Hong Kong Med J 2018 Jun;24(3):226–37 | Epub 4 Jun 2018
DOI: 10.12809/hkmj176939
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
The first pilot study of expanded newborn screening for
inborn errors of metabolism and survey of related knowledge and opinions
of health care professionals in Hong Kong
Chloe M Mak, MD, FHKAM (Pathology)1;
Eric CY Law, PhD, FHKAM (Pathology)2,3; Hencher HC Lee, MA,
FRCPA1; WK Siu, PhD, FHKAM (Pathology)1; KM Chow,
FRCOG, FHKAM (Obstetrics and Gynaecology)4; Sidney KC Au Yeung,
FRCOG, FHKAM (Obstetrics and Gynaecology)5; Hextan YS Ngan,
FRCOG, FHKAM (Obstetrics and Gynaecology)6; Niko KC Tse,
FHKCPaed, FHKAM (Paediatrics)7; NS Kwong, FHKCPaed, FHKAM
(Paediatrics)8; Godfrey CF Chan, FHKCPaed, FHKAM (Paediatrics)9;
KW Lee, FRCOG, FHKAM (Obstetrics and Gynaecology)4; WP Chan,
MB, ChB, FHKAM (Obstetrics and Gynaecology)4; SF Wong, FRCOG,
FHKAM (Obstetrics and Gynaecology)5; Mary HY Tang, FRCOG, FHKAM
(Obstetrics and Gynaecology)6; Anita SY Kan, MRCOG, FHKAM
(Obstetrics and Gynaecology)6; Amelia PW Hui, FRCOG, FHKAM
(Obstetrics and Gynaecology)6; PL So, FRCOG, FHKAM (Obstetrics
and Gynaecology)5; CC Shek, FHKCPaed, FHKAM (Paediatrics)7;
Robert SY Lee, FHKCPaed, FHKAM (Paediatrics)7; KY Wong, FHKCPaed, FHKAM (Paediatrics)9;
Eric KC Yau, FHKCPaed, FHKAM (Paediatrics)7; KH Poon, MRCP(UK),
FHKCPaed8; Sylvia Siu, MB, ChB, FHKAM (Paediatrics)8;
Grace WK Poon, FHKCPaed, FHKAM (Paediatrics)9; Anne MK Kwok,
FHKCPaed, FHKAM (Paediatrics)9; Judy WY Ng, BAppSc(Nurs), MSSc
(Counselling)4; Vera CS Yim, FHKAN (HKCMW), MSC5;
Grace GY Ma, BSN, MHSM (Health Services Management)6; CH Chu,
MS10; TY Tong, MSc1; YK Chong, FHKCPath, FHKAM
(Pathology)1; Sammy PL Chen, FRCPA, FHKAM (Pathology)1;
CK Ching, FRCPA, FHKAM (Pathology)1; Angel OK Chan, MD, FHKAM
(Pathology)3; Sidney Tam, FRCP, FHKAM (Pathology)4;
Ruth LK Lau, MB, ChB, FHKAM (Pathology)11; WF Ng, MB, ChB,
FHKAM (Pathology)11; KC Lee, MB, ChB, FHKAM (Pathology)1;
Albert YW Chan, MD, FHKAM (Pathology)1; CW Lam, PhD, FHKAM
(Pathology)2
1 Chemical Pathology Laboratory,
Department of Pathology, Princess Margaret Hospital, Kwai Chung, Hong Kong
2 Department of Pathology, The
University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
3 Division of Clinical Biochemistry,
Queen Mary Hospital, Pokfulam, Hong Kong
4 Department of Obstetrics and
Gynaecology, Princess Margaret Hospital, Kwai Chung, Hong Kong
5 Department of Obstetrics and
Gynaecology, Tuen Mun Hospital, Tuen Mun, Hong Kong
6 Department of Obstetrics and
Gynaecology, Queen Mary Hospital, Pokfulam, Hong Kong
7 Department of Paediatrics and
Adolescent Medicine, Princess Margaret Hospital, Kwai Chung, Hong Kong
8 Department of Paediatrics and
Adolescent Medicine, Tuen Mun Hospital, Tuen Mun, Hong Kong
9 Department of Paediatrics and
Adolescent Medicine, Queen Mary Hospital, Pokfulam, Hong Kong
10 Department of Pathology, United
Christian Hospital, Kwun Tong, Hong Kong
11 Department of Pathology, Yan Chai
Hospital, Tsuen Wan, Hong Kong
Corresponding author: Dr CW Lam (ching-wanlam@pathology.hku.hk)
Abstract
Introduction: Newborn screening
is important for early diagnosis and effective treatment of inborn
errors of metabolism (IEM). In response to a 2008 coroners’ report of a
14-year-old boy who died of an undiagnosed IEM, the OPathPaed service
model was proposed. In the present study, we investigated the
feasibility of the OPathPaed model for delivering expanded newborn
screening in Hong Kong. In addition, health care professionals were
surveyed on their knowledge and opinions of newborn screening for IEM.
Methods: The present prospective
study involving three regional hospitals was conducted in phases, from 1
October 2012 to 31 August 2014. The 10 steps of the OPathPaed model were
evaluated: parental education, consent, sampling, sample dispatch, dried
blood spot preparation and testing, reporting, recall and counselling,
confirmation test, treatment and monitoring, and cost-benefit analysis.
A fully automated online extraction system for dried blood spot analysis
was also evaluated. A questionnaire was distributed to 430 health care
professionals by convenience sampling.
Results: In total, 2440 neonates
were recruited for newborn screening; no true-positive cases were found.
Completed questionnaires were received from 210 respondents. Health care
professionals supported implementation of an expanded newborn screening
for IEM. In addition, there is a substantial need of more education for
health care professionals. The majority of respondents supported
implementing the expanded newborn screening for IEM immediately or
within 3 years.
Conclusion: The feasibility of
OPathPaed model has been confirmed. It is significant and timely that
when this pilot study was completed, a government-led initiative to
study the feasibility of newborn screening for IEM in the public health
care system on a larger scale was announced in the Hong Kong Special
Administrative Region Chief Executive Policy Address of 2015.
New knowledge added by this study
- The feasibility of the OPathPaed service model was evaluated in 2440 neonates. The main focus was on parental education, consent, sampling, sample dispatch, dried blood spot preparation and testing, reporting, recall, and counselling.
- Of 210 health care professionals who responded to a survey, 73.6% were unaware of newborn screening for inborn errors of metabolism (IEM), 87.6% urged for more education, and 91.3% supported implementing expanded newborn screening for IEM immediately or within 3 years.
- The OPathPaed service model for implementing expanded newborn screening for IEM is feasible for local public hospital settings.
- Health care professionals support implementation of newborn screening for IEM. In addition, there is a substantial need of more education.
Introduction
The expansion of newborn screening (NBS) for
various genetic disorders with a focus on inborn errors of metabolism
(IEM) has become a mandatory part of health care policy worldwide.
Multiplex testing by tandem mass spectrometry has extended the scope of
NBS far beyond the traditional ‘one test for one disease’ paradigm,
requiring only a tiny blood sample, obtained by a simple heel prick.1 2 As a result,
many inherited diseases are now screened for to allow early diagnosis and
intervention and thereby prevent permanent damage or potential deaths.
Inborn errors of metabolism are a group of rare
metabolic diseases with heterogeneous clinical presentations and genetic
aetiologies. They are individually rare but collectively common. In 2011,
Lee et al3 reported a 5-year
retrospective review on the laboratory diagnosis of amino acid disorders,
organic acidurias, and fatty acid beta-oxidation defects in three regional
hospitals. The overall local incidence of classical IEM was 1 in 4122 live
births.3 No phenylketonuria was
identified through the screening of 18 000 newborns in the early 1970s.4 Hyperphenylalaninaemia was the
second most common amino acid disorder reported by Lee et al,3 with an incidence of 1 in 29 542 live births. Another
study by Hui et al5 reported the
overall incidence of common IEM as 1 in 5400. According to the Hong Kong
Paediatric Metabolic Registry, there were two cohorts, the first one with
20 years from 1982 to 2002 with 89 IEM patients and the second one with 14
years from 1996 to 2010 with 120 IEM patients. The estimated incidence of
IEM was 1 in 7580 (unpublished data); however, as that was a voluntary
case-finding study from several hospitals, the incidence was likely to be
an underestimate. These figures are similar to those reported worldwide,
such as 1 in 5800 in mainland China,6
1 in 5882 in Taiwan,7 and 1 in 4000
in America.8
In 2000, a mandatory NBS programme for
hyperphenylalaninaemia, congenital hypothyroidism, and congenital deafness
was implemented in mainland China.9
In 2006, the American College of Medical Genetics recommended 29 metabolic
diseases (IEM) for which screening should be mandated.10 Since then, the scope of this recommendation has been
expanding (Recommended Uniform Screening Panel, the Secretary of the
Department of Health and Human Services11).12 In Hong Kong, population
screening for congenital hypothyroidism and glucose-6-phosphate
dehydrogenase (G6PD) deficiency using umbilical cord blood has been
mandatory since March 1984 under the Neonatal Screening Unit of the
Clinical Genetic Service, Department of Health. This programme has
resulted in a significant reduction in related morbidities and
mortalities.
In 2008, a coroner inquest was called to
investigate the sudden death of a 14-year-old boy with a postmortem
genetic diagnosis of glutaric acidaemia type II.13
The Coroners’ Report demanded that “The Department of Health, the Hospital
Authority, the Faculty of Medicine of various universities and others
concerned should carry out a feasibility study to see whether universal
check may be carried out on all newborn babies for congenital metabolism
defect.”14
To be effective, an expanded NBS programme needs to
be coupled with improved general awareness of IEM and NBS. Educational
support and training are required for frontline clinicians engaged in the
diagnosis and care of patients with IEM.15
Several studies have shown that health care professionals do not have
satisfactory awareness and knowledge of IEM.15
16 17
18 Therefore, a better
understanding of the awareness of IEM among health care professionals in
Hong Kong is needed.
We have conducted the first feasibility pilot study
on the expanded NBS service model in a hospital setting in Hong Kong and
the first survey on the knowledge and opinions on NBS for IEM among health
care professionals in Hong Kong.
Methods
This prospective pilot study was conducted in
phases from 1 October 2012 to 31 August 2014, involving three public
hospitals and The University of Hong Kong (HKU), with over 40
collaborators from departments of pathology, paediatrics, and obstetrics.
Phases 1 and 2 involved a single-site study conducted at Princess Margaret
Hospital from 1 October 2012 to 31 October 2013 and then at Tuen Mun
Hospital from 1 November 2013 to 31 March 2014. Phase 3 was university
(HKU)-based and the recruitment was open to the public from 3 March 2014
to 31 August 2014. Phase 4 was a two-site study at the Tuen Mun Hospital
and Queen Mary Hospital from 4 April 2014. Phase 5 was carried out at all
three hospitals from 2 July 2014 until 31 August 2014. The OPathPaed model
for expanded NBS was used for evaluation.19
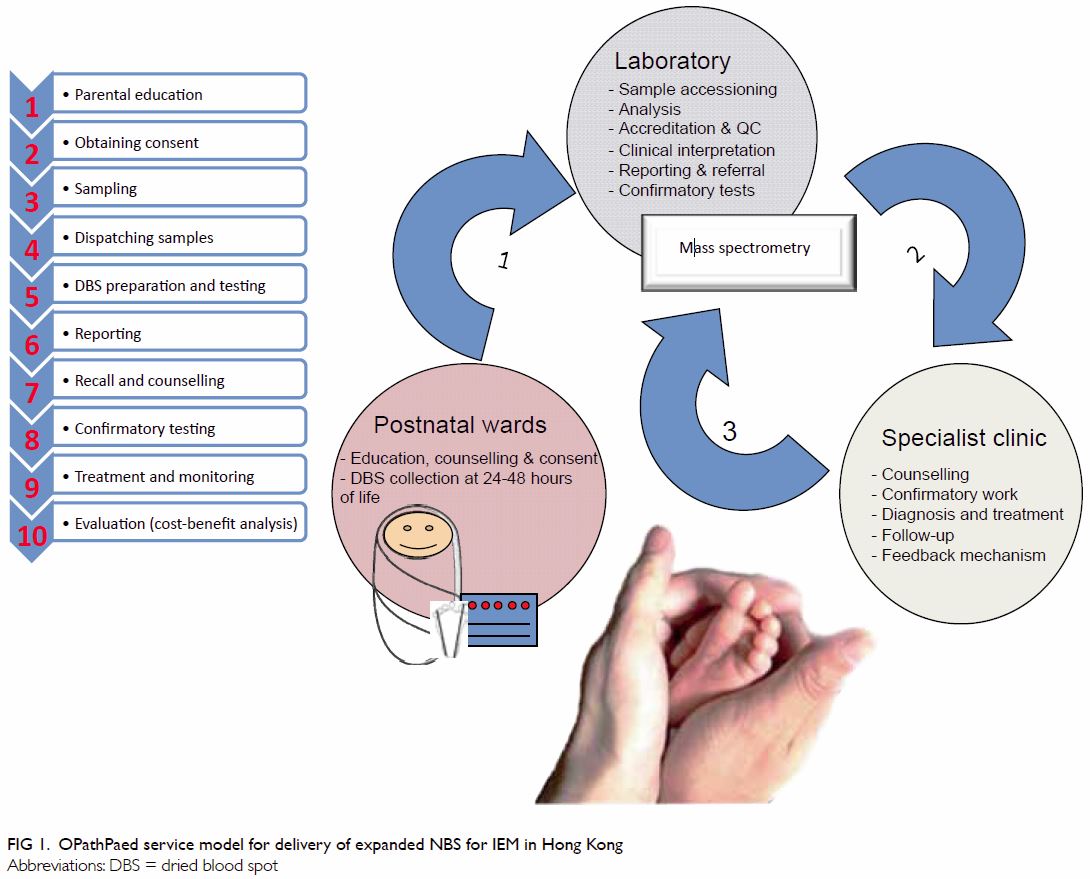
The OPathPaed model includes 10 steps: parental education, consent,
sampling, sample dispatch, dried blood spot (DBS) preparation and testing,
reporting, recall and counselling, confirmation test, treatment and
monitoring, and cost-benefit analysis (Fig 1).
Pilot study to investigate the feasibility of the
10-step OPathPaed model
Step 1: Parental education
Educational talks were delivered by chemical
pathologists during antenatal visits. With the help of the Save Babies
Through Screening Foundation, we added Chinese subtitles to the video
titled “Newborn Screening Saves Babies One Foot at a Time”. The video is
available online (https://www.youtube.com/watch?v=dxFit_a601w).
DVDs and a locally designed pamphlet with an email address and telephone
number for enquiries were distributed to expectant mothers (Fig
2). In order to raise public awareness, several interviews with the
media were arranged and reports were published in several newspapers20 21 22 and radio and television programmes.23 24

Figure 2. Chinese version of pilot study pamphlet on newborn screening for inborn errors of metabolism
Step 2: Obtaining consent
A consent form was designed for NBS for IEM (data
not shown). Educational videos and pamphlets were used to inform the
parents. Written informed consent was collected during a postnatal talk
after the education session. The talk was conducted in group presentation
for the mothers by chemical pathologists.
Step 3: Sampling
Paediatricians or pathologists organised training
for phlebotomists on the heel prick technique, in compliance with the
Clinical and Laboratory Standard Institute guidelines.25 An instruction sheet with photographs of valid and
invalid DBS samples was provided as guidance for the phlebotomists (Fig
3). Samples were collected from neonates aged between 24 hours and
28 days.
Step 4: Dried blood spot dispatching
Drying racks and special boxes designed for
specimen transport before complete drying were delivered to the testing
sites. Complete drying of blood spots was ensured for valid sample
integrity. The blood spot cards were dried perpendicular to each other
above and below the rack position to avoid contact contamination between
blood spots of different patients.
Step 5: Dried blood spot preparation and testing
Two commercial DBS assay kits: (1) MassChrom Amino
Acids and Acylcarnitines from Dried Blood/Non-derivatised (Chromsystems
Instruments & Chemicals GmBH, Gräfelfing, Germany); and (2) NeoBase
Non-derivatized MSMS kit (with succinylacetone assay; PerkinElmer, Waltham
[MA], US) were validated for use in the study. In addition to a manual
puncher and an autopuncher for DBS preparation, a fully automated online
extraction system (DBS-MS 500; CAMAG, Muttenz, Switzerland) was also
evaluated. The precision and local reference intervals of the commercial
assay kits are listed in Table 1. Our laboratory has participated in the
Newborn Screening Quality Assurance Programme organised by the US Centers
for Disease Control and Prevention (CDC) since 2011. The disease panel
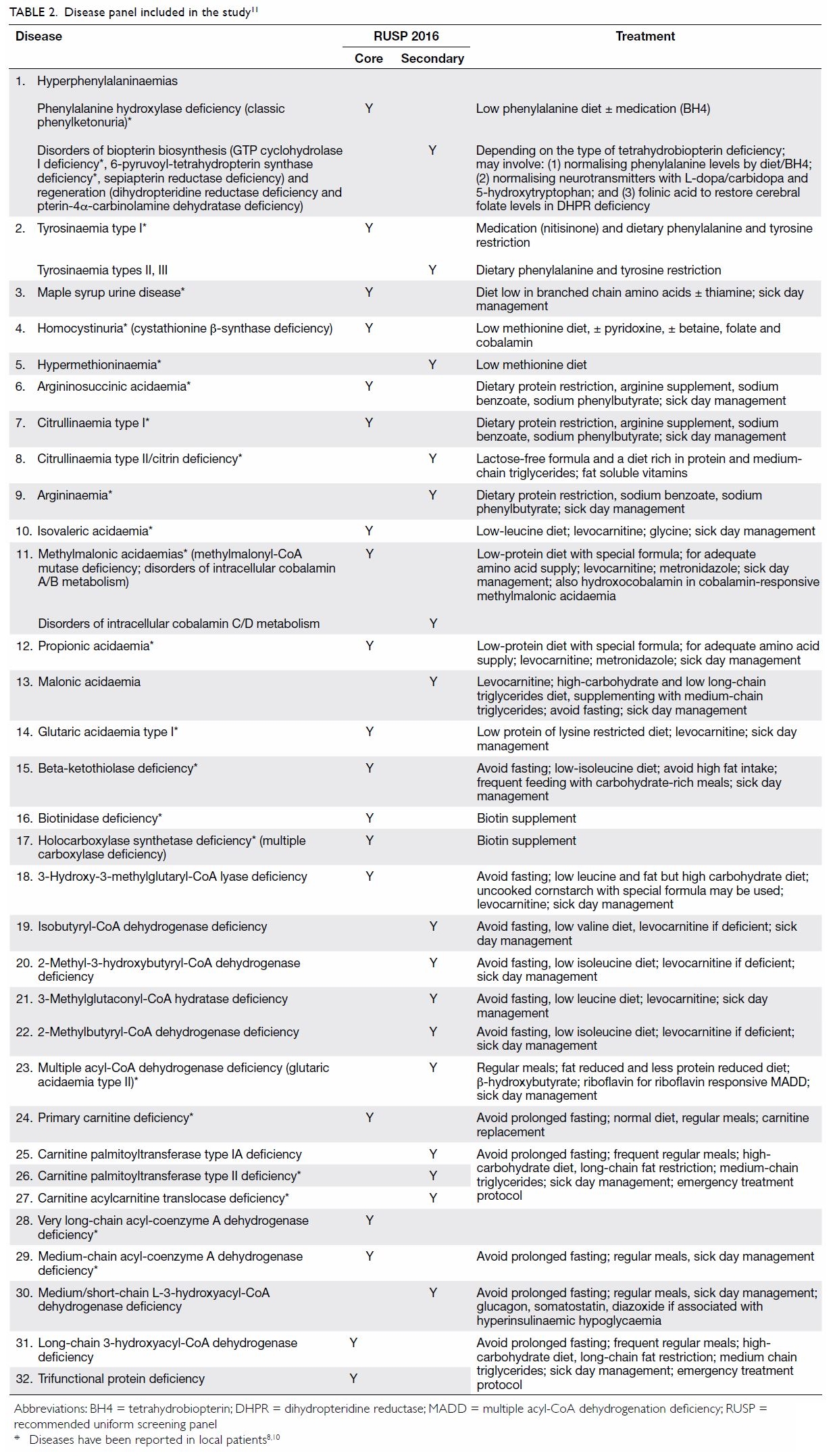
included in the study is shown in Table 2.8 10 11
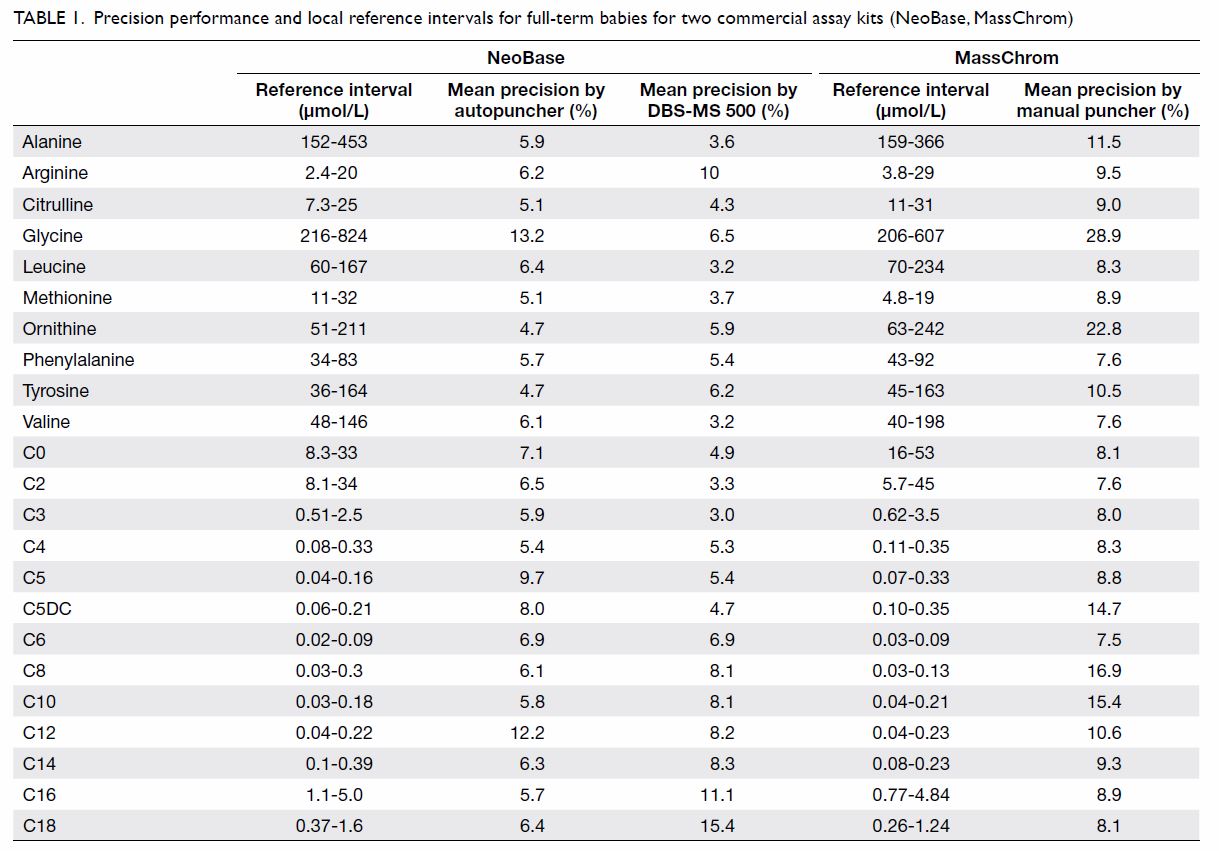
Table 1. Precision performance and local reference intervals for full-term babies for two commercial assay kits (NeoBase, MassChrom)
Step 6: Reporting
Chemical pathologists were responsible for
reporting of positive results to the paediatricians. The CDC cut-off for
clinical decision (https://wwwn.cdc.gov/NSQAP/Restricted/CDCCutOffs.aspx)
and the Region 4 Stork Collaborative Project (https://www.clir-r4s.org/)
data interpretation tools were applied during interpretation of the
results.
Step 7: Recall and counselling
Newborn Screening ACT Sheets and Confirmatory
Algorithms by the American College of Medical Genetics (https://www.ncbi.nlm.nih.gov/books/NBK55827/)
were followed for patient recall. All abnormal results were examined by
chemical pathologists. These chemical pathologists were also responsible
for contacting the parents for post-test counselling and for arranging
subsequent hospital referrals for care by paediatricians.
Step 8: Confirmation test
Confirmation of diagnosis was provided by regional
laboratories through measurements of functional metabolites (mainly plasma
amino acid levels, plasma acylcarnitine levels, and urine organic acid
levels) and genetic diagnosis by DNA sequencing wherever appropriate.
Step 9: Treatment and monitoring
Admission logistics and treatment protocols for
neonatal units with on-call rosters were established by hospital
paediatricians. The same regional laboratories mentioned in Step 8
continued to provide biochemical diagnostic services.
Step 10: Cost-benefit analysis
A cost-benefit analysis has been conducted and
published previously.26
Hyperphenylalaninaemia due to 6-pyruvoyl-tetrahydropterin synthase
deficiency was used as an example to evaluate the costs and benefits of
implementing an expanded NBS programme in Hong Kong. Assuming an annual
birth rate of 50 000 and hyperphenylalaninaemia incidence of 1 in 29 542
live births, the annual medical costs and adjusted loss of workforce would
be HK$20 773 207. The implementation and operational costs of an expanded
NBS programme are expected to be HK$10 473 848 annually. Thus,
implementing the expanded NBS programme is expected to result in an annual
saving of HK$9 632 750.26
Survey of health care professionals’ knowledge and
opinions of newborn screening for inborn errors of metabolism
A questionnaire was distributed by convenience
sampling to 430 health care professionals who worked in hospitals and were
not involved in the pilot study. These self-administered questionnaires
were distributed to local health care professionals including medical
doctors, nurses, and other allied health care professionals either in
person with returning envelopes or via email to department heads for
further distribution. The self-administered questionnaire in English was
modified from a previously published questionnaire that was tested among
parents.27 The self-administered
questionnaire included 13 questions that covered the local practice of the
existing NBS programme, as well as knowledge and opinions of an expanded
NBS programme. No personal identifiers were included in the questionnaire
and questions were mostly in a closed-ended format. Data analyses were
performed using Excel 2000 (Microsoft Corp. Redmond [WA], US) and GraphPad
QuickCalcs (http://graphpad.com/quickcalcs/ConfInterval1.cfm).
Percentages for each question were calculated as the number of replies
divided by the total number of respondents for that question. The
questions and corresponding responses are shown in Table 3.
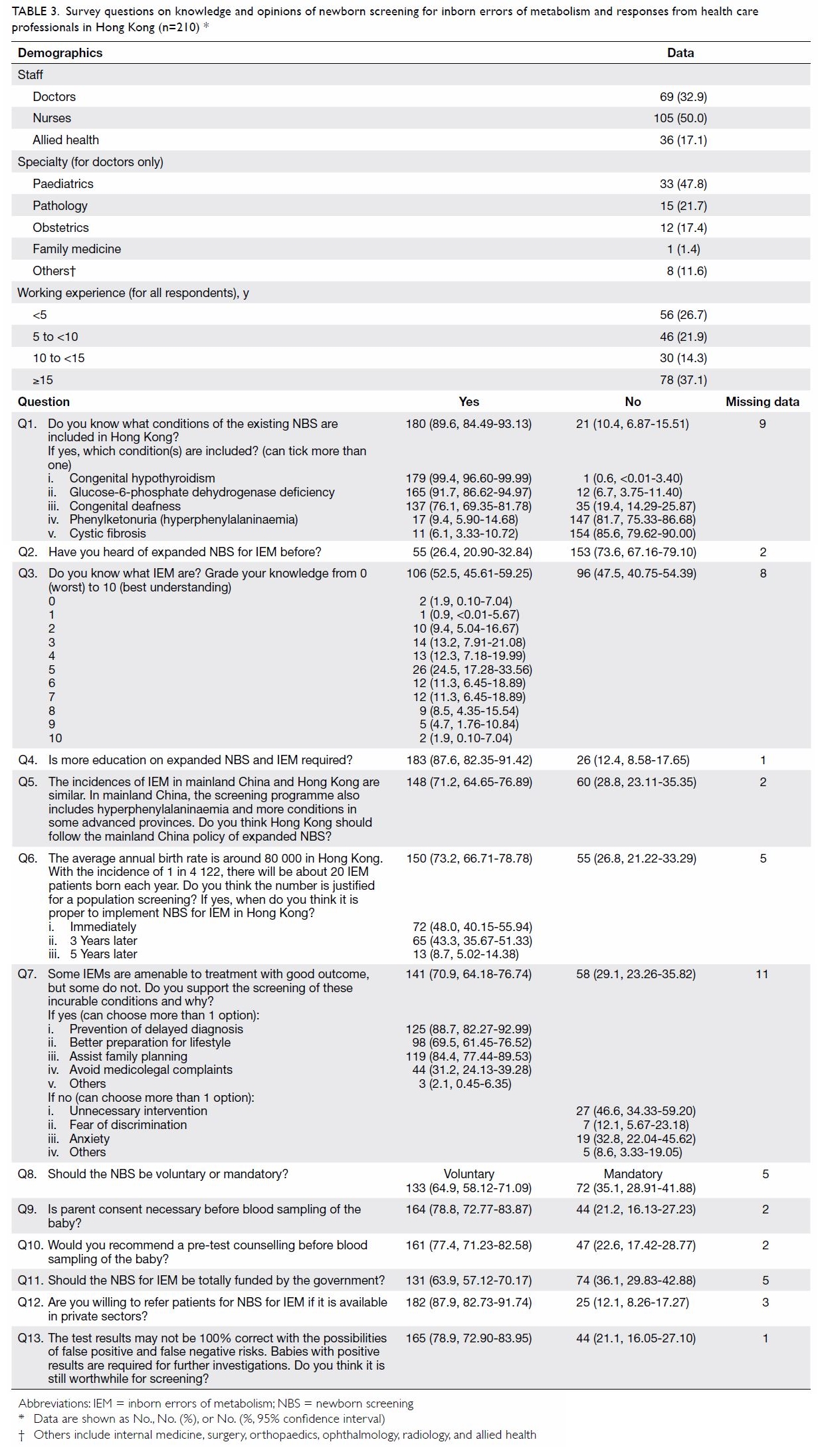
Table 3. Survey questions on knowledge and opinions of newborn screening for inborn errors of metabolism and responses from health care professionals in Hong Kong (n=210)
Results
Pilot study recruitment
By 31 August 2014, 2440 neonates had been
recruited. The DBSs were collected from neonates aged 24 to 48 hours
(n=2064, 84.6%), 3 to 5 days (n=331, 13.6%), 5 to 7 days (n=9, 0.4%), and
7 to 28 days (n=36, 1.5%). The participation rate was 86.6% on the days
when blood samples were collected. There were no recorded DBS sampling or
dispatch failures. The method validation and results of the DBS amino
acids and acylcarnitine assays have been published elsewhere28; further details are available from the corresponding
author on request. Overall, no true-positive cases were found in this
pilot study, likely because of the limited sample size. Six (0.25%)
false-positive cases were detected in 2440 neonates; of these, two had
mild elevations in long-chain acylcarnitine levels, two had high tyrosine
levels, one had a high citrulline level, and one had a low free carnitine
level. Subsequent laboratory findings were all normal. No false-negative
cases were reported from the IEM clinics of the involved hospitals within
2 years after project completion. However, patients who emigrated or
received treatment at private institutions could not be followed up.
Health care professionals’ knowledge and opinions of
newborn screening for inborn errors of metabolism
A total of 430 questionnaires were distributed and
210 (48.8%) completed responses were received. Results are shown in Table
3. Of the respondents, 50.0% were nurses and 32.9% were doctors. The
doctors worked mainly in departments of paediatrics (47.8%), pathology
(21.7%), and obstetrics (17.4%). Most (89.6%) respondents were aware of
the existing NBS programme for hypothyroidism and G6PD deficiency;
however, 47.5% did not know about IEM and 73.6% had not heard of expanded
NBS for IEM. Most (87.6%) respondents agreed that more education on IEM
and NBS is needed.
Discussion
This is the first prospective pilot study on NBS
for IEM in Hong Kong, and it has successfully evaluated the feasibility of
the OPathPaed model. This study is also the first to investigate the
knowledge and opinions on NBS for IEM of local health care professionals.
To implement an expanded NBS programme for IEM
successfully in Hong Kong, there are several important points that need to
be addressed. First, awareness and knowledge of NBS for IEM among the
general public and among health care professionals should be improved.27 Second, comprehensive data on the local disease
spectrum and incidence should be made available; such data were not
available until recently.3 5 Third, free flow of information and sharing of
experiences among colleagues working in the acute care and public health
sectors should be facilitated. Fourth, more emphasis should be given to
regular updates on NBS health care policy, confirmatory investigation
service support, and treatment protocols. Last, the use of umbilical cord
blood samples in the existing programme is unsuitable for an expanded NBS
programme for IEM because of unacceptably high false-negative rates.29 The metabolites associated with many amino acid
disorders, organic acid disorders, and fatty acid oxidation disorders are
not elevated in cord blood. In 2013, the hospital-based OPathPaed model
was published for the implementation of an expanded NBS programme suitable
for a local setting.19 The present
study further confirms the feasibility of the OPathPaed model for use on a
larger scale. The OPathPaed model integrates expert input from
obstetricians, pathologists, and paediatricians. Because babies born in
Hong Kong are normally delivered in hospitals, the OPathPaed model
approach should be able to achieve full coverage.
The success of an expanded NBS programme for IEM
would depend not only on the diagnostics but also on how well patients
diagnosed with IEM could be managed. It is difficult to accumulate
experience and the many metabolic diseases can easily cause confusion. In
addition, sophisticated management requires individualised drug
formulations, which may not be easily accessible or may involve off-label
prescriptions. Overseas studies have identified significant knowledge gaps
among clinicians involved in the follow-up care of newborns with IEM
identified by NBS.15 16 17 18 Some were poorly prepared to follow up the initial
diagnosis, provide appropriate counselling, or make appropriate clinical
referrals.17 In our study, 73.6%
of 210 health care professionals (who were not involved in the pilot
study) were unaware of the expanded NBS programme, and 47.5% of
respondents did not know what IEM were. The majority of respondents
(87.6%) agreed that better education was needed and 91.3% supported
expanding NBS for IEM immediately or within 3 years. According to a
parental survey among 172 parents regarding NBS for IEM,27 over 89% had never heard of NBS for IEM or metabolic
disorders. Although some IEM may be incurable, 97% of parents supported an
expanded NBS programme and 82.8% of parents supported implementation of
this expansion immediately or within 3 years.27
The present study also provides the first local
evaluation of the fully automated DBS-MS 500 system. The DBS is directly
eluted into the extraction chamber, with an online extraction system
connecting with the tandem mass spectrometer. There is no need for DBS
card punching. Together with the integrated optical card recognition and
barcode reading module, this automation minimises the risk of sample
misidentification during manual processing. The precision and accuracy
demonstrated are comparable to those of conventional procedures. However,
because the DBS-MS 500 system requires application of an internal standard
solution before extraction, the financial cost per extraction would be
higher than that for conventional methods. In addition, special DBS cards
are required for the extraction chamber. Third-party DBS cards of a
specific quality may not easily fit into the system. The throughput of up
to 500 DBS cards per run is more than adequate for local needs, as there
are about 50 000 live births annually in Hong Kong.
The limitations of the pilot study include small
and non-representative sample size, a relatively short study period that
may have been inadequate for follow-up to confirm true negatives, and the
convenience sampling and low response rate of the health care professional
survey.
Conclusion
The present pilot study investigated the
feasibility of an expanded NBS for IEM in Hong Kong, and surveyed health
care professionals for their knowledge and opinions on NBS for IEM. We
successfully evaluated the OPathPaed model on a larger scale than has been
attempted previously and demonstrated that health care professionals have
a favourable opinion of implementing an expanded NBS programme in Hong
Kong. It is timely that, as this pilot study was completed, the needs of
parents and health care workers were addressed in the Hong Kong Special
Administrative Region Chief Executive’s Policy Address of 2015, when a
government-led initiative was announced to study the feasibility of NBS
for IEM in the public health care system on a large scale.
Author contributions
All authors have made substantial contributions to
the concept or design of this study; acquisition of data; analysis or
interpretation of data; drafting of the article; and critical revision for
important intellectual content.
Acknowledgement
We acknowledge all collaborators, doctors, nurses,
medical technologists, phlebotomists, information technologists, and
parents for their efforts and support. We thank the Save Babies Through
Screening Foundation for allowing us to use their video for educational
purpose. We thank CAMAG Germany for providing technical support during the
evaluation of the DBS-MS 500. The CAMAG had no role in the study design,
data collection, analysis, reporting, or manuscript preparation.
Funding/support
This work was funded by the SK Yee Medical
Foundation. The funder had no role in study design, data collection,
analysis, interpretation, or manuscript preparation.
Declaration
All authors have no conflicts of interest to
disclose. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility
for its accuracy and integrity.
Ethical approval
Local ethical approval was obtained from each of
the regional hospitals involved in this study.
References
1. Millington DS, Kodo N, Norwood DL, Roe
CR. Tandem mass spectrometry: a new method for acylcarnitine profiling
with potential for neonatal screening for inborn errors of metabolism. J
Inherit Metab Dis 1990;13:321-4. Crossref
2. Carpenter KH, Wiley V. Application of
tandem mass spectrometry to biochemical genetics and newborn screening.
Clin Chim Acta 2002;322:1-10. Crossref
3. Lee HC, Mak CM, Lam CW, et al. Analysis
of inborn errors of metabolism: disease spectrum for expanded newborn
screening in Hong Kong. Chin Med J (Engl) 2011;124:983-9.
4. Davies DP. Hong Kong Reflections:
Health, Illness and Disability in Hong Kong Children. Hong Kong: The
Chinese University Press; 1995.
5. Hui J, Tang NL, Li CK, et al. Inherited
metabolic diseases in the Southern Chinese population: spectrum of
diseases and estimated incidence from recurrent mutations. Pathology
2014;46:375-82. Crossref
6. Gu X, Wang Z, Ye J, Han L, Qiu W.
Newborn screening in China: phenylketonuria, congenital hypothyroidism and
expanded screening. Ann Acad Med Singapore 2008;37(12 Suppl):107-10.
7. Niu DM, Chien YH, Chiang CC, et al.
Nationwide survey of extended newborn screening by tandem mass
spectrometry in Taiwan. J Inherit Metab Dis 2010;33(Suppl 2):S295-305. Crossref
8. Chace DH, Kalas TA, Naylor EW. The
application of tandem mass spectrometry to neonatal screening for
inherited disorders of intermediary metabolism. Annu Rev Genomics Hum
Genet 2002;3:17-45. Crossref
9. Zheng S, Song M, Wu L, et al. China:
public health genomics. Public Health Genomics 2010;13:269-75. Crossref
10. American College of Medical Genetics
Newborn Screening Expert Group. Newborn screening: toward a uniform
screening panel and system—executive summary. Pediatrics 2006;117(5 Pt
2):S296-307. Crossref
11. Recommended Uniform Screening Panel,
The Advisory Committee on Heritable Disorders in Newborns and Children, US
Department of Health and Human Services. Available from:
https://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendedpanel/.
Accessed 1 Aug 2017.
12. Therrell BL, Johnson A, Williams D.
Status of newborn screening programs in the United States. Pediatrics
2006;117(5 Pt 2):S212-52. Crossref
13. Lee HC, Lai CK, Siu TS, et al. Role of
postmortem genetic testing demonstrated in a case of glutaric aciduria
type II. Diagn Mol Pathol 2010;19:184-6. Crossref
14. Coroners’ Report 2008. Available from:
http://www.judiciary.hk/en/publications/coroner_report_july08.pdf.
Accessed 1 Aug 2017.
15. Gennaccaro M, Waisbren SE, Marsden D.
The knowledge gap in expanded newborn screening: survey results from
paediatricians in Massachusetts. J Inherit Metab Dis 2005;28:819-24. Crossref
16. Wells AS, Northrup H, Crandell SS, et
al. Expanded newborn screening in Texas: a survey and educational module
addressing the knowledge of pediatric residents. Genet Med 2009;11:163-8.
Crossref
17. Kemper AR, Uren RL, Moseley KL, Clark
SJ. Primary care physicians’ attitudes regarding follow-up care for
children with positive newborn screening results. Pediatrics
2006;118:1836-41. Crossref
18. Dunn L, Gordon K, Sein J, Ross K.
Universal newborn screening: knowledge, attitudes, and satisfaction among
public health professionals. South Med J 2012;105:218-22. Crossref
19. Mak CM, Lam C, Siu W, et al. OPathPaed
service model for expanded newborn screening in Hong Kong SAR, China. Br J
Biomed Sci 2013;70:84-8. Crossref
20. 一滴血驗出罕見遺傳病. Oriental Daily 2010 Sep
12. Available from:
http://orientaldaily.on.cc/cnt/news/20100912/00176_002.html. Accessed 1
Aug 2017.
21. 二代新生嬰兒篩檢代謝疾病. am730 2013 May 6.
Available from: http://archive.am730.com.hk/column-153216. Accessed 1 Aug
2017.
22. 篩查防智障代謝病, 社會可年省近千萬. Ming Pao 2014 Jun
9. Available from: https://news.mingpao.com/pns/篩查防智障代謝病%20社會可年省近千萬/web_tc/article/20140609/s00002/1402257010439. Accessed 1 Aug 2017.
23. 精靈一點 (RTHK radio programme, 2014 Apr
15). Available from:
http://programme.rthk.hk/channel/radio/programme.php?name=radio1/adwiser&d=2014-04-15&p=1147&e=259149&m=episode.
Accessed 1 Aug 2017.
24. 星期二檔案:這幾滴血 (TVB programme, 2014 Feb
25). Available from:
http://programme.tvb.com/news/tuesdayreport/episode/20140225/#page-1.
Accessed 1 Aug 2017.
25. NBS01-A6, Blood Collection on Filter
Paper for Newborn Screening Programs; Approved Standard—Sixth Edition.
Available from:
https://clsi.org/standards/products/newborn-screening/documents/nbs01/.
Accessed 1 Aug 2017.
26. Lee HH, Mak CM, Poon GW, Wong KY, Lam
CW. Cost-benefit analysis of hyperphenylalaninemia due to
6-pyruvoyl-tetrahydropterin synthase (PTPS) deficiency: for consideration
of expanded newborn screening in Hong Kong. J Med Screen 2014;21:61-70. Crossref
27. Mak CM, Lam CW, Law CY, et al.
Parental attitudes on expanded newborn screening in Hong Kong. Public
Health 2012;126:954-9. Crossref
28. Mak M. Chemical pathology analysis of
inborn errors of metabolism for expanded newborn screening in Hong Kong
[thesis]. The University of Hong Kong; 2012. Available from:
http://hub.hku.hk/handle/10722/180075. Accessed 1 Aug 2017.
29. Walter JH, Patterson A, Till J, Besley
GT, Fleming G, Henderson MJ. Bloodspot acylcarnitine and amino acid
analysis in cord blood samples: efficacy and reference data from a large
cohort study. J Inherit Metab Dis 2009;32:95-101. Crossref