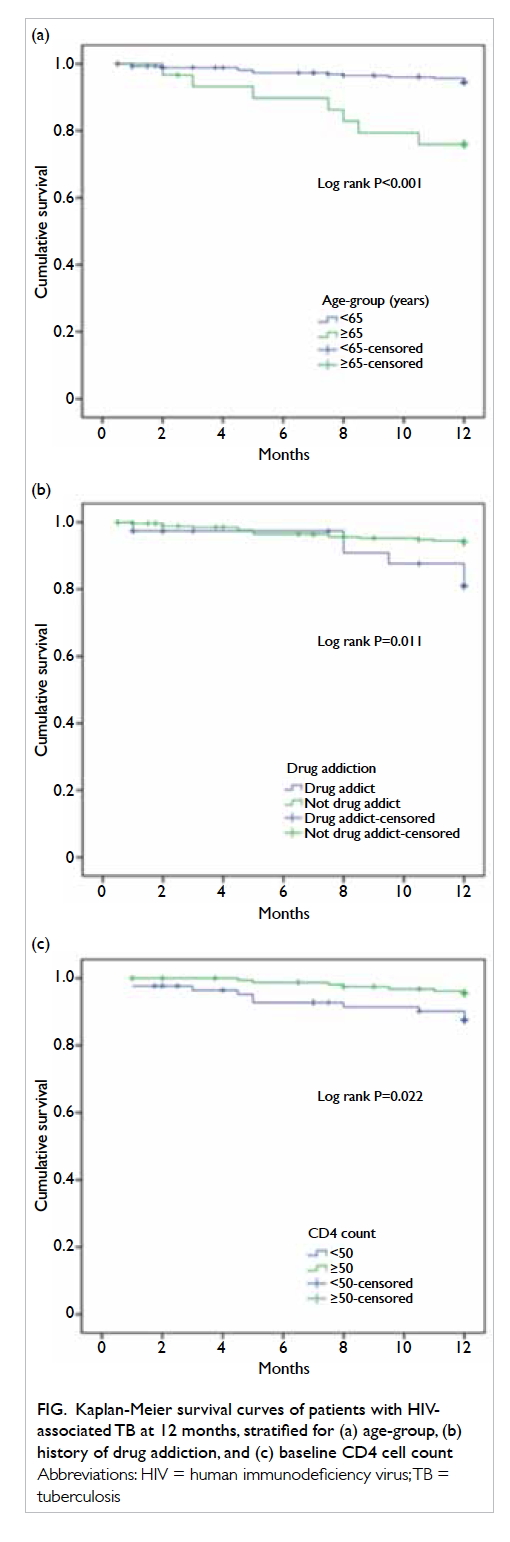
Figure. Kaplan-Meier survival curves of patients with HIVassociated TB at 12 months, stratified for (a) age-group, (b) history of drug addiction, and (c) baseline CD4 cell count
Discussion
This retrospective cohort study was designed to
examine the mortality pattern and factors associated with 1-year mortality
among patients with HIV-associated TB reported to the Hong Kong TB-HIV
Registry. In our cohort, 7.0% died within 12 months of anti-TB treatment
(median [IQR], 7.5 months [3.8-10 months]). The median age of death was 54
(IQR, 40.5-75.0) years. The cause of death was unrelated to TB in >75%
of the cohort. Older age, history of drug addiction, and a low CD4 cell
count (<50/μL) at the time of TB diagnosis were independent risk
factors for death within 12 months.
The 1-year mortality rate of 7.0% among patients
with HIV-associated TB in our cohort was lower than the rates in Eastern
Europe (29%) and Latin America (11%) reported in a study by Podlekareva et
al.2 The mortality rate in our study was also lower than the rates
reported in other countries in the Western Pacific region, such as
Thailand (17%)7 and Cambodia
(22.5%)8 among enrolled patients
with HIV-associated TB. Nonetheless, the rate was higher than the 4%
reported for Western Europe.2
Although these rates might not be directly comparable because of
differences in the study periods and methodologies used, the results from
our study add important data to the current body of literature regarding
mortality risk among patients with HIV-associated TB in the Western
Pacific region.
In our cohort, 76.2% of deaths were unrelated to
TB. This differed from the proportion reported among patients with
HIV-associated TB in Eastern Europe; TB was reported as the cause of death
in >60% of those who died.2
Differences in patient profile, degree of immunosuppression, proportion of
patients receiving ART, and proportion of patients with other
opportunistic infections might have contributed to the differences
observed with respect to the cause(s) of death.
The proportion of patients who died within the
first 2 months of anti-TB treatment due to TB was similar to that among
patients who died after 2 months (25.0% vs 23.5%). This finding differed
from the outcome of a prospective observational study that examined the
causes of death among patients with HIV-associated TB in Thailand: 18 of
33 deaths (55%) within 60 days after initiation of TB treatment were
caused by TB, compared with 11 of 41 deaths (27%) beyond 60 days after
initiation of TB treatment (P=0.02).7
However, the patients in that study exhibited a greater degree of
immunosuppression: the median CD4 count for patients in that study was
55/μL (IQR, 18-142/μL), compared with a median CD4 count of 100/μL (IQR,
36-204/μL) in our cohort.
The finding that older age constitutes a risk
factor for mortality in our cohort is consistent with findings in some
other studies.4 5 12 In a
recently reported retrospective 10-year electronic record review of
patients with TB in a South African province, although the large majority
of patients with TB that died were aged 18 to 49 years, the odds of dying
were incrementally higher in older age-groups: 8 to 17 years (adjusted
odds ratio [AOR]=2.0; 95% CI=1.5-2.7), 18 to 49 years (AOR=5.8; 95%
CI=4.0-8.4), 50 to 64 years (AOR=7.7; 95% CI=4.6-12.7), and ≥65 years
(AOR=14.4; 95% CI=10.3-20.2).4 A
similar finding was observed in another South African study.5 In our cohort, the median age of death was 54 years.
Patients aged ≥65 years had an adjusted hazard ratio of 4.5 for dying,
compared with younger patients. Atypical presentation, increased
comorbidity, and a higher proportion of drug-related adverse events might
have contributed to the increased odds of death in the older age-group.
In our cohort, a history of drug addiction was
significantly associated with death at 12 months (adjusted hazard
ratio=4.6; 95% CI=1.6-13.0). In the study by The TB:HIV Study Writing
Group, injection drug use was reported as a significant predictor of death
(hazard ratio=2.11; 95% CI=1.04-4.26); notably, this became
non-significant after adjustment due to high correlation with the region
of residence.3 The association
between a history of drug abuse and mortality in our cohort—also reported
in some other studies—is not surprising. Poor access to and uptake of
health services, poor adherence, and treatment default are well-known to
pose unique challenges for treatment of drug users.13 In a study of 291 patients with smear- and
culture-positive pulmonary TB presenting for retreatment in Morocco,
substance users were 2.7 times more likely to default treatment, compared
with non-substance users (AOR=2.73; 95% CI=1.04-7.15).14 Because injection drug users exhibit a high
prevalence of chronic viral hepatitis and alcohol abuse, hepatotoxic
reactions to anti-TB drugs may be more common.15
In a study that examined the role of hepatitis C virus and HIV in anti-TB
drug-induced hepatotoxicity, Ungo et al16
found that patients with TB exhibited a four-to-five-fold increased risk
of drug-induced hepatitis if coinfected with viral hepatitis or HIV;
moreover, they exhibited a 14-fold increased risk if coinfected with both.
In addition, efavirenz, nevirapine, and rifampicin may increase methadone
clearance, thus causing methadone withdrawal; this may dissuade drug users
from continuing treatment.15
In a study that examined TB-related mortality in
people living with HIV in Eastern Europe and Latin America, a CD4 cell
count of <50/μL was associated with increased TB-related mortality
(hazard ratio=3.46; 95% CI=2.02-5.95; P<0.0001) after adjustment for
multidrug-resistant TB status.2 In
another study conducted in Ethiopia, a CD4 cell count of <75/μL was
also associated with increased mortality (adjusted hazard ratio=4.83; 95%
CI=1.98-11.77).6 In our cohort,
consistent with the findings from prior studies, patients with
HIV-associated TB with a low CD4 cell count (<50/μL) at the time of TB
diagnosis had an adjusted hazard ratio of 2.9 (95% CI=1.1-7.7; P=0.03) for
mortality at 1 year. The findings from our study and other studies support
the recommendation that ART be initiated early during TB treatment in
patients with HIV.
Case category other than new cases was associated
with increased mortality at 12 months in univariate analysis in our study.
However, the association disappeared in the adjusted model. Of note, prior
history of TB or retreatment has been associated with increased mortality
in some other studies.5 17 Because our study was a retrospective case review, it
is uncertain whether misclassification or inadequate control for other
unknown confounding factors might have obscured an association between
case category and mortality.
Studies regarding the association between sputum
smear status and death have reported conflicting results. In a
retrospective cohort study of predictors of TB mortality in Khayelitsha,
South Africa, an area with high HIV prevalence, no association was found
between smear status and death.5
Similarly, in a study of patients coinfected with TB and HIV at a teaching
hospital in Ghana, no differences were reported in clinical outcome
between patients with smear-negative TB status and those with
smear-positive TB status.18 In a
prospective study of 827 adult TB in-patients registered at Zomba
Hospital, Malawi, of whom 77% were HIV-seropositive, patients with
smear-negative pulmonary TB exhibited the highest death rates during 32
months of follow-up (hazard ratio=2.7; 95% CI=2.1-3.5; P<0.001,
compared to smear-positive patients).19
Similar findings were reported in studies of patients with HIV-associated
TB in South Africa and in Cameroon.17
20 Conversely, in a study that
examined patients with HIV with culture-confirmed pulmonary TB in the US
from 1993 to 2006, patients with smear-negative TB exhibited better
survival than patients with smear-positive disease, both before (hazard
ratio=0.82; 95% CI=0.75-0.90) and after (hazard ratio=0.81; 95%
CI=0.71-0.92) the introduction of combination ART.21 In our study, a
positive sputum smear was associated with mortality in univariate
analysis, but not in multivariate analysis. The results from our study
regarding the effect of AFB smear status on survival were similar to those
from the study in the US. The reasons for observed differences in survival
among patients with HIV with sputum smear-negative TB between
resource-rich and resource-limited settings are likely complex; these
might have been a result of differences in study populations, the
availability of diagnostics, misdiagnosis, or differences in treatment
regimens.21
Of note, ART was not a prognostic factor for
mortality in our study, either for the entire cohort or when the analysis
was restricted to the subgroup with a CD4 cell count of <50/μL (results
for the latter not shown). In addition, the mortality rate among patients
in the later cohort (2011 to 2015) remained similar compared to the
earlier cohort (2006 to 2010), although the proportion of patients
receiving ART at 8 weeks had doubled. This conflicts with the finding that
ART reduces mortality, reported in some other studies.3 5 The failure
of ART to affect mortality in our study might have been because ART was
selectively initiated for patients with more advanced HIV-associated TB
(ie, by attending physicians) during the study period. High age-related
mortality in the cohort, as discussed above, and inadequate control for
other confounding factors might have masked the protective effect of ART.
The strength of this study is that it included a
relatively complete set of data regarding clinical features of all
patients, as well as the cause(s) and timing of death among patients who
died. Such data have been less frequently reported in previous studies.
The primary limitation is that it was a retrospective study conducted
under programme conditions. Our risk factor evaluation was limited by the
availability of data present in the database of the TB-HIV Registry and in
clinical records. Information regarding case categories, drug addiction,
and CD4 cell count was unavailable for a small subset of patients.
Classifying patients with missing case category information as new cases
and those with missing substance use data as non-users might have biased
our analyses of these risk factors such that no significant differences
were found. The time lag between HIV diagnosis and initiation of ART could
not be determined because information regarding the date of HIV diagnosis
was missing for some patients in the earlier portion of the study period,
as well as for patients who were diagnosed outside Hong Kong. Furthermore,
information regarding the time lag between presentation of patients to the
health care system and the initiation of anti-TB treatment was not
captured in the TB-HIV Registry. Therefore, the potential effect of such
delay in the initiation of anti-TB treatment on mortality could not be
examined. Another limitation is that the accuracy of the data in the
programme record forms (on which the database was constructed) and death
certificates for coding TB as the underlying cause of death was unknown.
Nonetheless, for those patients who died, relevant clinical records from
chest clinics and hospitals were traced for review. Misclassification
regarding the cause(s) of death should be minimal. Finally, the small
number of patients who died, limited statistical power, and inadequate
controls for other unknown confounding factors may have concealed the
effects of significant variables, as discussed above.
Notwithstanding the aforementioned limitations, we
demonstrated that older age, history of drug addiction, and low CD4 cell
count (<50/μL) at TB diagnosis were independent risk factors for death
within 12 months. This study complements previous studies by providing
information regarding risk factors associated with mortality among
patients with HIV-associated TB in an area in the Western Pacific region
with intermediate TB burden and low HIV prevalence. The results from our
study may have important implications for health care programmes; they may
aid in formulating innovative strategies and new guidelines to guide
targeted measures for the management of disease among subgroups of
patients at risk of death from TB or HIV during the course of anti-TB
treatment. As such, they may help optimise TB and HIV management in the
Western Pacific region.
Author contributions
Concept or design of study: CK Chan, ECC Leung, CC
Leung, KH Wong.
Acquisition of data: CK Chan, IKY Mak, WK Chan, KCW Chan, MP Lee.
Analysis or interpretation of data: ECC Leung, CC Leung, CK Chan, MP Lee, KCW Chan, IKY Mak.
Drafting of the article: CK Chan, ECC Leung.
Critical revision for important intellectual content: All authors.
Acquisition of data: CK Chan, IKY Mak, WK Chan, KCW Chan, MP Lee.
Analysis or interpretation of data: ECC Leung, CC Leung, CK Chan, MP Lee, KCW Chan, IKY Mak.
Drafting of the article: CK Chan, ECC Leung.
Critical revision for important intellectual content: All authors.
Acknowledgement
The authors would like to thank Dr CM Tam, previous
Consultant Chest Physician in-charge of Tuberculosis and Chest Service,
Centre for Health Protection, Department of Health of Hong Kong, for his
contributions to the conception and design of this work, as well as his
valuable comments on the early draft of this article.
Declaration
All authors have disclosed no conflicts of
interest. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity. The findings of this study were presented
in part at the 13th Hong Kong, Macau, Taiwan, Shanghai and Guangdong
Tuberculosis Conference, Hong Kong, 2 November 2017.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
This study was approved by the Ethics Committee of
the Department of Health of Hong Kong.
References
1. World Health Organization. Global
Tuberculosis Report 2017. Geneva: World Health Organization; 2017.
2. Podlekareva DN, Efsen AM, Schultze A, et
al. Tuberculosis-related mortality in people living with HIV in Europe and
Latin America: an international cohort study. Lancet HIV 2016;3:e120-31. Crossref
3. TB:HIV Study Writing Group. One-year
mortality of HIV-positive patients treated for rifampicin- and
isoniazid-susceptible tuberculosis in Eastern Europe, Western Europe, and
Latin America. AIDS 2017;31:375-84. Crossref
4. Heunis JC, Kigozi NG, Chikobvu P, Botha
S, van Rensburg HD. Risk factors for mortality in TB patients: a 10-year
electronic record review in a South African province. BMC Public Health
2017;17:38. Crossref
5. Pepper DJ, Schomaker M, Wilkinson RJ, de
Azevedo V, Maartens G. Independent predictors of tuberculosis mortality in
a high HIV prevalence setting: a retrospective cohort study. AIDS Res Ther
2015;12:35. Crossref
6. Sileshi B, Deyessa N, Girma B, Melese M,
Suarez P. Predictors of mortality among TB-HIV co-infected patients being
treated for tuberculosis in Northwest Ethiopia: a retrospective cohort
study. BMC Infect Dis 2013;13:297. Crossref
7. Cain KP, Anekthananon T, Burapat C, et
al. Causes of death in HIV-infected persons who have tuberculosis,
Thailand. Emerg Infect Dis 2009;15:258-64. Crossref
8. Marcy O, Laureillard D, Madec Y, et al.
Causes and determinants of mortality in HIV-infected adults with
tuberculosis: an analysis from the CAMELIA ANRS 1295-CIPRA KH001
randomized trial. Clin Infect Dis 2014;59:435-45. Crossref
9. Tuberculosis and Chest Service, Centre
for Health Protection, Department of Health, Hong Kong SAR Government.
Annual Report 2015. Available from:
http://www.info.gov.hk/tb_chest/doc/Annual_Report_2015.pdf. Accessed 18
Sep 2018.
10. Special Preventive Programme, Centre
for Health Protection, Department of Health, Hong Kong SAR Government. HIV
surveillance report-2015 update. Available from:
http://www.info.gov.hk/aids/english/surveillance/sur_report/hiv15.pdf.
Accessed 18 Sep 2018.
11. Chan CK, Wong KH, Leung CC, et al.
Treatment outcomes after early initiation of antiretroviral therapy for
human immunodeficiency virus-associated tuberculosis. Hong Kong Med J
2013;19:474-83. Crossref
12. Waitt CJ, Squire SB. A systematic
review of risk factors for death in adults during and after tuberculosis
treatment. Int J Tuberc Lung Dis 2011;15:871-85. Crossref
13. Deiss RG, Rodwell TC, Garfein RS.
Tuberculosis and illicit drug use: review and update. Clin Infect Dis
2009;48:72-82. Crossref
14. Dooley KE, Lahlou O, Ghali I, et al.
Risk factors for tuberculosis treatment failure, default, or relapse and
outcomes of retreatment in Morocco. BMC Public Health 2011;11:140. Crossref
15. World Health Organization. Integrating
collaborative TB and HIV services within a comprehensive package of care
for people who inject drugs. Geneva: World Health Organization; 2016.
16. Ungo JR, Jones D, Ashkin D, et al.
Antituberculosis drug-induced hepatotoxicity. The role of hepatitis C
virus and the human immunodeficiency virus. Am J Respir Crit Care Med
1998;157:1871-6. Crossref
17. Mabunda TE, Ramalivhana NJ, Dambisya
YM. Mortality associated with tuberculosis/HIV co-infection among patients
on TB treatment in the Limpopo province, South Africa. Afr Health Sci
2014;14:849-54. Crossref
18. Mudd J, Jaramillo L, Kwara E, et al.
Impact of smear-negative results on tuberculosis outcome in HIV
co-infected patients at a teaching hospital in Ghana. Ann Glob Health
2016;82:516.
19. Kang’ombe C, Harries AD, Banda H, et
al. High mortality rates in tuberculosis patients in Zomba Hospital,
Malawi, during 32 months of follow-up. Trans R Soc Trop Med Hyg
2000;94:305-9. Crossref
20. Djouma FN, Noubom M, Ngomba AV,
Donfack H, Kouomboua PS, Saah MA. Determinants of death among tuberculosis
patients in a semi urban diagnostic and treatment centre of Bafoussam,
West Cameroon: a retrospective case-control study. Pan Afr Med J
2015;22:253. Crossref
21. Cavanaugh JS, Shah NS, Cain KP,
Winston CA. Survival among patients with HIV infection and smear-negative
pulmonary tuberculosis–United States, 1993-2006. PLoS One 2012;7:e47855. Crossref

