Hong
Kong Med J 2018 Feb;24(1):11–7 | Epub 29 Dec 2017
DOI: 10.12809/hkmj176820
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Characteristics and clinical outcomes of living renal
donors in Hong Kong
YL Hong, MSc1; CH Yee, FHKAM (Surgery)1;
CB Leung, FHKAM (Surgery)2; Jeremy YC Teoh, FHKAM (Surgery)1;
Bonnie CH Kwan, FHKAM (Medicine)2; Philip KT Li, FHKAM
(Medicine)2; Simon SM Hou, FHKAM (Surgery)1; CF Ng,
FHKAM (Surgery)1
1 SH Ho Urology Centre, Department of
Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong,
Shatin, Hong Kong
2 Division of Nephrology, Department of
Medicine and Therapeutics, Prince of Wales Hospital, The Chinese
University of Hong Kong, Shatin, Hong Kong
Corresponding author: Prof CF Ng (ngcf@surgery.cuhk.edu.hk)
Abstract
Introduction: In Asia, few
reports are available on the outcomes for living renal donors. We report
the short- and long-term clinical outcomes of individuals following
living donor nephrectomy in Hong Kong.
Methods: We retrospectively
reviewed the characteristics and clinical outcomes of all living renal
donors who underwent surgery from January 1990 to December 2015 at a
teaching hospital in Hong Kong. Information was obtained from hospital
records and territory-wide electronic patient records.
Results: During the study
period, 83 individuals underwent donor nephrectomy. The mean (± standard
deviation) follow-up time was 12.0 ± 8.3 years, and the mean age at
nephrectomy was 37.3 ± 10.0 years. A total of 44 (53.0%), four (4.8%),
and 35 (42.2%) donors underwent living donor nephrectomy via an open,
hand-port assisted laparoscopic, and laparoscopic approach,
respectively. The overall incidence of complications was 36.6%, with
most being grade 1 or 2. There were three (9.4%) grade 3a complications;
all were related to open donor nephrectomy. The mean glomerular
filtration rate was 96.0 ± 17.5 mL/min/1.73 m2 at baseline
and significantly lower at 66.8 ± 13.5 mL/min/1.73 m2 at
first annual follow-up (P<0.01). The latest mean glomerular
filtration rate was 75.6% ± 15.1% of baseline. No donor died or
developed renal failure. Of the donors, 14 (18.2%) developed
hypertension, two (2.6%) had diabetes mellitus, and three (4.0%) had
experienced proteinuria.
Conclusion: The overall
perioperative outcomes are good, with very few serious complications.
The introduction of a laparoscopic approach has decreased perioperative
blood loss and also shortened hospital stay. Long-term kidney function
is satisfactory and no patients developed end-stage renal disease. The
incidences of new-onset medical diseases and pregnancy-related
complications were also low.
New knowledge added by this study
- The overall perioperative outcomes are good, with very few serious complications, among living renal donors. The introduction of a laparoscopic approach has decreased perioperative blood loss and also shortened hospital stay.
- Long-term kidney function was satisfactory and no patients developed end-stage renal disease (ESRD).
- The incidences of new-onset medical disease and pregnancy-related complications were also low.
- Medical practitioners should encourage relatives of patients with ESRD to consider the possibility of kidney donation.
Introduction
Chronic kidney disease (CKD) is the progressive
loss of kidney function over a period of time. End-stage renal disease
(ESRD) is the final stage of CKD. Patients with ESRD require renal
replacement therapy that includes haemodialysis, peritoneal dialysis, and
renal transplantation.
Currently, there are approximately 7000 patients on
various forms of renal replacement therapy being cared for in the public
sector in Hong Kong. As of 31 December 2016, 2047 patients were on the
renal transplant waiting list. Nonetheless, between 2007 and 2016, only 58
to 87 cadaveric renal transplants were performed in Hong Kong each year.1 With the long waiting list and low
number of cadaveric kidneys available, living donor renal transplant is
the only possible alternative. It offers advantages over other renal
replacement therapies, as it provides better long-term results, shortens
the waiting time for an organ, lowers the risk of complications or
rejection, and provides better quality of life after recovery. Despite
these advantages, only seven to 15 living donor transplants were performed
each year between 2007 and 2015 at the hospitals of the Hong Kong Hospital
Authority.1
One of the major fears of an individual who is
considering living organ donation concerns possible clinical outcomes.
Although studies show that living donors have a similar to or better life
expectancy than the general population, they are nevertheless at increased
risk of developing ESRD, hypertension, gestational hypertension, and
pre-eclampsia.2 3 4
In Hong Kong, few reports on the perioperative,
short-term, and long-term clinical outcomes are available, especially
those related to the minimally invasive surgical approach now employed for
donor nephrectomy. This study reports our observation of characteristics
of donors, and the short- and long-term clinical outcomes following living
donor nephrectomy in Hong Kong.
Methods
Study design
We retrospectively reviewed the characteristics and
short- and long-term clinical outcomes of all patients who underwent
living donor nephrectomy at the Prince of Wales Hospital in Hong Kong
between January 1990 and December 2015. Information was obtained from the
Clinical Management System that includes the majority of electronic
patient records—including consultation histories, operation records,
radiology results, laboratory results, and medication records—collected
and filed under the Hospital Authority since 2000. Medical records before
2000 and pregnancy-related information were reviewed manually by formally
trained medical students and cross-checked by a urologist, and retrieved
from the medical records of the involved patients.
The study was conducted in accordance with the
principles outlined in the Declaration of Helsinki, and approved by the
Joint Chinese University of Hong Kong–New Territories East Cluster
Clinical Research Ethics Committee, with the requirement of patient
informed consent waived because of its retrospective nature.
Study measures
Baseline demographics including sex, age at
donation, ethnicity, relationship with recipient, diabetes mellitus
status, hypertension status, body mass index, and serum creatinine level
were obtained. Glomerular filtration rate (GFR) was derived from the serum
creatinine level using a modified equation from the Modification of Diet
in Renal Disease (MDRD) study.5
Operation details, including surgical approach, laterality of donated
kidney, operating time, warm ischaemia time, blood loss, and need for
transfusion were retrieved.
Short-term complications within 30 days of surgery
were classified according to the Clavien-Dindo classification of surgical
complications.6 Long-term outcomes
were also assessed, with particular reference to development of
hypertension, diabetes mellitus, renal stones, proteinuria, and renal
failure. Serial changes in GFR were also assessed.
For female donors, pregnancy-related variables were
recorded and included any pregnancy after surgery, records of
pregnancy-related hydronephrosis, pregnancy-related urinary tract
infection, pre-eclampsia, gestational diabetes mellitus, gestational
hypertension, and any fetal loss.
Statistical analyses
All statistical analyses were performed using the
SPSS (Windows version 23.0; IBM Corp, Armonk [NY], United States).
Categorical variables were presented in counts and percentages while
continuous variables were presented as mean ± standard deviation. Outcomes
following open and laparoscopic techniques were compared by Chi squared
test or Fisher’s exact test for categorical variables, and independent t
test or Mann-Whitney U test for continuous variables. Paired t
test or Wilcoxon rank sum test, whichever was appropriate, was used to
evaluate the pre- and post-difference in GFR. A P value of <0.05 was
considered statistically significant. Missing data were excluded from
analysis.
Results
Donor characteristics
Between 1 January 1990 and 31 December 2015, a
total of 83 donors underwent unilateral nephrectomy at the Prince of Wales
Hospital. In one donor, records could not be traced, with only information
about the sex, age at nephrectomy, and type of surgical technique.
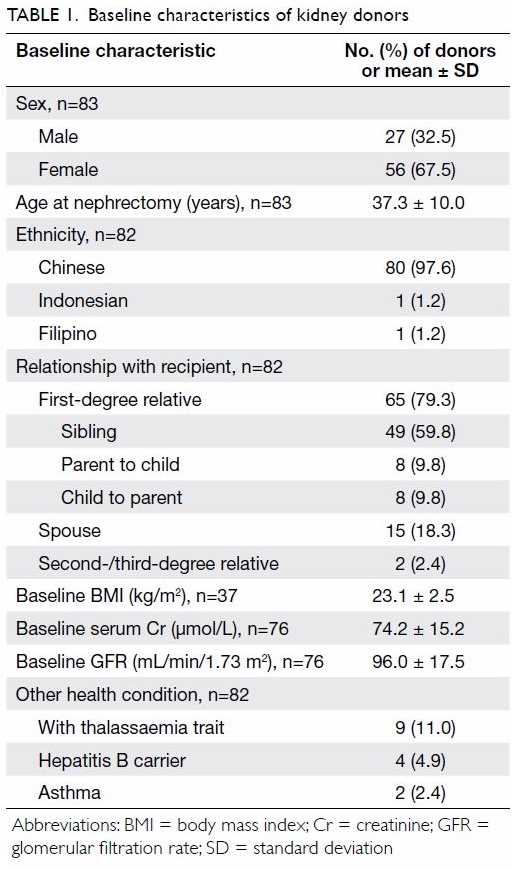
Of the 83 donors, 56 (67.5%) were female. The mean
age at nephrectomy was 37.3 ± 10.0 years. The majority were Chinese
(97.6%) and a first-degree relative of the recipient (79.3%). None had
hypertension or diabetes mellitus. The mean preoperative GFR was 96.0 ±
17.5 mL/min/1.73 m2. Nine (11.0%) donors had thalassaemia
trait, four (4.9%) had hepatitis B, and two (2.4%) had asthma (Table
1).
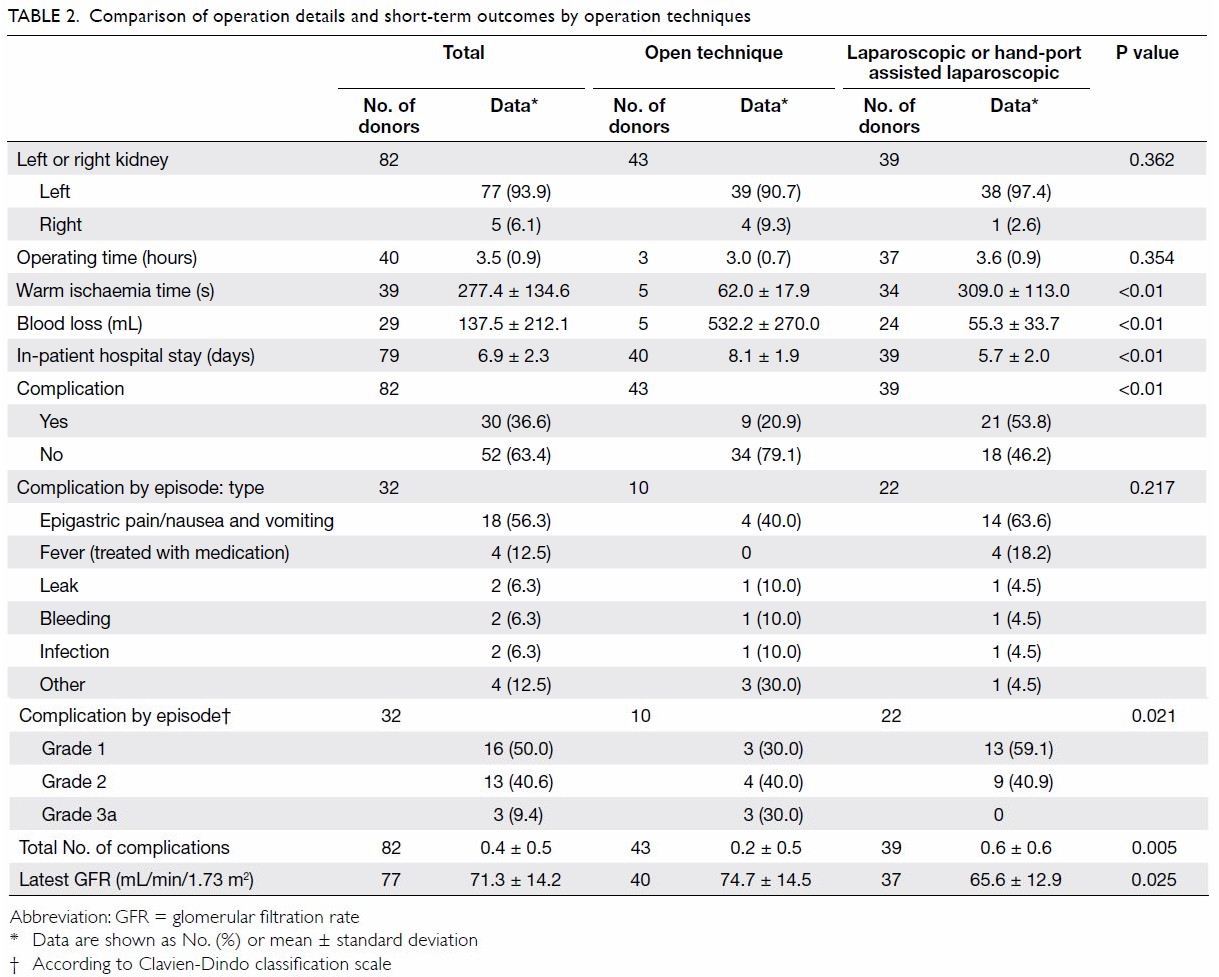
Operation details and short-term outcomes
Around half (n=44, 53.0%) of the donors underwent
open living donor nephrectomy, as this was the only technique used at our
centre until 2002. After 2002, a hand-port assisted laparoscopic approach
(n=4, 4.8%) and later a laparoscopic approach (n=35, 42.2%) were adopted.
In most instances, the left kidney was donated (n=77, 93.9%) [Table
2].
Comparing laparoscopic or hand-port assisted
laparoscopic living donor nephrectomy (LDN) with open donor nephrectomy
(ODN), LDN was associated significantly with longer warm ischaemia time
(309.0 ± 113.0 s vs 62.0 ± 17.9 s; P<0.01), less blood loss (55.3 ±
33.7 mL vs 532.2 ± 270.0 mL; P<0.01), and shorter hospital stay (5.7 ±
2.0 days vs 8.1 ± 1.9 days; P<0.01). In addition, LDN was associated
significantly with more short-term complications (53.8% vs 20.9%;
P<0.01). The most commonly experienced complication was epigastric
pain/nausea and vomiting (n=18, 56.3%), followed by fever requiring
medication (n=4, 12.5%). Most complications were grade 1 on the
Clavien-Dindo classification scale (n=16, 50.0%), only three (9.4%) were
grade 3a and all were related to ODN. The grade 3a complications were
wound dehiscence that required a second operation for re-suturing,
persistent pancreatic fluid discharge that required insertion of a
pancreatic stent, and pneumothorax with chest drain inserted.
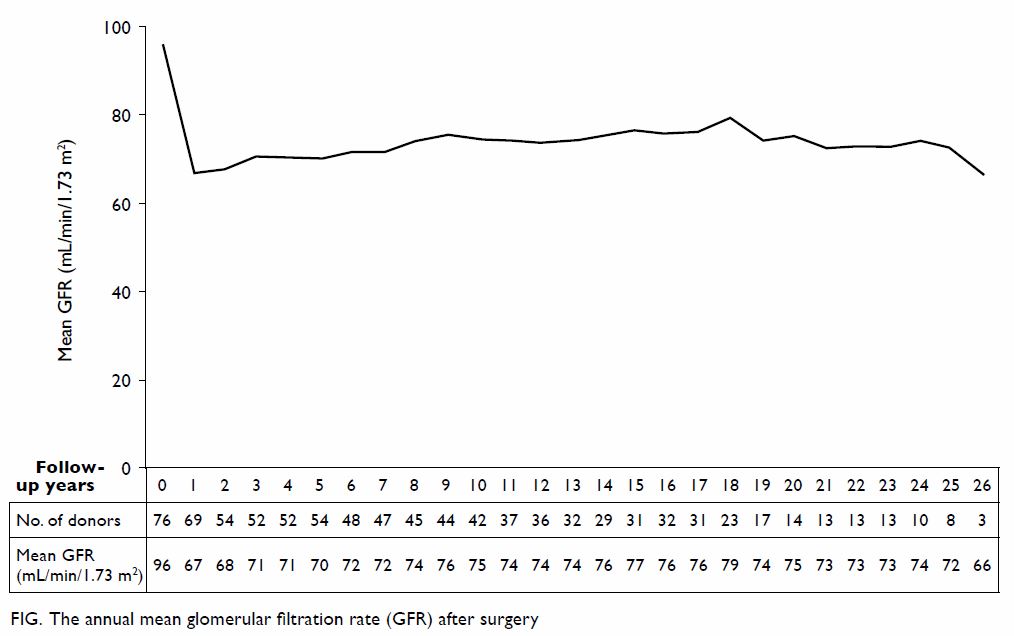
Long-term outcomes
The mean follow-up time was 12.0 ± 8.3 years. The
mean GFR was 96.0 ± 17.5 mL/min/1.73 m2 at baseline and it
dropped significantly to 66.8 ± 13.5 mL/min/1.73 m2 at 1-year
follow-up (P<0.01). The GFR then gradually improved until 8 years after
surgery and became stable (Fig). Of 73 living donors with at least one
follow-up (mean follow-up time, 12.0 ± 8.2 years) and baseline serum
creatinine level available, the latest GFR was 75.6% ± 15.1% of baseline
GFR with the mean latest GFR being 71.3 ± 14.2 mL/min/1.73 m2.
The mean GFR was 70.4% ± 12.3% of baseline level 1 year after surgery.
Comparison of latest GFR with that 1 year after surgery revealed that it
was stable (± 10% change) in 23 (39.0%) of 59 patients and higher (>10%
increment) in 29 (49.2%) patients. None of the donors had died or
developed ESRD. Fourteen (18.2%) donors developed hypertension, two (2.6%)
had diabetes mellitus, and three (4.0%) had experienced proteinuria (Table
3).
Pregnancy-related complications
Of 56 female donors, 11 (19.6%) became pregnant
after kidney donation: 17 pregnancies were reported. None of the pregnant
donors experienced gestational hydronephrosis or gestational hypertension.
Three donors each had gestational diabetes mellitus, pre-eclampsia, and
post-delivery urinary tract infection. Two donors had experienced fetal
loss, one in the first trimester and another one at an unknown gestational
age (Table 4).
Discussion
Postoperative morbidity and mortality are the prime
concerns when making a decision about kidney donation. Our results confirm
that living donor nephrectomy is a relatively safe procedure, with a low
incidence of major complications and mortality. In addition, the incidence
of developing any other major disease was not particularly high in our
series. This form of renal replacement therapy should be further promoted
in Hong Kong to benefit more people with ESRD.
Results from previous studies have shown that
living renal donors have a similar to or better life expectancy than the
general population.7 8 9 10 Mjøen et al,11
however, reported that compared with healthy matched individuals, living
renal donors had an increased risk of death. In Hong Kong, Chu et al12 reported one death related to multiple myeloma among
95 living renal donors with active follow-up and a mean follow-up period
of 13.4 years. There were no deaths recorded in our study with a mean
follow-up of 12 years.
Long-term renal function is another major concern
of renal donors. Our results revealed that 1 year after living donor
nephrectomy, the mean GFR of the kidney donors dropped significantly from
96.0 ± 17.5 mL/min/1.73 m2 at baseline to 66.8 ± 13.5
mL/min/1.73 m2. Nonetheless, it then gradually improved. This
is probably partly related to the adaptation of the remaining kidney with
hyper-filtration. From our series, the mean GFR was 70.4% ± 12.3% of
baseline level 1 year after surgery but improved to 75.6% ± 15.1% of
baseline level at the last follow-up. In the majority (88.2%) of donors,
the last available GFR was static or higher than that 1 year after
donation. This is comparable with the report of Rook et al13 in which GFR usually reached 64% ± 7% of the
pre-donation level 1 year after donation.
Despite these changes in GFR, ESRD in renal donors
is very rare, with an incidence of less than 0.5% in 15 years after
donation.11 14 15 Ibrahim
et al8 reported that survival and
risk of ESRD in kidney donors appeared to be similar to those in the
general population. Our study and that of Chu et al12 observed no ESRD in local kidney donors.
The effect of kidney donation on the development of
hypertension is controversial. Although reports suggest that the incidence
of hypertension among kidney donors increases,16
17 18
19 others have not confirmed this
observation.20 21 22 23 24 In Hong
Kong, the prevalence of hypertension in the general population was 12.6%
in 2014,25 which is lower than our
reported figure of 18.2%. With the progression of time after surgery,
however, the prevalence of hypertension among living donors is expected to
increase as age is a known influence in hypertension. Without a comparable
control group, we cannot conclude if there is any actual discrepancy in
the prevalence of hypertension among living donors compared with the
general population.
Young female potential donors may have concerns
about the impact of kidney donation on any future pregnancy. Garg et al4 reported that gestational
hypertension or pre-eclampsia was more common among living donors than
non-donors. Although our study showed an alarmingly high percentage (11%)
of pre-eclampsia and absence of gestational hypertension, the small sample
size (11 donors reported one or more pregnancies) undermines the ability
to infer the actual percentage.
Perioperative complications may also deter
potential living donors. Based on the US data, Lentine et al26 reported that 16.8% of donors experience a
perioperative complication; most commonly gastrointestinal (4.4%). Our
study showed a higher complication rate of 36.6%, with epigastric pain or
nausea and vomiting being the major complication (56.3%). We further
examined the techniques used and established that the complication rates
of 20.9% or 53.8% respectively in donors who underwent ODN or LDN were
significantly different (P<0.01). Despite the above mean complication
rates, all complications of LDN were mild and of grade 1 or 2 according to
the Clavien-Dindo classification, while three patients who underwent ODN
had grade 3a complications. This is contrary to the majority of previous
findings that suggest a lower perioperative complication rate for LDN and
increased risk of more serious complications than during an ODN,27 although other indicators such as longer warm
ischaemia time, less blood loss, and shorter hospital stays were still in
line with previous findings. Further analysis of the differences between
our local data and those of previous studies is warranted.
This study has several limitations. First, this was
a retrospective study and the total number of living donors was
restricted. Second, data quality could not be controlled and some data
were incomplete, in particular for the obstetric records at other
hospitals. Some data were also lost either because records were too old
and pre-dated the electronic system or donors were no longer followed up
at our centre. The oldest record included in the study was from 1990. At
that time, record keeping was not always accurate, resulting in some
baseline records from the early 1990s being missing. For example, the
baseline GFR level of seven (8.4%) patients was not found, and might have
affected the overall data quality as well as the analysis and conclusion.
Third, although the urologist endeavoured to ensure accurate data entry,
initial interpretation of the raw records was by medical students so
certain inaccuracies might have occurred. Lastly, it is known that GFR
might be underestimated when derived from the MDRD equation.
Conclusion
Living donor kidney transplantation is an important
approach to improve the quality of life of patients with ESRD. Good short-
and long-term outcome for kidney donors is important for promoting kidney
donation. Our results suggest that the overall perioperative outcomes are
good, with only very few serious (grade III) complications after surgery,
occurring following an open approach. Long-term kidney function of donors
was satisfactory and no patients developed ESRD. Although we had no
control arm in our study, the overall incidences of new-onset medical
diseases and pregnancy-related complications were low. The introduction of
a laparoscopic approach for kidney harvesting has helped to decrease blood
loss during surgery and also shorten hospital stay. Based on this
encouraging result, relatives of patients with ESRD should be encouraged
to consider the possibility of kidney donation.
Acknowledgements
Sincere thanks are given to Ms Karen Man-ting Chuk,
Ms Tracy Lok-sze Chiu, Mr Wing-tung Leung, and Mr On-wa Ng for assisting
with the data collection.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Statistics (Milestones of Hong Kong
organ transplantation): organ donation. Available from:
http://www.organdonation. gov.hk/eng/statistics.html. Accessed 27 Dec
2017.
2. Reese PP, Boudville N, Garg AX. Living
kidney donation: outcomes, ethics, and uncertainty. Lancet
2015;385:2003-13. Crossref
3. Delanaye P, Weekers L, Dubois BE, et al.
Outcome of the living kidney donor. Nephrol Dial Transplant 2012;27:41-
50. Crossref
4. Garg AX, Nevis IF, Mcarthur E, et al.
Gestational hypertension and preeclampsia in living kidney donors. N Engl
J Med 2015;372:124-33. Crossref
5. Levey AS, Greene T, Kusek JW, Beck GJ. A
simplified equation to predict glomerular filtration rate from serum
creatinine. J Am Soc Nephrol 2000;11:155A0828.
6. Dindo D, Demartines N, Clavien PA.
Classification of surgical complications: a new proposal with evaluation
in a cohort of 6336 patients and results of a survey. Ann Surg
2004;240:205-13. Crossref
7. Segev DL, Muzaale AD, Caffo BS, et al.
Perioperative mortality and long-term survival following live kidney
donation. JAMA 2010;303:959-66. Crossref
8. Ibrahim HN, Foley R, Tan L, et al.
Long-term consequences of kidney donation. N Engl J Med 2009;360:459-69. Crossref
9. Okamoto M, Akioka K, Nobori S, et al.
Short- and long-term donor outcomes after kidney donation: analysis of 601
cases over a 35-year period at Japanese single center. Transplantation
2009;87:419-23. Crossref
10. Garg AX, Meirambayeva A, Huang A, et
al. Cardiovascular disease in kidney donors: matched cohort study. BMJ
2012;344:e1203. Crossref
11. Mjøen G, Hallan S, Hartmann A, et al.
Long-term risks for kidney donors. Kidney Int 2014;86:162-7. Crossref
12. Chu KH, Poon CK, Lam CM, et al.
Long-term outcomes of living kidney donors: a single centre experience of
29 years. Nephrology (Carlton) 2012;17:85-8. Crossref
13. Rook M, Hofker HS, van Son WJ, Homan
van der Heide JJ, Ploeg RJ, Navis GJ. Predictive capacity of pre-donation
GFR and renal reserve capacity for donor renal function after living
kidney donation. Am J Transplant 2006;6:1653-9. Crossref
14. Muzaale AD, Massie AB, Wainwright J,
McBride MA, Wang M, Segev DL. Long-term risk of ESRD attributable to live
kidney donation: matching with healthy non-donors. Am J Transplant
2013;13:204-5.
15. Fehrman-Ekholm I, Nordén G, Lennerling
A, et al. Incidence of end-stage renal disease among live kidney donors.
Transplantation 2006;82:1646-8. Crossref
16. Kasiske BL, Ma JZ, Louis TA, Swan SK.
Long-term effects of reduced renal mass in humans. Kidney Int
1995;48:814-9. Crossref
17. Gossmann J, Wilhelm A, Kachel HG, et
al. Long-term consequences of live kidney donation follow-up in 93% of
living kidney donors in a single transplant center. Am J Transplant
2005;5:2417-24. Crossref
18. Garg AX, Prasad GV, Thiessen-Philbrook
HR, et al. Cardiovascular disease and hypertension risk in living kidney
donors: an analysis of health administrative data in Ontario, Canada.
Transplantation 2008;86:399-406. Crossref
19. Doshi MD, Goggins MO, Li L, Garg AX.
Medical outcomes in African American live kidney donors: a matched cohort
study. Am J Transplant 2012;13:111-8. Crossref
20. Fehrman-Ekholm I, Dunér F, Brink B,
Tydén G, Elinder CG. No evidence of accelerated loss of kidney function in
living kidney donors: results from a cross-sectional follow-up.
Transplantation 2001;72:444-9. Crossref
21. Macdonald D, Kukla AK, Ake S, et al.
Medical outcomes of adolescent live kidney donors. Pediatric Transplant
2014;18:336-41. Crossref
22. Janki S, Klop KW, Dooper IM, Weimar W,
Ijzermans JN, Kok NF. More than a decade after live donor nephrectomy: a
prospective cohort study. Transpl Int 2015;28:1268-75. Crossref
23. Tavakol MM, Vincenti FG, Assadi H, et
al. Long-term renal function and cardiovascular disease risk in obese
kidney donors. Clin J Am Soc Nephrol 2009;4:1230-8. Crossref
24. El-Agroudy AE, Wafa EW, Sabry AA, et
al. The health of elderly living kidney donors after donation. Ann
Transplant 2009;14:13-9.
25. Census and Statistics Department, Hong
Kong SAR Government. Thematic Household Survey Report No. 58. Available
from: http://www.statistics.gov.hk/pub/ B11302582015XXXXB0100.pdf.
Accessed 28 Oct 2016.
26. Lentine KL, Lam NN, Axelrod D, et al.
Perioperative complications after living kidney donation: a national
study. Am J Transplant 2016;16:1848-57. Crossref
27. Fonouni H, Mehrabi A, Golriz M, et al.
Comparison of the laparoscopic versus open live donor nephrectomy: an
overview of surgical complications and outcome. Langenbecks Arch Surg
2014;399:543-51. Crossref