DOI: 10.12809/hkmj154501
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Electroconvulsive therapy for new-onset super-refractory
status epilepticus
Eric LY Chan, MB, BS, FRCP; WC Lee, MB, BS, FHKAM (Medicine); CK Koo, MB, BS, FHKCA;
Horace ST King, BNHS, FPHKAN; CT Woo, FPHKAN; SH Ng, MB, BS, FHKAM (Medicine)
Department of Medicine and Geriatrics, Tuen Mun Hospital, Tuen Mun, Hong Kong
Corresponding author: Dr Eric LY Chan (chanlye@ha.org.hk)
Introduction
Despite the advances in neuroscience and medical
therapy for epilepsy, status epilepticus, especially
when refractory or super-refractory (defined as
seizure that continues or recurs ≥24 hours after onset
of anaesthetic therapy, including cases that recur on
reduction or withdrawal of anaesthesia),1 remains an
enormous challenge. Multiple and high-dose drug
loading is usually prescribed but may be futile. New
modalities of treatment including hypothermia and
ketogenic diets have been tried with some success
in reported case series.2 We report a case of new-onset
super-refractory status epilepticus treated
successfully with electroconvulsive therapy (ECT).
Case presentation
A 31-year-old male with a history of childhood asthma
presented to Tuen Mun Hospital in November 2012
following onset of generalised tonic-clonic seizure
at home. He had upper respiratory symptoms with
fever, myalgia, and cough for a week previously.
There was no history of recent travel or drug abuse.
Physical examination revealed no focal
neurological abnormalities. Investigations showed
a normal routine blood picture and renal
function except mild liver impairment with
alanine aminotransferase level of 72 U/L. General
autoimmune (antinuclear antibodies, anti–early
nuclear antigen antibodies, C3/C4 and antithyroid
antibodies) and toxicology screening were negative.
Dried blood spot test for neurometabolic screening
was also negative. Examination of cerebrospinal fluid
showed white blood cell count 9 per mm3, red blood
cell 2 per mm3, protein 0.54 g/dL, and glucose 5.4 g/dL.
Microbiological investigations (herpes simplex virus,
human immunodeficiency virus, Japanese encephalitis
virus, varicella zoster virus, and enteroviruses) were
negative. Serology for neurosyphilis and leptospirosis
was also negative. Serum anti-CASPR2 Ab, anti-LGI1
Ab, anti-VGKC Ab, and anti-NMDAR Ab (serum and
cerebrospinal fluid) were all negative.
He was initially treated with intravenous
acyclovir and ceftriaxone for presumed acute infectious
meningoencephalitis. Routine electroencephalogram
(EEG) showed a generalised slow background of 4 to
6 Hz without epileptiform discharges. He developed
a clustering of generalised tonic-clonic seizures 2
days later and was admitted to the intensive care
unit. He underwent mechanical ventilation and
aggressive treatment with medication at the maximal
tolerable dosage including intravenous phenytoin
(300 mg/d), valproate (1200 mg/d), midazolam (~60
mg/h), propofol (up to 110 mg/h), phenobarbitone
(300 mg/d), and levetiracetam (3000 mg/d). Despite
treatment he remained convulsive with seizures
evident on EEG. Intravenous immunoglobulin was
first given 8 days following admission for possible
autoimmune encephalitis but was unsuccessful.
Electroencephalogram showed generalised epilepti-form
discharges and runs of EEG seizure activity
over the bitemporal and bifrontocentral regions. His
condition was later complicated by rhabdomyolysis
and renal failure (creatine phosphokinase up to 47 000
U/L) that was controlled by aggressive intravenous
fluid administration.
Magnetic resonance imaging of the brain
(Fig 1) showed multiple patchy areas of cortical
T2 hyperintensity bilaterally that were more
indicative of epileptic changes with the possibility of
encephalitis. Electroencephalogram finally reached
burst suppression and seizure suppression following
infusion of thiopentone (300 mg/h) and ketamine
(220 mg/h). The former was withdrawn because of
sepsis that was treated with ticarcillin/sulbactam
and meropenem/ertapenem. The generalised
epileptiform discharges and seizures returned 8 days
later despite such aggressive treatment.
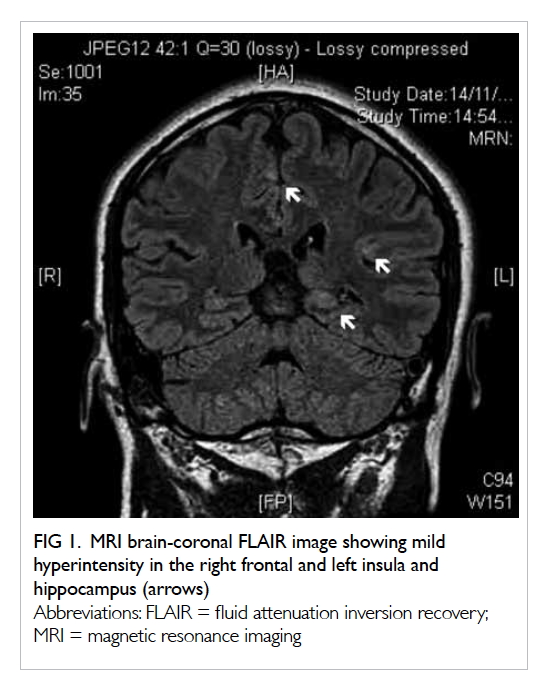
Figure 1. MRI brain-coronal FLAIR image showing mild hyperintensity in the right frontal and left insula and hippocampus (arrows)
Hypothermia by external cooling (18 days
after admission) with body temperature reduced to
32°C with a ketogenic diet (81% lipid, 4.7% Chinese
hamster ovary and 13.9% protein) and urine ketosis
had no effect. Plasmapheresis was attempted on day
22 but also failed.
Finally, ECT was attempted using the
spECTrum 5000Q (Techsan, Czech Republic) and
followed the standard psychiatric protocol for
treatment of refractory major depression. Ketamine
and propofol continued throughout the procedure.
The first course of ECT commenced 30 days after
admission, and was administered 3 times per day for
3 days:
- Day 1: pulse width at 0.5 ms, frequency 40 Hz × 1 and 60 Hz × 2, duration of 8 seconds, current 800 mA, 200 J
- Day 2: pulse width at 0.5 ms, frequency 60 Hz × 2 and 80 Hz × 1, duration of 8 seconds, current 800 mA, 200 J (tonic seizure, EEG seizure, and R arm clonus-induced)
- Day 3: pulse width at 0.5 ms, frequency 80 Hz × 3, duration of 8 seconds, current 800 mA, 200 J (tonic seizure–induced and EEG showed spindle coma)
Attenuation and abolition of continuous
lateralised epileptiform discharges and seizures were
achieved with interictal focal epileptiform discharge
over the right frontal region only. The EEG seizure
induced by stimulation comprised generalised fast
beta activities different to the patient’s own seizure
activities.
The EEG from the first day of the first course
stimulation is shown in FIgure 2.3 4 The second
course was given 8 days later (again thrice per day for
3 days) as there was no sustainable improvement. In
this course, all therapies were given with pulse width
0.5 ms, frequency 80 Hz, duration of 8 seconds,
and current 800 mA, 200 J after referencing the
EEG response of the last stimulation. Arm clonus,
with one arm paralysed with muscle relaxants and
the other for observing EEG-induced seizure and
threshold titration, was observed in 10 of the 15
stimulations.
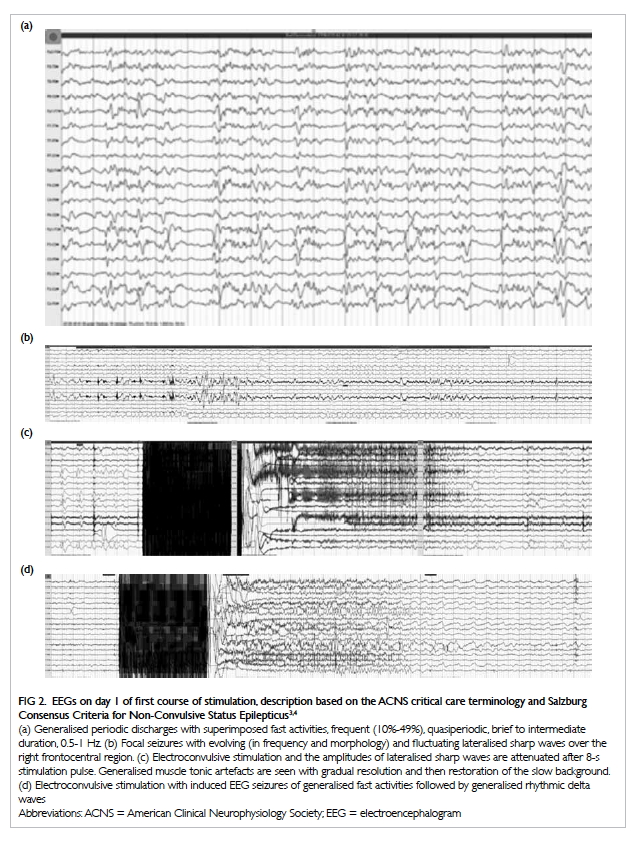
Figure 2. EEGs on day 1 of first course of stimulation, description based on the ACNS critical care terminology and Salzburg Consensus Criteria for Non-Convulsive Status Epilepticus3 4
(a) Generalised periodic discharges with superimposed fast activities, frequent (10%-49%), quasiperiodic, brief to intermediate duration, 0.5-1 Hz. (b) Focal seizures with evolving (in frequency and morphology) and fluctuating lateralised sharp waves over the right frontocentral region. (c) Electroconvulsive stimulation and the amplitudes of lateralised sharp waves are attenuated after 8-s stimulation pulse. Generalised muscle tonic artefacts are seen with gradual resolution and then restoration of the slow background. (d) Electroconvulsive stimulation with induced EEG seizures of generalised fast activities followed by generalised rhythmic delta waves
Electroencephalogram 1 week after
completion of the second course showed a triphasic
wave pattern rather than the previous generalised
periodic discharges with EEG seizures over the
right frontocentral and right hemisphere. The
patient had hyperammonaemia, likely secondary to
hepatotoxicity due to the prolonged use of multiple
antiepileptics and anaesthetics, and was treated with
sodium benzoate.
Oxcarbazepine and lacosamide were added for
focal electrographic seizures. Electroencephalogram
10 days after ECT continued to show generalised
continuous slow waves with intermittent rhythmic
slowing of 1 Hz. There was some eye blinking but no
ictal EEG changes.
Electroencephalogram 1 month after ECT
showed an improved background of 6 to 8 Hz and
occasional EEG seizures over the right frontocentral
region, as well as clinically automotor seizures.
The patient was transferred back to the
general ward 1 month later and commenced
active rehabilitation. He was discharged home 3
months later, although he continued to require a
frame for walking and experienced short duration
of breakthrough seizures. His positron emission
tomography scan later showed no evidence of
malignancy. One year later, the patient remained
ambulatory with aids but with cognitive decline and
personality changes. He was able to self-care, but his
seizures remained pharmacoresistant.
Discussion
This is the first case of super-refractory status
epilepticus, defined as status epilepticus that
continues or recur ≥24 hours after the onset of
anaesthetic therapy including those cases that recur
on reduction or withdrawal of anaesthesia,1 that has
been treated with ECT successfully in our locality.
There are only individual reports describing
the use of ECT for status epilepticus over the last 30
years,5 6 although its use was first described in the
1940s. It was not until the introduction of super-refractory
status epilepticus7 that the role of multiple
exploratory therapies (those without support from
systemic investigations or clinical trials including
use of ketamine, hypothermia, ketogenic diet, and
ECT) were added to the management protocol.8 The
most promising news for this specific seizure status
nonetheless comes from the recent discovery of the
treatable autoimmune encephalitic nature of many
such cases with specific identifiable antibodies such
as anti-NMDAR Ab, anti-LGI1 Ab, and anti-VGKC
Ab.
The term NORSE (new-onset refractory status
epilepticus) was introduced in 20059 for patients
with refractory status epilepticus and no history
of seizures and no identifiable aetiology. Reviewing
the limited literature, these cases reported
usually have features suggestive of an infectious
or inflammatory nature with febrile episodes or
abnormal cerebrospinal fluid pleocytosis.10 These
cases are most likely to be autoimmune encephalitis,
but the antibodies are not available or have not yet
been identified. Our patient was likely true NORSE,
although the possibility of a postinfectious or
autoimmune mechanism cannot be excluded as the
panel of testing has not been exhausted.
Electroconvulsive therapy in status epilepticus
was first described by Carrasco González et al in 1997
and Viparelli and Viparelli in 1992.5 11 Since then,
there have been other case reports or series reporting
success of this therapy, both in adult and paediatric
patients.12 13 It is usually applied with the withdrawal
of anticonvulsants or anaesthetics. Mechanisms
suggested include enhanced gamma-aminobutyric
acid inhibition, the effect of paradoxical stimulation
of status epilepticus and electrical modulation.14 In
our patient, anticonvulsants or anaesthetic agents
were given without an end date and we applied ECT
in addition to, not instead of, such drug therapy. The
EEG epileptiform discharges showed immediate
attenuation following electrical stimulation, and
supports the possibility of enhancing the seizure
threshold or an inhibitory mechanism. The later
EEG changes were related to significant metabolic
encephalopathy (hyperammonaemia) rather than
previous runs of epileptiform discharges, also
suggested the modulatory effect of ECT when a
course was given rather than just a few shots. Of
course, one would also argue that the improvement
could be the late effect of previous intravenous
immunoglobulin or plasmapheresis although these
had no immediate effect on the EEG or clinical
seizures or epileptiform discharges.
Despite the apparent successful outcome
for our patient following the addition of ECT, we
require more cases, both adult and paediatric, with
such treatment applied as well as a clear definition
of the status epilepticus stages (early, refractory or
super-refractory) and specific categorisation of the
syndrome and aetiology (autoimmune or cryptogenic
to be NORSE) before we can confidently support the
role and effectiveness of this physical therapy.
Declaration
The authors have no conflicts of interest to disclose.
References
1. Shorvon S, Trinka E. The London-Innsbruck Status
Epilepticus Colloquia 2007-2011, and the main advances
in the topic of status epilepticus over this period. Epilepsia
2013;54(Suppl 6):11-3. Crossref
2. Cervenka MC, Hocker S, Koenig M, et al. Phase I/II
multicenter ketogenic diet study for adult superrefractory
status epilepticus. Neurology 2017;88:938-43. Crossref
3. Hirsch LJ, LaRoche SM, Gaspard N, et al. American
Clinical Neurophysiology Society’s Standardized Critical
Care EEG Terminology: 2012 version. J Clin Neurophysiol
2013;30:1-27. Crossref
4. Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG
terminology and criteria for nonconvulsive status
epilepticus. Epilepsia 2013;54(Suppl. 6):28-9. Crossref
5. Carrasco González MD, Palomar M, Rovira R.
Electroconvulsive therapy for status epilepticus. Ann
Intern Med 1997;127:247-8. Crossref
6. Griesemer DA, Kellner CH, Beale MD, Smith GM.
Electroconvulsive therapy for treatment of intractable
seizures. Initial findings in two children. Neurology
1997;49:1389-92. Crossref
7. Shorvon S. Super-refractory status epilepticus: an approach
to therapy in this difficult clinical situation. Epilepsia
2011;52(Suppl 8):53-6. Crossref
8. Shorvon S, Ferlisi M. The treatment of super-refractory
status epilepticus: a critical review of available therapies
and a clinical treatment protocol. Brain 2011;134:2802-18. Crossref
9. Wilder-Smith EP, Lim EC, Teoh HL, et al. The NORSE (new-onset
refractory status epilepticus) syndrome: defining a
disease entity. Ann Acad Med Singapore 2005;34:417-20.
10. Gall CR, Jumma O, Mohanraj R. Five cases of new onset
refractory status epilepticus (NORSE) syndrome: outcomes
with early immunotherapy. Seizure 2013;22:217-20. Crossref
11. Viparelli U, Viparelli G. ECT and grand mal epilepsy.
Convuls Ther 1992;8:39-42.
12. Kamel H, Cornes SB, Hegde M, Hall SE, Josephson SA.
Electroconvulsive therapy for refractory status epilepticus:
a case series. Neurocrit Care 2010;12:204-10. Crossref
13. Lambrecq V, Villéga F, Marchal C, et al. Refractory status
epilepticus: electroconvulsive therapy as a possible
therapeutic strategy. Seizure 2012;21:661-4. Crossref
14. Walker MC. The potential of brain stimulation in status
epilepticus. Epilepsia 2011;52(Suppl 8):61-3. Crossref

