Hong
Kong Med J 2017 Dec;23(6):570–8 | Epub 13 Oct 2017
DOI: 10.12809/hkmj176240
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Clinical utility of late-night and post-overnight
dexamethasone suppression salivary cortisone for the investigation of
Cushing’s syndrome
CM Ng, FRCP (Edin), FHKAM (Medicine)1;
TK Lam, MB, BS, MRCP1; YC Au Yeung, MRCP, FHKAM (Medicine)1;
CH Choi, FRCP (Edin), FHKAM (Medicine)1; YP Iu, BSc, MSc2;
CC Shek, MB, BS, FHKAM (Pathology)2; SC Tiu, MD, FHKAM
(Medicine)1
1 Department of Medicine, Queen
Elizabeth Hospital, Jordan, Hong Kong
2 Department of Pathology, Queen
Elizabeth Hospital, Jordan, Hong Kong
Corresponding author: Dr CM Ng (ngcm2@ha.org.hk)
Abstract
Introduction: There is a
pressing need to identify diagnostic testing for Cushing’s syndrome that
can be achieved with ease and at low cost. This study aimed to explore
the usefulness of late-night and post-overnight 1-mg dexamethasone
suppression salivary cortisone, as measured by liquid
chromatography–tandem mass spectrometry, for investigation of
hypercortisolism.
Methods: Salivary cortisone data
of subjects were investigated according to a pre-specified protocol.
Subjects were classified as having ‘hypercortisolism’ or ‘eucortisolism’
on the basis of histological or biochemical criteria. Receiver operating
characteristic curves were drawn to identify the cut-off values and
study their performance characteristics. We measured 24-hour urinary
free cortisol; late-night salivary cortisol and cortisone; and
post-overnight 1-mg dexamethasone suppression serum cortisol, and
salivary cortisol and cortisone. Saliva and urine samples were assayed
by liquid chromatography–tandem mass spectrometry.
Results: In this study, 21
subjects were classified as having hypercortisolism and 78 as having
eucortisolism. A late-night salivary cortisone cut-off of 13.50 nmol/L
had a sensitivity of 94.7% and a specificity of 87.2%. After taking 1-mg
dexamethasone the night before, a salivary cortisol cut-off of 0.85
nmol/L had a sensitivity of 76.2% and a specificity of 96.2%; a salivary
cortisone cut-off of 7.45 nmol/L had a sensitivity of 85.7% and a
specificity of 94.9%, while a salivary cortisone cut-off of 3.25 nmol/L
had a sensitivity of 95.2% and a specificity of 79.5%. Many salivary
cortisol samples were below the detection limit of liquid
chromatography–tandem mass spectrometry. In comparison with salivary
cortisol, salivary cortisone had a better correlation with total serum
cortisol and better diagnostic performance following dexamethasone
suppression.
Conclusions: Both late-night and
post-overnight dexamethasone suppression salivary cortisone levels are
of diagnostic value in the investigation of hypercortisolism.
New knowledge added by this study
- Compared with salivary cortisol, salivary cortisone has better diagnostic performance after dexamethasone suppression.
- Both late-night and post-overnight dexamethasone suppression salivary cortisone levels are of diagnostic value in the investigation of hypercortisolism.
- Using the cut-off value generated from this study, late-night and post-overnight dexamethasone suppression salivary cortisone, measured by liquid chromatography–tandem mass spectrometry, can be added to the panel of diagnostic tests for hypercortisolism.
Introduction
The diagnosis of Cushing’s syndrome, especially
when hypersecretion is mild, is plagued by uncertainties. Most clinical
features—such as diabetes mellitus, hypertension, obesity,
hyperlipidaemia, osteoporosis, or depression—are non-specific and highly
prevalent in the general population. More specific features such as
myopathy or easy bruising may be absent even in subjects with florid
biochemical hypercortisolism. In addition, no single test can diagnose
Cushing’s syndrome with 100% sensitivity and specificity.1 It is a common phenomenon that tests for
hypercortisolism—for examples, 24-hour urinary free cortisol (UFC),
late-night salivary cortisol (SalFLN) or serum cortisol level
after 1-mg overnight dexamethasone suppression test (SerFDex)—often
produce discordant results. Each test has its own caveats, affected by the
level of binding proteins, completeness of urine collection, and
absorption and metabolism of dexamethasone. In recent years, increased
awareness of the metabolic and cardiovascular consequences of Cushing’s
syndrome2 has led to a pressing
need to identify tests that are easy to perform and can provide useful
information at a low cost.
Collection of saliva samples is non-invasive and
stress-free.3 4 Salivary cortisol is not affected by the levels of
binding proteins so it provides a reliable indication of the biologically
active free serum cortisol level.5
Significant advances have been made with the use of salivary cortisol in
the investigation of hypercortisolism.3
6 7
8 9
The availability of liquid chromatography–tandem mass spectrometry
(LC-MS/MS) also enables the measurement of other glucocorticoid analytes.
Among these and of particular interest is cortisone that is present in the
saliva at a higher concentration—the salivary cortisone–to–cortisol ratio
is up to 6-8:110 11 12—due to
conversion of cortisol to cortisone by the 11 beta-hydroxysteroid
dehydrogenase 2 (11β-HSD2) enzyme in the salivary glands.13 This higher concentration makes it more detectable
than salivary cortisol.10 Salivary
cortisone has been shown by some investigators to have a better and more
linear relationship with serum total cortisol14
and serum free cortisol10 than
salivary cortisol.
In this study, we reviewed the late-night salivary
cortisone (SalELN) and post-overnight dexamethasone suppression
salivary cortisone (SalEDex) values of subjects being
investigated for hypercortisolism in our centre, in an attempt to define
cut-off values with reasonable sensitivity and specificity.
Methods
Subjects
All subjects referred to the Endocrine Unit of
Queen Elizabeth Hospital in Hong Kong for suspected endogenous
hypercortisolism were evaluated according to a pre-specified protocol.
Written informed consent was obtained from all subjects. The results of
subjects investigated during May 2013 (when salivary measurement by
LC-MS/MS became available) to September 2016 were reviewed. This study was
approved by the Hospital Ethics Committee. No patient was receiving
medical treatment for Cushing’s syndrome at the time of assessment.
Subjects who were taking medication (such as rifampicin, phenytoin,
phenobarbital, and alcohol) that might interfere with dexamethasone
metabolism, or were night or shift workers, were excluded.
Investigations performed
Detailed oral and written instructions were given
to all subjects by an endocrine specialist nurse. For the collection of
saliva sample, subjects were instructed to refrain from smoking, brushing
teeth, and eating or drinking anything but water for at least 30 minutes
before collection. Saliva samples were collected using a cotton swab in
Salivette tubes (Sarstedt, Nümbrecht, Germany). Salivettes were kept at
4°C in a home refrigerator and sent to the laboratory within 24 hours.
According to the pre-specified protocol, on day 1,
a 24-hour urine sample was collected for UFC measurement. On day 2, a
0900h saliva sample was collected at the Endocrine Centre, under nurse
supervision. The patient was then instructed to collect a late-night
(between 2300h and 2400h) saliva sample that evening, after which he/she
was to take dexamethasone 1 mg orally. On day 3, subjects returned to the
Endocrine Centre for a simultaneous blood and saliva sample at 0900h. The
blood sample was sent for serum cortisol assay. Saliva samples were
assayed for both cortisol and cortisone.
Laboratory assays
Serum cortisol was measured by competitive
chemiluminescent microparticle immunoassay using the Abbott ARCHITECT
i2000SR system (Abbott Diagnostics, Illinois, US). The coefficient of
variation of the assay for serum cortisol was 4.0%-6.2% at low levels and
3.3%-4.3% at high levels. Salivary cortisol and salivary cortisone were
measured by LC-MS/MS using the Waters Xevo TQ MS system (Waters
Corporation, Milford [MA], US). Cortisol and cortisone were extracted from
saliva using the organic solvent methyl tert-butyl ether after addition of
a deuterium-labelled internal standard mixture of cortisol-d4 and
cortisone-d8 (CDN isotopes). The organic supernatant was dried under
nitrogen at a temperature below 45°C and dissolved in the initial mobile
phase for LC-MS/MS analysis. The steroid analytes were separated from the
matrix background in a reversed-phase chromatography that employed a sub-2
μm analytical column (ACQUITY UPLC HSS T3 Column, 2.1 x 100 mm, 1.8 mm;
Waters Corporation, Milford [MA], US) and a 6-minute elution method
consisting of a gradient mixture of 0.1% glacial acetic acid, 0.2 mM
ammonium acetate in water and methanol. Negative electrospray mode was
used for analyte ionisation. The charged acetate adducts were monitored by
multiple reaction monitoring mode with two stable mass transitions for
cortisol (421>331; 421>297) and cortisone (419>329; 419>301)
and one multiple reaction monitoring for each of the corresponding
deuterated internal standards (cortisol-d4: 425>335; cortisone-d8:
427>337). Quantitative measurement was derived using the linear least
squares regression method with origin excluded and 1/x weighting for
better accuracy at a low concentration level. The coefficient of variation
of the assay for salivary cortisol and cortisone was 5%-7% and 7%-10%,
respectively across the analyte reporting ranges up to 250 nmol/L. The
lower limit of detection was 0.5 nmol/L for both salivary cortisol and
cortisone. Urinary cortisol was also measured by LC-MS/MS.
Adrenocorticotropic hormone (ACTH) was measured by Immulite 2000 XPi
(Siemens Healthcare GmbH, Erlangen, Germany) chemiluminescent immunometric
assay. The upper reference limit of ACTH was 10.2 pmol/L.
Definition of hypercortisolism
Subjects were classified as having hypercortisolism
if either the biochemical or the histological criterion was fulfilled. The
biochemical criterion was defined as having an abnormal value in at least
two of the following three tests: (1) SerFDex >138 nmol/L,
or >50 nmol/L in the context of adrenal incidentaloma15; (2) UFC >157 nmol/day; and (3) SalFLN
≥3 nmol/L. The categorisation of hypercortisolism was made without
knowledge (ie blinded) of the three outcome parameters being evaluated for
diagnostic accuracy, namely SalELN, post-overnight
dexamethasone suppression salivary cortisol (SalFDex), and SalEDex.
The reference range for UFC in our laboratory, established locally from
the 2.5th to 97.5th percentile of 112 healthy adults, was 22-157 nmol/day.
The reference level for SalFLN in our laboratory, derived from
the 97.5th percentile of 61 normal individuals, was <3 nmol/L. The
histological criterion was defined as histological proof and postoperative
improvement in biochemical and clinical parameters. Subjects not
fulfilling either of these criteria were classified as having
eucortisolism.
Statistical analyses
For calculation purposes, results below the
detection limit of the assay were set to the lowest detection value.
Continuous data were expressed as mean ± standard deviation if parametric,
and median (range) if non-parametric. Chi squared test was used to detect
any difference between categorical data. Unpaired t test was used
to compare continuous variables, and Mann-Whitney test was used for
non-parametric data. A P value of <0.05 was considered statistically
significant. Correlation between serum and salivary values were performed
using Pearson correlation coefficients.
For estimation of the optimal diagnostic cut-off
value, receiver operating characteristic (ROC) curves were drawn using
data from the subjects classified as hypercortisolism or eucortisolism.
The optimal cut-off was chosen where the Youden’s index (sensitivity +
specificity–1) was maximal. The test performance characteristics were
calculated to assess their utility. The quality of diagnostic tests was
expressed as the area under ROC curve (AUC). For sample size requirement
estimation, for an estimated AUC of 0.8, a minimum of nine positive cases
was required.16 Statistical
analysis was performed using the Statistical Package for Social Science
(Windows version 20.0; IBM Corp, Armonk [NY], US).
Results
A total of 115 subjects were referred to our
Endocrine Clinic for assessment of hypercortisolism during the study
period. Of them, 14 subjects with a history of transsphenoidal surgery or
adrenalectomy who had been referred for postoperative assessment of
endocrine function were excluded. One patient with ongoing investigations
and pending re-evaluation and another with end-stage renal failure were
also excluded. No patient was taking exogenous steroids. All subjects had
normal renal and liver function tests.
A total of 115 sets of biochemical investigations
were performed in 99 subjects (40 males, 59 females; mean age, 55.3 ± 14.3
years; range, 19-81 years). The primary indications for testing were
adrenal incidentaloma in 52 subjects, suspected secondary hypertension or
diabetes mellitus in 25, Cushingoid features in 21, and pituitary mass in
one. Eleven subjects had two or more sets of investigations performed (two
patients had 4 sets, one patient had 3 sets, and eight patients had 2
sets). For these subjects, only the data set with the highest SerFDex
was chosen for analysis. In two subjects, the volume of the late-night
salivary sample was inadequate for measurement.
In this study, 21 subjects were found to have
hypercortisolism according to the above criteria—20 subjects met the
biochemical criterion; eight subjects met the histological criterion,
including one whose set of tests did not fulfil the biochemical criterion
(SerFDex 135 nmol/L; normal UFC and SalFLN, ACTH 1.1
pmol/L) and who underwent surgery because of an adrenal adenoma that
enlarged from 1.6 cm to 2.5 cm over 2 years, postoperative spot cortisol
was <28 nmol/L and hydrocortisone replacement was required for 6 months
before axis recovery. Among the 21 subjects who had hypercortisolism, 7
had adrenal Cushing’s, 4 pituitary Cushing’s, 3 ectopic ACTH syndrome, 1
adrenocortical carcinoma, and 6 subclinical Cushing’s. Of these subjects,
14 (67%) had elevated UFC, 18 (86%) had non-suppressed SerFDex,
and 17 (81%) had elevated SalFLN. Among the eight subjects with
histological proof (6 adrenalectomies, 1 transsphenoidal surgery, 1
enucleation of pancreatic neuroendocrine tumour), all had clinical and
biochemical improvement after operation. Eucortisolism was diagnosed in 78
subjects according to the aforementioned criteria.
The baseline characteristics of the subjects are
shown in Table 1. There were no statistically significant
differences between the hypercortisolism and the eucortisolism groups with
respect to age, gender, and prevalence of obesity, diabetes mellitus, or
hypertension. There was a statistically significantly higher prevalence of
Cushingoid features and of proximal myopathy in the hypercortisolism
group.
The biochemical test results are shown in Table
2. The hypercortisolism group had statistically significantly higher
levels of SerFDex, UFC, SalFLN, SalELN,
SalFDex, and SalEDex. No SalFLN sample in
the hypercortisolism group and 17 (21.8%) SalFLN samples in the
eucortisolism group had levels below the detection limit of 0.5 nmol/L. No
SalELN sample in the hypercortisolism group had levels below
the detection limit of 0.5 nmol/L. Four (19.0%) SalFDex samples
in the hypercortisolism group and 68 (87.2%) SalFDex samples in
the eucortisolism group had a level below the detection limit of 0.5
nmol/L. One (1.3%) SalEDex sample in the eucortisolism group
had a level below the detection limit of 0.5 nmol/L.
The ROC analysis (Fig 1) and Table 3 reveal that the optimal cut-off for SalELN
was 13.50 nmol/L. Setting the specificity at a level of 95%, the cut-off
for SalELN would be 20.50 nmol/L.
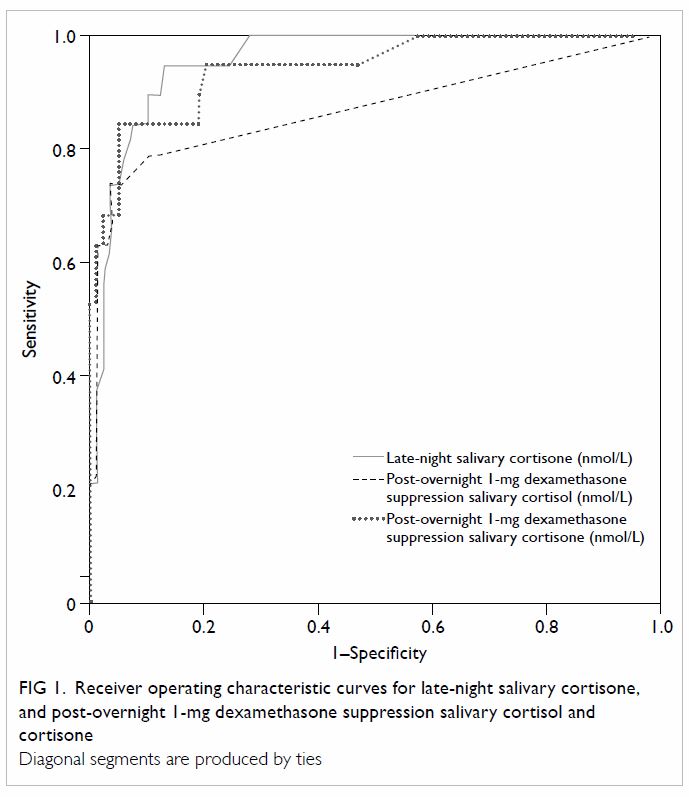
Figure 1. Receiver operating characteristic curves for late-night salivary cortisone, and post-overnight 1-mg dexamethasone suppression salivary cortisol and cortisone
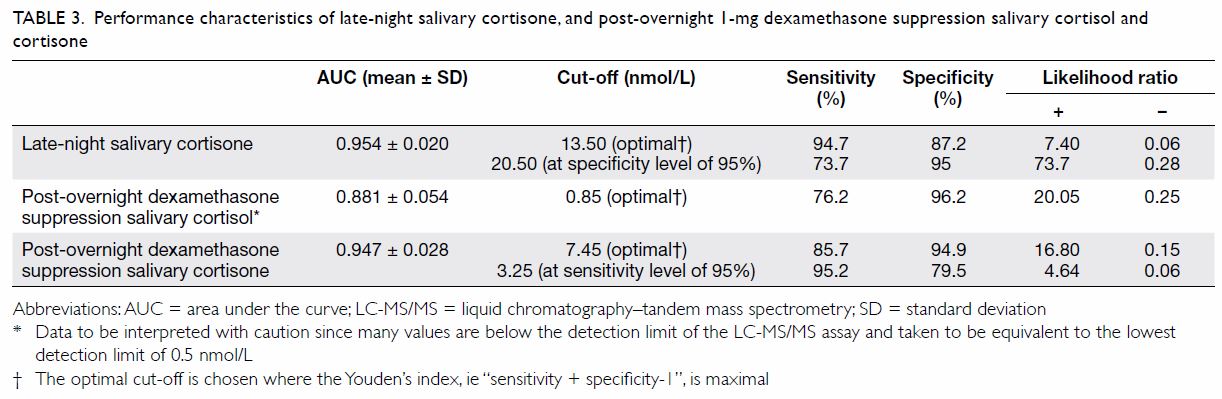
Table 3. Performance characteristics of late-night salivary cortisone, and post-overnight 1-mg dexamethasone suppression salivary cortisol and cortisone
After overnight 1-mg dexamethasone suppression, the
optimal cut-off for SalFDex was 0.85 nmol/L (Table
3). Nonetheless, these values should be interpreted with caution,
since many SalFDex values in both the eucortisolism (87.2%) and
hypercortisolism (19.0%) groups were below the detection limit of the
LC-MS/MS assay and were consequently presumed to be equivalent to the
lowest detection limit of 0.5 nmol/L.
After 1-mg overnight dexamethasone suppression, the
optimal cut-off for SalEDex was 7.45 nmol/L. Setting the
sensitivity at a level of 95%, the cut-off for SalEDex would be
3.25 nmol/L (Table 3).
The correlation between 0900h serum cortisol and
salivary cortisol was 0.81 (P<0.01); and that between 0900h serum
cortisol and salivary cortisone was 0.88 (P<0.01). The correlation
between SerFDex and SalFDex was 0.90 (P<0.01);
and that between SerFDex and SalEDex was 0.94
(P<0.01) [Fig 2].
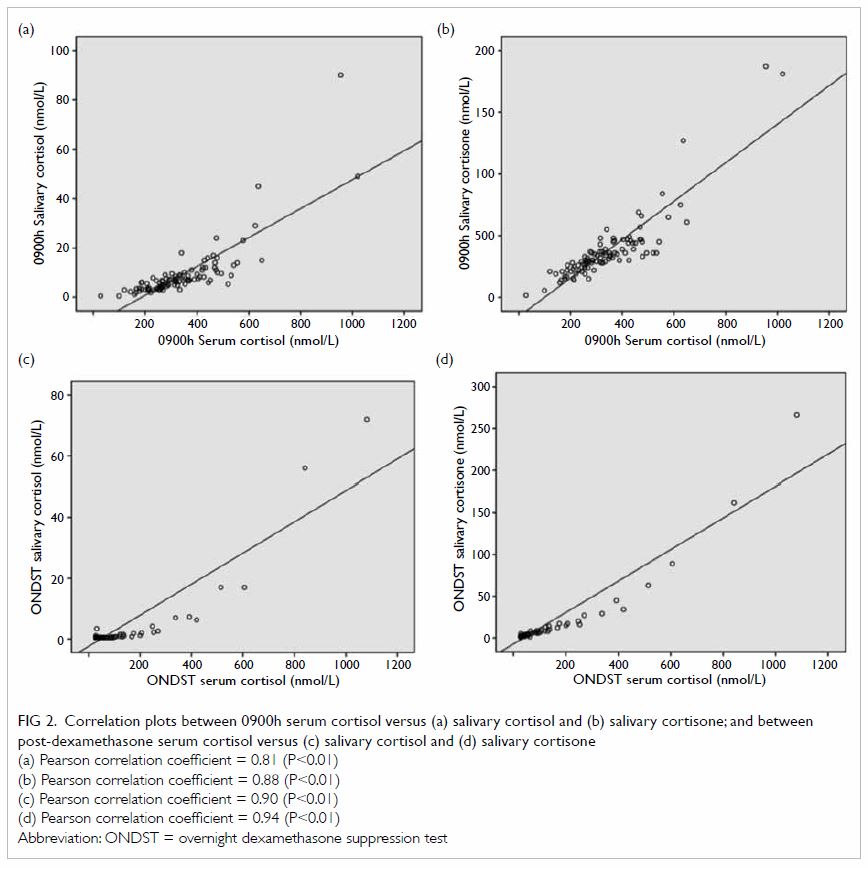
Figure 2. Correlation plots between 0900h serum cortisol versus (a) salivary cortisol and (b) salivary cortisone; and between post-dexamethasone serum cortisol versus (c) salivary cortisol and (d) salivary cortisone
Discussion
All investigators who study Cushing’s syndrome are
confronted with the conundrum of accurately diagnosing or excluding the
condition with no gold standard test.1
In our study, in addition to the histological criterion, we considered it
appropriate to include a set of biochemical criteria in which subjects
with two positive results among the three commonly used tests—namely the
SerFDex, the UFC, and the SalFLN—were considered to
have hypercortisolism. This is in agreement with the Endocrine Society
Clinical Practice Guideline17 that
recommends performing one or two other tests if one of these is abnormal;
and if results from two different tests are concordant, to proceed with
investigations to establish the cause of Cushing’s syndrome. One
well-recognised contentious point in the interpretation of the SerFDex
is the optimal cut-off: <140 nmol/L is a widely cited normal response,
but can lead to false-negative results in up to 15% of subjects with
Cushing’s syndrome.18 19 The more stringent cut-off of <50 nmol/L
sacrifices specificity for sensitivity.20
21 In this study, we adopted a
double cut-off as proposed by the European Society of Endocrinology
Clinical Practice Guideline in collaboration with the European Network for
the Study of Adrenal Tumors15; the
rationale being that a more sensitive cut-off should be employed in those
with a higher pretest probability of Cushing’s syndrome, such as the
presence of an adrenal adenoma on imaging studies.22 A more specific cut-off can be employed in general to
avoid overdiagnosis.
The loss of circadian rhythm with absence of a
late-night cortisol nadir is a well-established feature of Cushing’s
syndrome. Midnight serum cortisol is, however, difficult to obtain. When
SalFLN was shown to correlate well with serum cortisol levels,
with sensitivity of 92%-100% and specificity of 93%-100% for the diagnosis
of Cushing’s syndrome,17 it
rapidly became one of the most popular tests in investigating endogenous
hypercortisolism. In view of the theoretical advantages of salivary
cortisone, we also attempted to explore the performance characteristics of
SalELN. Our data showed that it had a good sensitivity of 94.7%
and a specificity of 87.2% at the cut-off of 13.50 nmol/L, as measured by
LC-MS/MS.
We could not compare the utility of SalFLN
with cortisone in this study, since SalFLN was one of the
criteria applied to define Cushing’s syndrome. Simultaneous measurement of
salivary cortisol and salivary cortisone can nonetheless alert clinicians
to certain caveats encountered when measuring salivary cortisol alone.
When the salivary cortisol-to-cortisone ratio is exceptionally high,
direct contamination of the oral sample by topical or oral hydrocortisone
must be excluded. Ingestion of glycyrrhetinic acid (eg in licorice,
carbenoxolone), which competitively inhibits 11β-HSD2, or rare cases of
genetic 11β-HSD2 defect, can also lead to the same anomaly.
A number of other investigators have explored the
utility of SalFDex in the diagnosis of Cushing’s syndrome.
Apart from its convenience, salivary values are not affected by conditions
that affect corticosteroid-binding globulin (CBG) or albumin levels, such
as acute and chronic illness, pregnancy or oestrogen treatment, or genetic
variants of CBG. A sensitivity varying between 97% and 100% and a
specificity between 77% and 100% have been variously reported, with
cut-off level varying between 1.7 nmol/L and 2 nmol/L.6 7 8 9 In the
current study, we found that the sensitivity of SalFDex was
only 76.2% at the optimal cut-off of 0.85 nmol/L. Although this
discrepancy with other studies might be due to a number of factors, such
as the means of defining normal ranges and the criteria for diagnosing
Cushing’s syndrome, one important factor that is evident from our data is
the method used for assaying salivary cortisol: some used
electrochemiluminescence assay,9
others used radioimmunoassay6 7 8; but we
measured SalFDex with LC-MS/MS.12
Unlike immunoassays, LC-MS/MS measurement of analytes is more specific,
with less cross-reactivity among different cortisol precursors and
metabolites.23 The concentration
of SalFDex was very low: 19% of our patients with
hypercortisolism and 87% of those with eucortisolism had SalFDex
below the detection limit of 0.5 nmol/L, leading to uncertainty in
establishing the cut-off, since all those with results of <0.5 nmol/L
could only be considered to have salivary cortisol level equal to 0.5
nmol/L in the analysis. Immunoassays, by measuring other cortisol
precursors or metabolites in varying degrees in addition, could have
bypassed this problem. Other studies have also reported that SalFLN
has poorer diagnostic performance characteristics if measured by LC-MS/MS,
in comparison with the less-specific immunoassays such as chemiluminescent
assays or radioimmunoassays.24 25
Nevertheless, LC-MS/MS is analytically more
superior and is expected to become the steroid assay of choice in the
future.26 Values generated by
studies using LC-MS/MS have greater inter-centre and long-term
generalisability in view of the lack of assay-specific steroid
cross-reactivity. Adoption of cut-offs generated by studies in which
salivary cortisol was assayed using antibody-based methods into clinical
practice is known to be problematic.27
Individual centres are often advised to generate their own references and
cut-offs although this is often not feasible. In a meta-analysis on the
use of SalFLN for investigation of Cushing’s syndrome,25 the recommended cut-offs varied widely, from 3.59 to
15.17 nmol/L. To overcome the problem of lower performance characteristics
due to low levels of salivary cortisol, instead of going for immunoassays,
a better solution may be to measure salivary cortisone that is present in
a much higher concentration than salivary cortisol. At a serum cortisol
below 74 nmol/L, Debono et al28
showed that salivary cortisol could become undetectable by LC-MS/MS, while
salivary cortisone was always detected. Similarly, our data showed that
after dexamethasone suppression, when the salivary cortisol became too low
to be measured with LC-MS/MS in many subjects, salivary cortisone could
still be measured in all but one subject in the eucortisolism group.
Between salivary cortisol and salivary cortisone,
this study showed that salivary cortisone would be the preferred test
because it is present at a higher concentration in the saliva; and at
comparable specificity levels, SalEDex appears to have better
accuracy (as reflected by the higher AUC of the ROC curves), sensitivity,
and negative LR than SalFDex.
Apart from the optimal cut-off, clinically it is
often useful to have two cut-offs, one for ruling in a diagnosis (high
specificity) and another one for excluding a diagnosis (high sensitivity),
depending on clinicians’ preference. If we arbitrarily define an
acceptable and useful cut-off as having a 95% level of either sensitivity
or specificity, the two useful cut-offs for SalELN as derived
from our study were 13.50 and 20.50 nmol/L, respectively; those for SalEDex
were 3.25 nmol/L and 7.45 nmol/L, respectively.
Traditionally, in the algorithm for the workup for
Cushing’s syndrome, late-night levels (serum or salivary cortisol) have
been used for screening (excluding Cushing’s syndrome), whereas the
post-dexamethasone level (serum cortisol) has been used for diagnosis
(ruling in Cushing’s syndrome). When used as such, the cut-off of 13.50
nmol/L can be used for SalELN; whereas 7.45 nmol/L can be used
for SalEDex. For the time being, SalEDex data can
supplement serum cortisol measurement as a confirmatory test when
concordant, or alert the clinician to the potential pitfalls with serum
cortisol (eg variations in CBG levels) when discordant. With more
experience, SalEDex may even ultimately replace the need to
measure serum cortisol.
The strength of this study lies in the rigour with
which a pre-specified protocol was adhered to. A high success rate of
sample collection was achieved, with little missing data. Insufficient
salivary volume collected in the Salivette tubes was the most common
reason for unsuccessful salivary collection, because LC-MS/MS requires a
larger saliva volume (100-250 µL) than immunoassay (40-50 µL).29 Two thirds of our study subjects were referred either
because of an adrenal incidentaloma or common clinical conditions such as
diabetes mellitus and hypertension, and had no clinical features of
Cushing’s syndrome. This population was quite representative of cases
referred to an endocrine centre for workup of Cushing’s syndrome.
A notable limitation of this study is the small
number of subjects with hypercortisolism. The cut-off for the SerFDex
was adopted from the literature rather than from data derived from our own
healthy volunteers. The Endocrine Society Clinical Practice Guideline17 recommended two separate measurements of SalFLN
or UFC. Only one sample for each was collected in our study. Although we
might have consequently missed some cases of episodic hypercortisolism, we
assumed that if less than two out of the relatively sensitive tests were
positive at the time of sampling, the subjects would likely be in a phase
of normal cortisol secretion even if they had episodic Cushing’s syndrome.
Conclusions
Our study showed that salivary cortisone can become
the analyte of choice for investigating Cushing’s syndrome in the era of
LC-MS/MS. Our data suggest using 13.50 nmol/L for SalELN, and
either 7.45 nmol/L (more specific) or 3.25 nmol/L (more sensitive) for
SalEDex, as cut-offs.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Stewart PM. Is subclinical Cushing’s
syndrome an entity or a statistical fallout from diagnostic testing?
Consensus surrounding the diagnosis is required before optimal treatment
can be defined. J Clin Endocrinol Metab 2010;95:2618-20. Crossref
2. Ferraù F, Korbonits M. Metabolic
comorbidities in Cushing’s syndrome. Eur J Endocrinol
2015;173:M133-57. Crossref
3. Viardot A, Huber P, Puder JJ, Zulewski
H, Keller U, Müller B. Reproducibility of nighttime salivary cortisol and
its use in the diagnosis of hypercortisolism compared with urinary free
cortisol and overnight dexamethasone suppression test. J Clin Endocrinol
Metab 2005;90:5730-6. Crossref
4. Graham UM, Hunter SJ, McDonnell M,
Mullan KR, Atkinson AB. A comparison of the use of urinary cortisol to
creatinine ratios and nocturnal salivary cortisol in the evaluation of
cyclicity in patients with Cushing’s syndrome. J Clin Endocrinol Metab
2013;98:E72-6. Crossref
5. Vining RF, McGinley RA, Maksvytis JJ, Ho
KY. Salivary cortisol: a better measure of adrenal cortical function than
serum cortisol. Ann Clin Biochem 1983;20(Pt 6):329-35. Crossref
6. Barrou Z, Guiban D, Maroufi A, et al.
Overnight dexamethasone suppression test: comparison of plasma and
salivary cortisol measurement for the screening of Cushing’s syndrome. Eur
J Endocrinol 1996;134:93-6. Crossref
7. Cardoso EM, Arregger AL, Tumilasci OR,
Contreras LN. Diagnostic value of salivary cortisol in Cushing’s syndrome
(CS). Clin Endocrinol (Oxf) 2009;70:516-21.
8. Castro M, Elias PC, Quidute AR, Halah
FP, Moreira AC. Out-patient screening for Cushing’s syndrome: the
sensitivity of the combination of circadian rhythm and overnight
dexamethasone suppression salivary cortisol tests. J Clin Endocrinol Metab
1999;84:878-82. Crossref
9. Deutschbein T, Broecker-Preuss M,
Flitsch J, et al. Salivary cortisol as a diagnostic tool for Cushing’s
syndrome and adrenal insufficiency: improved screening by an automatic
immunoassay. Eur J Endocrinol 2012;166:613-8. Crossref
10. Perogamvros I, Keevil BG, Ray DW,
Trainer PJ. Salivary cortisone is a potential biomarker for serum free
cortisol. J Clin Endocrinol Metab 2010;95:4951-8. Crossref
11. Wood P. Salivary steroid
assays—research or routine? Ann Clin Biochem 2009;46(Pt 3):183-96. Crossref
12. Antonelli G, Ceccato F, Artusi C,
Marinova M, Plebani M. Salivary cortisol and cortisone by LC-MS/MS:
validation, reference intervals and diagnostic accuracy in Cushing’s
syndrome. Clin Chim Acta 2015;451(Pt B):247-51.
13. Smith RE, Maguire JA, Stein-Oakley AN,
et al. Localization of 11 beta-hydroxysteroid dehydrogenase type II in
human epithelial tissues. J Clin Endocrinol Metab 1996;81:3244-8. Crossref
14. Perogamvros I, Owen LJ, Newell-Price
J, Ray DW, Trainer PJ, Keevil BG. Simultaneous measurement of cortisol and
cortisone in human saliva using liquid chromatography-tandem mass
spectrometry: application in basal and stimulated conditions. J Chromatogr
B Analyt Technol Biomed Life Sci 2009;877:3771-5. Crossref
15. Fassnacht M, Arlt W, Bancos I, et al.
Management of adrenal incidentalomas: European Society of Endocrinology
Clinical Practice Guideline in collaboration with the European Network for
the Study of Adrenal Tumors. Eur J Endocrinol 2016;175:G1-G34. Crossref
16. Hanley JA, McNeil BJ. The meaning and
use of the area under a receiver operating characteristic (ROC) curve.
Radiology 1982;143:29-36. Crossref
17. Nieman LK, Biller BM, Findling JW, et
al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical
Practice Guideline. J Clin Endocrinol Metab 2008;93:1526-40. Crossref
18. Findling JW, Raff H, Aron DC. The
low-dose dexamethasone suppression test: a reevaluation in patients with
Cushing’s syndrome. J Clin Endocrinol Metab 2004;89:1222-6. Crossref
19. Görges R, Knappe G, Gerl H, Ventz M,
Stahl F. Diagnosis of Cushing’s syndrome: re-evaluation of midnight plasma
cortisol vs urinary free cortisol and low-dose dexamethasone suppression
test in a large patient group. J Endocrinol Invest 1999;22:241-9. Crossref
20. Ceccato F, Barbot M, Zilio M, et al.
Screening tests for Cushing’s syndrome: urinary free cortisol role
measured by LC-MS/MS. J Clin Endocrinol Metab 2015;100:3856-61. Crossref
21. Nieman LK. Cushing’s syndrome: update
on signs, symptoms and biochemical screening. Eur J Endocrinol
2015;173:M33-8. Crossref
22. Lopez D, Luque-Fernandez MA, Steele A,
Adler GK, Turchin A, Vaidya A. “Nonfunctional” adrenal tumors and the risk
for incident diabetes and cardiovascular outcomes: a cohort study. Ann
Intern Med 2016;165:533-42. Crossref
23. Raff H, Auchus RJ, Findling JW, Nieman
LK. Urine free cortisol in the diagnosis of Cushing’s syndrome: is it
worth doing and, if so, how? J Clin Endocrinol Metab 2015;100:395-7. Crossref
24. Erickson D, Singh RJ, Sathananthan A,
Vella A, Bryant SC. Late-night salivary cortisol for diagnosis of
Cushing’s syndrome by liquid chromatography/tandem mass spectrometry
assay. Clin Endocrinol (Oxf) 2012;76:467-72. Crossref
25. Carroll T, Raff H, Findling JW.
Late-night salivary cortisol for the diagnosis of Cushing syndrome: a
meta-analysis. Endocr Pract 2009;15:335-42. Crossref
26. Handelsman DJ, Wartofsky L.
Requirement for mass spectrometry sex steroid assays in the Journal of
Clinical Endocrinology and Metabolism. J Clin Endocrinol Metab
2013;98:3971-3. Crossref
27. Beko G, Varga I, Glaz E, et al. Cutoff
values of midnight salivary cortisol for the diagnosis of overt
hypercortisolism are highly influenced by methods. Clin Chim Acta
2010;411:364-7. Crossref
28. Debono M, Harrison RF, Whitaker MJ, et
al. Salivary cortisone reflects cortisol exposure under physiological
conditions and after hydrocortisone. J Clin Endocrinol Metab
2016;101:1469-77. Crossref
29. Raff H. Cushing’s syndrome: diagnosis
and surveillance using salivary cortisol. Pituitary 2012;15:64-70. Crossref