DOI: 10.12809/hkmj177060
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
The current treatment landscape of irritable
bowel syndrome in adults in Hong Kong:
consensus statements
Justin CY Wu, MB, ChB, MD1;
Annie OO Chan, MB, ChB, PhD2;
Yawen Chan, MSocSci3;
Gordon CL Cheung, MPhil, RD (UK)4;
TK Cheung, MB, BS, PhD5;
Ambrose CP Kwan, MB, BS5;
Vincent KS Leung, MB, BS6;
Arthur DP Mak, MB, BS, MRCPsych7;
WC Sze, MB, BS, GradDFM5;
Raymond Wong, MD, PhD5
1 Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Gastroenterology and Hepatology, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
3 Hong Kong Institute of Integrative Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong
4 Hong Kong Nutrition Association, Hong Kong
5 Private specialist in Gastroenterology and Hepatology, Hong Kong
6 Department of Gastroenterology and Hepatology, Hong Kong Baptist Hospital, Kowloon Tong, Hong Kong
7 Department of Psychiatry, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Prof Justin CY Wu (justinwu@cuhk.edu.hk)
Abstract
Objective: The estimated prevalence of irritable
bowel syndrome in Hong Kong is 6.6%. With the
increasing availability of pharmacological and
non-pharmacological treatments, the Hong Kong
Advisory Council on Irritable Bowel Syndrome has
developed a set of consensus statements intended
to serve as local recommendations for clinicians
about diagnosis and management of irritable bowel
syndrome.
Participants: A multidisciplinary group of
clinicians constituting the Hong Kong Advisory
Council on Irritable Bowel Syndrome—seven
gastroenterologists, one clinical psychologist, one
psychiatrist, and one nutritionist—convened on 20
April 2017 in Hong Kong.
Evidence: Published primary research articles, meta-analyses,
and guidelines and consensus statements
issued by different regional and international
societies on the diagnosis and management of
irritable bowel syndrome were reviewed.
Consensus Process: An outline of consensus
statements was drafted prior to the meeting.
All consensus statements were finalised by the
participants during the meeting, with 100%
consensus.
Conclusions: Twenty-four consensus statements
were generated at the meeting. The statements
were divided into four parts covering: (1) patient
assessment; (2) patient’s psychological distress; (3)
dietary and alternative approaches to managing
irritable bowel syndrome; and (4) evidence to
support pharmacological management of irritable
bowel syndrome. It is recommended that primary
care physicians assume the role of principal care
provider for patients with irritable bowel syndrome.
The current statements are intended to guide primary
care physicians in diagnosing and managing patients
with irritable bowel syndrome in Hong Kong.
Introduction
Irritable bowel syndrome (IBS) is a common
condition encountered by primary care physicians,
with an estimated local prevalence of 6.6%,1 yet it
remains poorly understood. Irritable bowel syndrome
is believed to be a multifactorial disease involving
motility dysfunction, visceral hypersensitivity,
psychiatric co-morbidity, neuroendocrine
dysfunction, genetics and epigenetics, dysbiosis,
diet, and immune activation.2
First-line pharmacological treatment for IBS
may include smooth muscle relaxants, antidiarrhoeal
drugs, or laxatives. Nonetheless significant recent
advances have been made in the understanding of IBS
and new treatment modalities have emerged, such
as dietary modifications and the use of probiotics,
as well as pharmacological therapies, including
antidepressants, non-systemic antibiotics, serotonin-receptor
modulators, chloride channel activators,
guanylate cyclase C receptor agonists, mixed µ- and
κ-opioid receptor agonist and δ antagonists, and
alpha 2 δ ligands. Traditional Chinese medicine,
including herbal medicine and acupuncture, which
is valued by many Hong Kong Chinese people as an
important form of complementary medical care, has
also been investigated as a treatment for IBS.
The current standard of diagnosis and
management of IBS is based principally on data from
western studies; nonetheless cultural differences
must be considered in local practice, including
the differences in perception of symptoms, dietary
trends, and treatment goals within the Hong Kong
Chinese population.
For this reason, the Hong Kong Advisory
Council on IBS developed a set of consensus
statements offering guidance on the diagnosis and
management of IBS in Hong Kong.
Methods
A multidisciplinary group of clinicians constituting
the Hong Kong Advisory Council on IBS—seven
gastroenterologists, one clinical psychologist, one
psychiatrist, and one nutritionist—convened on 20
April 2017 in Hong Kong. An outline of consensus
statements was created prior to the meeting; this
was divided into four parts covering: (1) patient
assessment; (2) psychological distress; (3) dietary
and alternative approaches to managing IBS; and (4)
evidence for pharmacological management of IBS.
Published primary research articles, meta-analyses,
and guidelines and consensus statements issued by
different regional and international societies on the
diagnosis and management of IBS were reviewed
during the meeting. All consensus statements were
finalised by the participants during the meeting,
with 100% unanimity.
Results
Patient assessment—from primary care to diagnosis
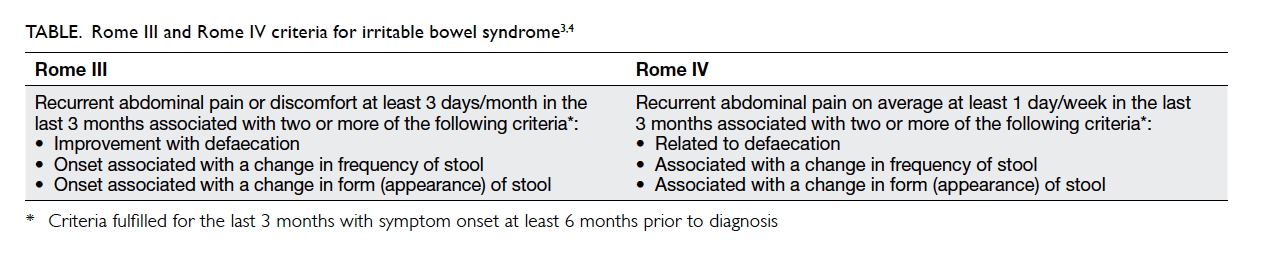
Statement 1: The Rome IV criteria allow for an
objective diagnosis of IBS. The long-term duration
of symptoms required by the criteria to make a
diagnosis, however, is too restrictive. Patients with a
shorter duration of symptoms should also be treated
for IBS.
The revised Rome IV criteria specify abdominal
pain as a requirement for diagnosis of IBS,3 while the
former Rome III criteria specified abdominal pain
or discomfort (Table).4 Due to cultural differences
and connotations of the word ‘pain’ in Chinese
languages, Chinese patients are more likely to
complain of ‘bloating’ and ‘discomfort’ than ‘pain’
when they were describing their symptoms.5 Patients
with abdominal discomfort or bloating without pain
as the dominant symptom should also be considered
for diagnosis of IBS in real-life clinical practice.
Statement 2: Physicians should make a positive
symptom-based clinical diagnosis; there are no
confirmatory diagnostic tests for IBS.3
Physicians should exercise clinical judgement
in determining appropriate investigations (eg blood
tests, stool tests, diagnostic imaging), considering
age, family history, and the presence of alarming
symptoms.6 Specific investigations such as
colonoscopy or abdominal imaging are not routinely
recommended in patients younger than 50 years
without specific risk factors.7
Statement 3: Inflammatory bowel disease
(Crohn’s disease and ulcerative colitis) and colorectal
cancer are the most important differential diagnoses
of IBS and should be actively excluded in patients
who present with IBS-related symptoms.6 8
Additional differential diagnoses include
enteric infections, medications, gynaecological
pathologies in female patients (eg endometriosis,
uterine fibroids, pelvic inflammatory disease),
pancreatic disorders, metabolic diseases (eg
hypercalcaemia), and ischaemic bowel disease in
elderly patients.8 9
Statement 4: Primary care providers should
be the principal physicians to diagnose and manage
IBS.
Compared with specialists, primary care
providers have the advantage of being more familiar
with a patient and are able to provide medical
care with a holistic approach, which is important
when managing a multifaceted disease such as
IBS.6 Psychological well-being and lifestyle factors
(eg exercise, diet, stress-coping strategies, sleep)
should be addressed.6 Primary care providers
should also educate patients about the disease,
provide reassurance about prognosis, and manage
expectations of treatment.6 10 Primary care providers
should recognise alarming symptoms or indicators
suggestive of organic pathology of the gastrointestinal
tract (eg anaemia, weight loss, bleeding) or significant
health problems; referral to other disciplines should
be made where appropriate.6 11 It is also important
to recognise the risk of (unwarranted) frequent
consultations and specialist referrals that will cause
unnecessary stress for the patient and prolonged
anxiety regarding their health.10
Statement 5: The main treatment objectives
in IBS are: symptomatic relief, improved quality of
life, reduced functional impairment, education, and
empowerment.6
It is important for physicians to verbally
acknowledge to patients that they have a clinical
condition with bothersome symptoms, while
reassuring them about their fears of severe
underlying conditions or deterioration of health; or
to reassure patients about their prognosis in the case
of post-infectious IBS.10
Understanding the patient’s psychological
distress
Statement 6: Anxiety and depressive disorders are
mental morbidities that are commonly observed in
patients with IBS and should be actively screened for
and managed. Sleep disturbances may be a symptom
of more severe mental distress.
In a community-based survey in Hong Kong,
the prevalence of generalised anxiety disorder was
16.5% in patients with IBS, compared with 3.3%
in the general population (odds ratio [OR]=5.8).12
A Taiwanese cohort of 4689 patients also found
increased risks of depressive disorder (hazard ratio
[HR]=2.89; 95% confidence interval [CI], 2.30-3.19),
anxiety disorder (HR=2.89; 95% CI, 2.42-3.46),
and sleep disorder (HR=2.47; 95% CI, 2.02-3.02) in
patients with IBS.13 In a cross-sectional study of 201
subjects with IBS, 67.2% were poor sleepers.14 The
correlation between sleep score and IBS severity was
independent of anxiety and depression; nonetheless
the prevalence of sleep disturbances was higher in
patients with co-morbid anxiety and depression.14
Statement 7: Mental health morbidities in
patients with IBS should be screened for in the
primary care setting. Patients with mental health
morbidities should be encouraged to consult mental
health professionals. Referral to a psychiatrist is
indicated for psychosis, suicidal ideation, violent
behaviour, or other life-threatening conditions.6
Hints of mental morbidities include14 15;
• persistently low mood and/or reduced enjoyment of pleasurable activities;
• multiple and extra-intestinal somatic symptoms;
• stress-related gastrointestinal symptoms;
• family history of mental illness;
• suicidal ideation or a history of such attempts;
• sleep disturbance;
• significant functional impairment; and
• health anxiety10:
o repeated investigations
o relentless search for health information
• persistently low mood and/or reduced enjoyment of pleasurable activities;
• multiple and extra-intestinal somatic symptoms;
• stress-related gastrointestinal symptoms;
• family history of mental illness;
• suicidal ideation or a history of such attempts;
• sleep disturbance;
• significant functional impairment; and
• health anxiety10:
o repeated investigations
o relentless search for health information
Standardised instruments (eg Patient Health
Questionnaire [PHQ]) can easily be administered to
facilitate clinical assessments. The PHQ is available
online in Cantonese and is appropriate for use in
primary care clinics.16 Mild-to-moderate anxiety
and depression can be managed in the primary
care setting. Physicians should routinely counsel
patients on the importance of mental health in the
management of IBS.
Counselling and face-to-face psychological
interventions have been found to be efficacious in
the management of IBS. A study of 149 patients
with moderate or severe IBS resistant to the
antispasmodic agent mebeverine found that the
addition of cognitive behavioural therapy, delivered
by primary care nurses, had a considerable initial
benefit on symptom severity compared with
mebeverine alone, with the benefit persisting after 3
and 5 months.17 Cognitive behavioural therapy also
showed a significant benefit on the work and social
adjustment scale that persisted 12 months after
therapy (mean reduction of 2.8 points).17 A meta-analysis
also demonstrated similar positive benefits
of cognitive behavioural therapy (HR=0.60; 95% CI,
0.44-0.83), dynamic psychotherapy (HR=0.60; 95%
CI, 0.39-0.93), hypnotherapy (HR=0.74; 95% CI,
0.63-0.87), and multi-component psychotherapy
(HR=0.72; 95% CI, 0.62-0.83) compared with control
treatment.18
Dietary and alternative approaches to
irritable bowel syndrome
Statement 8: A short trial of low-FODMAP diet
has been shown to improve symptoms of IBS.19
Involvement of dieticians may improve accuracy
and adherence to the low-FODMAP diet or other
specific diets.20
In a randomised, controlled, single-blind,
crossover trial, a low-FODMAP (fermentable
oligosaccharides, disaccharides, monosaccharides,
and polyols) diet for 21 days led to a significant
improvement in symptoms of IBS (including
abdominal pain, bloating, passage of gas and
dissatisfaction with stool consistency) and quality
of life compared with a standard Australian diet.19
Possible mechanisms include a decrease in osmotic
diarrhoea, fermentation and altered gut microbiota,
immune activation and visceral sensitivity.21 22
Statement 9: Other dietary concerns that may
affect IBS include lactose intolerance, high-fat diet,
high-fibre diet, chilli, and gluten.
These can all be potential aggravators of IBS,
but do not apply to all patients.23 Coeliac disease and
non-coeliac gluten sensitivity are rare in Chinese
populations and therefore trial of a gluten-free diet
is not warranted in Chinese patients.24
Statement 10: Health care practitioners should
exercise caution in recommending excessively
restrictive diets that could lead to malnutrition,
quality of life impairment, or psychological distress
(as a result of the difficulty of adherence).
For selected patients on long-term restrictive
diets or with multiple food intolerances, referral to
a dietician may help to minimise the risk of nutrient
deficiency.20
Statement 11: The role of food allergy in the
pathophysiology of IBS in unclear. Routine food
allergy testing is not recommended.25
Statement 12: Herbal medicine has been shown
to be effective only if an individualised approach
is taken. This requires assessment by a Chinese
medicine practitioner.
The benefit of individualised herbal medicine
was shown in a 1998 study in which 116 patients
with IBS were randomised to receive placebo
(n=35), individualised (n=38), or a standard (n=43)
Chinese herbal medicine for 16 weeks.26 Only
the individualised treatment group maintained
improvement at 14 weeks after completion of
treatment.26 This was confirmed by a 2006 Hong Kong
study by Leung et al27 in 199 diarrhoea-predominant
IBS patients randomised to receive placebo (n=59)
or standard (n=60) Chinese herbal formula for 16
weeks. No differences in global or individual IBS
symptoms or quality of life were observed at any
follow-up visits.
Statement 13: The current evidence does not
support acupuncture as an effective treatment for
IBS.
A meta-analysis that evaluated evidence
from 17 randomised controlled trials reported
that acupuncture is not more effective than sham
treatment for improving symptom severity (P=0.36)
or quality of life (P=0.83).28
Pharmacological management of irritable
bowel syndrome
Statement 14: The currently approved drug classes
for treatment of IBS are antispasmodics, laxatives,
and antidiarrhoeal drugs.
Statement 15: There are good efficacy and
safety data to support antispasmodics as first-line
therapy for IBS.
A 2008 meta-analysis evaluated data from
22 randomised controlled trials comparing
antispasmodics (including otilonium bromide,
cimetropium, hyoscine, pinaverium, trimebutine,
rociverine, alverine, dicycloverine, mebeverine,
pirenzepine, prifinium, and propinox) with placebo.
Of 905 patients assigned to antispasmodics, 350
(39%) had persistent symptoms after treatment
compared with 485 (56%) of 873 allocated to placebo
(relative risk [RR]=0.68; 95% CI, 0.57-0.81; P<0.001).29
Otilonium bromide (RR=0.55; 95% CI, 0.31-0.97)
and hyoscine (RR=0.63; 95% CI, 0.51-0.78) were the
only antispasmodics to show consistent evidence of
efficacy. The most frequent adverse events were dry
mouth, dizziness and blurred vision, but none of the
trials reported any serious adverse events.29
It is important to recognise that not all
antispasmodics share the same efficacy and safety
profile. Moreover, there are additional safety concerns
(eg blurred vision, mental confusion, aggravation of
prostatism, tachycardia) with antispasmodics of the
anticholinergic subclass; additional monitoring is
required with such agents.30
Statement 16: There is a lack of head-to-head
studies comparing the efficacy and safety of different
antispasmodics. Moreover, antispasmodics have
varying mechanisms of action.
Hyoscine is an antispasmodic that blocks the
action of muscarinic and nicotinic acetylcholine
receptors in smooth muscle and secretory glands
causing decreased motility of the gastrointestinal
tract.31
Otilonium bromide is an antispasmodic with
several modes of action that are not shared by other
antispasmodics. It works by blocking L-type calcium
channels on smooth muscle cells thereby restoring
physiological motility. It also exhibits an antisecretory
effect and reduces spasm through inhibition of
muscarinic M3 receptor–coupled calcium signals.
Finally, otilonium bromide antagonises tachykinin
receptors on the intestinal smooth muscle cells and
afferent nervous terminations, thus modulating the
development of intestinal hyperalgesia and reducing
visceral hypersensitivity by enhancing sensory
thresholds to rectosigmoid distension.32
Statement 17: Otilonium bromide can be
prescribed by primary care physicians as first-line
therapy for IBS.
Data from a total of 883 patients with IBS from
three randomised controlled trials were included in
a pooled analysis. A significant therapeutic effect
of otilonium bromide was observed after 10 and 15
weeks of treatment compared with placebo, with
reference to intensity and frequency of abdominal
pain, severity of bloating, and rate of responders
as evaluated by patients and physicians.33 The
most common treatment-emergent adverse
events associated with otilonium bromide were
gastrointestinal events (abdominal pain, flatulence,
worsening IBS) and infections. Nearly all were mild
to moderate (99% in the otilonium bromide group
and 98% in the placebo group) and were considered
unrelated to the study treatment (92% in the
otilonium bromide group and 94% in the placebo
group).34
Statement 18: Further study is warranted to
establish an optimal treatment period for otilonium
bromide.
Many patients use otilonium bromide on an
as-needed basis or as prophylaxis prior to known
triggering events (eg travel, large meals). Others use
otilonium bromide on a long-term basis (eg those
with frequent daily symptoms). A randomised,
double-blind clinical trial demonstrated a lower
rate of symptom relapse (P=0.009) and higher
relapse-free probability (P=0.038) in patients treated
with otilonium bromide for 15 weeks compared
with patients treated with placebo.34 A 2-year
study demonstrated a significant improvement in
abdominal pain, abdominal distension, and bowel
movements in patients treated with otilonium
bromide, compared with a high-roughage diet.35
Statement 19: Antispasmodics are also
commonly prescribed in combination with
antidiarrhoeal drugs or laxatives. No clinical data,
however, are available on combination therapy.
Statement 20: Selective serotonin reuptake
inhibitors (SSRIs) are used in patients with co-morbid
anxiety or depressive disorder or as off-label
treatment for patients who do not respond to
first-line treatment for IBS. Treatment with SSRIs
requires close monitoring for efficacy and safety.
Selective serotonin reuptake inhibitors have
proven efficacy for IBS, anxiety and depressive
disorders, and should be considered when organ-based
treatment and psychological treatment are
not accessible or effective. A meta-analysis of five
randomised controlled trials found that SSRIs were
more effective and better tolerated than placebo
as treatment for IBS (RR=0.62; 95% CI, 0.45-0.87).36 Nonetheless, SSRIs should be prescribed
by physicians or mental health professionals with
experience and training in antidepressant drug
treatment. Potential adverse events, including
suicidal ideation in non-suicidal patients,
warrants careful attention to patients taking
antidepressants.37
Statement 21: Probiotics have demonstrated
positive results in the treatment of IBS.
A 2013 meta-analysis found that probiotics
consisting of Lactobacillus, Bifidobacterium,
Escherichia, Streptococcus or combination probiotics
had beneficial effects on the persistence of IBS
symptoms (RR=0.79; 95% CI, 0.70-0.89), global IBS,
abdominal pain, bloating and flatulence scores, and
led to an increase in the number of stools per week.38
The exact mechanism of action, optimal regimen and
delivery mode, and durability of efficacy remains to
be determined. Moreover, although adverse events
with probiotics are rare, there are little long-term
safety data available.38 Finally, the efficacy of different
probiotic strains is variable and limits their use as a
first-line treatment.38
Statement 22: Short-term rifaximin has been
found to be effective in relieving bloating symptoms.
Rifaximin is a poorly absorbed, luminally active
antibiotic. A meta-analysis of five studies found that
short-term use of rifaximin was effective in relieving
bloating symptoms (OR=1.55; 95% CI, 1.23-1.96)
and led to global IBS symptom improvement
(OR=1.57; 95% CI, 1.22-2.01).39 The role of rifaximin
has not been fully acknowledged in the management
algorithm of IBS owing to the concern of antibiotic
resistance, risk of Clostridium difficile infection, and
long-term effectiveness.
Statement 23: Other novel therapies that are
Food and Drug Administration–approved based
on positive results in patients with IBS, but are not
yet available in the primary care setting in Hong
Kong include: serotonin receptor modulators,
secretagogues, and peripherally acting opioid
receptor modulators.
Statement 24: There are insufficient efficacy
and safety data to justify the clinical use of faecal
microbiota transplantation in the management of
IBS.
Conclusions
Irritable bowel syndrome is a common disorder
encountered in general practice, yet effective
treatment remains a challenge for primary care
physicians and gastroenterologists. This is the first
consensus statement on the appropriate approach
to diagnosis and management of IBS in Hong Kong.
This paper summarises important considerations
in managing patients with IBS, along with clinical
efficacy and safety data on pharmacological
treatments. These consensus statements aimed to
provide local general practitioners with information
to counsel and manage patients with IBS in Hong
Kong.
The treatment of IBS depends on patient
symptoms.6 After actively excluding relevant and
serious pathologies, psychological and dietary
aspects of IBS should first be addressed.6
Food allergy testing is not recommended in
patients with IBS25; nonetheless important dietary
considerations include FODMAPs, fibre, chilli,
lactose, and gluten.23 Coeliac disease is rare in the
Chinese population; data suggest that wheat is not
completely absorbed in the small bowel and may
produce gastrointestinal symptoms.23 Although the
primary carbohydrate in the Chinese diet is rice,
there is a strong influence of western cuisine in Hong
Kong and wheat is found in many traditional Hong
Kong–style foods.
Anxiety and depression are common in
patients with IBS. Psychological interventions such
as counselling, cognitive behavioural therapy, and
hypnotherapy are effective treatments for patients
with mental morbidities and IBS.17 18 Physicians
should routinely counsel patients on the importance
of mental health in the management of IBS.
Motivated patients may consider traditional
Chinese medicine but an individualised approach
must be taken.26 At this time, there is insufficient
evidence to recommend acupuncture for patients
with IBS.28
The currently approved drug classes for
treatment of IBS are antispasmodics, laxatives,
and antidiarrhoeal drugs. Antispasmodics are a
heterogeneous drug class with varying mechanisms
of action. Otilonium bromide and hyoscine are the
only antispasmodics to show consistent evidence
of efficacy but the anticholinergic side-effect of
hyoscine has limited its frequent use in IBS.29 31
Acknowledgements
English language editing and writing support,
funded by an unrestricted educational grant from
A. Menarini Hong Kong Limited, was provided by
Cassandra Thomson of MIMS (Hong Kong) Limited.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Kwan AC, Hu WH, Chan YK, Yeung YW, Lai TS, Yuen H.
Prevalence of irritable bowel syndrome in Hong Kong. J
Gastroenterol Hepatol 2002;17:1180-6. Crossref
2. Camilleri M. Peripheral mechanisms in irritable bowel
syndrome. N Engl J Med 2012;367:1626-35. Crossref
3. Lacy BE, Mearin F, Chang L, et al. Bowel disorders.
Gastroenterology 2016 Feb 18. Epub ahead of print. Crossref
4. Shih DQ, Kwan LY. All roads lead to Rome: update on Rome
III criteria and new treatment options. Gastroenterol Rep
2007;1:56-65.
5. Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable
bowel syndrome in Asia: something old, something
new, something borrowed. J Gastroenterol Hepatol
2009;24:1601-7. Crossref
6. Khanbhai A, Singh Sura D. Irritable bowel syndrome for
primary care physicians. Br J Med Pract 2013;6:a608.
7. Hong Kong Department of Health. About colorectal
cancer 2016. Available from: http://www.colonscreen.gov.
hk/en/public/about_crc/who_should_be_screened_and_who_need_not_be_screened.html. Accessed 1 Jun 2017.
8. Zammit E. The irritable bowel syndrome. Malta Med J
2009;21:34-40.
9. Cartwright SL, Knudson MP. Evaluation of acute abdominal
pain in adults. Am Fam Physician 2008;77:971-8.
10. O’Sullivan MA, Mahmud N, Kelleher DP, Lovett E,
O’Morain CA. Patient knowledge and educational needs
in irritable bowel syndrome. Eur J Gastroenterol Hepatol
2000;12:39-43. Crossref
11. Hookway C, Buckner S, Crosland P, Longson D. Irritable
bowel syndrome in adults in primary care: summary of
updated NICE guidance. BMJ 2015;350:h701. Crossref
12. Lee S, Wu J, Ma YL, Tsang A, Guo WJ, Sung J. Irritable
bowel syndrome is strongly associated with generalized
anxiety disorder: a community study. Aliment Pharmacol
Ther 2009;30:643-51. Crossref
13. Lee YT, Hu LY, Shen CC, et al. Risk of psychiatric disorders
following irritable bowel syndrome: a nationwide population-based
cohort study. PLoS One 2015;10:e0133283. Crossref
14. Moradian-Shahrbabaki M, Vahedi H, Sadeghniiat-Haghighi K, Shamsipour M. Tracing the relationships
between sleep disturbances and symptoms of irritable
bowel syndrome. J Sleep Sci 2016;1:101-8.
15. Fadgyas-Stanculete M, Buga AM, Popa-Wagner A,
Dumitrascu DL. The relationship between irritable bowel
syndrome and psychiatric disorders: from molecular
changes to clinical manifestations. J Mol Psychiatry
2014;2:4. Crossref
16. Yu X, Stewart SM, Wong PT, Lam TH. Screening for
depression with the Patient Health Questionnaire–2
(PHQ-2) among the general population in Hong Kong. J
Affect Disord 2011;134:444-7. Crossref
17. Kennedy T, Jones R, Darnley S, Seed P, Wessely S, Chalder T.
Cognitive behaviour therapy in addition to antispasmodic
treatment for irritable bowel syndrome in primary care:
randomised controlled trial. BMJ 2005;331:435. Crossref
18. Ford AC, Quigley EM, Lacy BE, et al. Effect of antidepressants
and psychological therapies, including hypnotherapy, in
irritable bowel syndrome: systematic review and meta-analysis.
Am J Gastroenterol 2014;109:1350-65. Crossref
19. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A
diet low in FODMAPs reduces symptoms of irritable bowel
syndrome. Gastroenterology 2014;146:67-75.e5. Crossref
20. Nanayakkara WS, Skidmore PM, O’Brien L, Wilkinson TJ,
Gearry RB. Efficacy of the low FODMAP diet for treating
irritable bowel syndrome: the evidence to date. Clin Exp
Gastroenterol 2016;9:131-42.
21. Hayes PA, Fraher MH, Quigley EM. Irritable bowel
syndrome: the role of food in pathogenesis and management.
Gastroenterol Hepatol (N Y) 2014;10:164-74.
22. De Palma G, Reed DE, Pigrau M, et al. Diet-microbiota
interactions underlie symptoms’ generation in IBS.
Gastroenterology 2017;152(5 Suppl 1):S160. Crossref
23. Gonlachanvit S. Are rice and spicy diet good for functional
gastrointestinal disorders? J Neurogastroenterol Motil
2010;16:131-8. CrossRef
24. Jiang LL, Zhang BL, Liu YS. Is adult celiac disease really
uncommon in Chinese? J Zhejiang Univ Sci B 2009;10:168-71. Crossref
25. Kennedy DA, Lewis E, Cooley K, Fritz H. An exploratory
comparative investigation of Food Allergy/Sensitivity
Testing in IBS (the FAST study): a comparison between
various laboratory methods and an elimination diet. Adv
Integr Med 2014;1:124-30. Crossref
26. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu
M. Treatment of irritable bowel syndrome with Chinese
herbal medicine: a randomized controlled trial. JAMA
1998;280:1585-9. Crossref
27. Leung WK, Wu JC, Liang SM, et al. Treatment of diarrhea-predominant
irritable bowel syndrome with traditional
Chinese herbal medicine: a randomized placebo-controlled
trial. Am J Gastroenterol 2006;101:1574-80. Crossref
28. Manheimer E, Wieland LS, Cheng K, et al. Acupuncture
for irritable bowel syndrome: systematic review and meta-analysis.
Am J Gastroenterol 2012;107:835-47. Crossref
29. Ford AC, Talley NJ, Spiegel BM, et al. Effect of fibre,
antispasmodics, and peppermint oil in the treatment of
irritable bowel syndrome: systematic review and meta-analysis.
BMJ 2008;337:a2313. Crossref
30. Hesch K. Agents for treatment of overactive bladder:
a therapeutic class review. Proc (Bayl Univ Med Cent)
2007;20:307-14.
31. Samuels LA. Pharmacotherapy update: hyoscine
butylbromide in the treatment of abdominal spasms. Clin
Med Ther 2009;1:647-55. Crossref
32. Triantafillidis JK, Malgarinos G. Long-term efficacy
and safety of otilonium bromide in the management of
irritable bowel syndrome: a literature review. Clin Exp
Gastroenterol 2014;7:75-82. Crossref
33. Clavé P, Tack J. Efficacy of otilonium bromide in
irritable bowel syndrome: a pooled analysis. Therap Adv
Gastroenterol 2017;10:311-22. Crossref
34. Clavé P, Acalovschi M, Triantafillidis JK, et al. Randomised
clinical trial: otilonium bromide improves frequency
of abdominal pain, severity of distention and time to
relapse in patients with irritable bowel syndrome. Aliment
Pharmacol Ther 2011;34:432-42. Crossref
35. Villagrasa M, Boix J, Humbert P, Quer JC. Aleatory clinical
study comparing otilonium bromide with a fiber-rich
diet in the treatment of irritable bowel syndrome. Ital J
Gastroenterol 1991;23(8 Suppl 1):67-70.
36. Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi
P. Efficacy of antidepressants and psychological therapies
in irritable bowel syndrome: systematic review and meta-analysis.
Gut 2009;58:367-78. Crossref
37. Bielefeldt AØ, Danborg PB, Gøtzsche PC. Precursors to
suicidality and violence on antidepressants: systematic
review of trials in adult healthy volunteers. J R Soc Med
2016;109:381-92. Crossref
38. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics,
probiotics, and synbiotics in irritable bowel syndrome and
chronic idiopathic constipation: systematic review and
meta-analysis. Am J Gastroenterol 2014;109:1547-61. Crossref
39. Menees SB, Maneerattannaporn M, Kim HM, Chey WD.
The efficacy and safety of rifaximin for the irritable bowel
syndrome: a systematic review and meta-analysis. Am J
Gastroenterol 2012;107:28-35. Crossref