DOI: 10.12809/hkmj176288
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
Spinal cord stimulation for chronic non-cancer pain: a
review of current evidence and practice
Stanley SC Wong, MB, BS, FHKAM (Anaesthesiology)1;
CW Chan, MB, BS, FHKAM (Anaesthesiology)2; CW Cheung, MD,
FHKAM (Anaesthesiology)1
1 Laboratory and Clinical Research
Institute for Pain, Department of Anaesthesiology, The University of Hong
Kong, Pokfulam, Hong Kong
2 Department of Anaesthesiology,
Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: CW Cheung (cheucw@hku.hk)
Abstract
Spinal cord stimulation provides analgesia through
electrical stimulation of the dorsal column of the spinal cord via
electrode leads placed into the epidural space. In traditional tonic
stimulation, a painful sensation is replaced with paraesthesia. Spinal
cord stimulation is effective in reducing neuropathic pain, enhancing
function, and improving quality of life in different chronic pain
conditions. Currently, there is most evidence to support its use for
failed back surgery syndrome when multidisciplinary conventional
management is unsuccessful. Temporary trial leads are inserted in
carefully selected patients to test their responsiveness prior to
permanent implantation. Newer neuromodulation modalities are now
available. These include burst stimulation, high-frequency stimulation,
and dorsal root ganglion stimulation. Results are encouraging to date, and
they may provide superior analgesia and cover for deficiencies of
traditional tonic stimulation. Although complications are not uncommon,
they are rarely life threatening or permanently disabling. Nonetheless,
device removal is occasionally needed.
Introduction
Neuromodulation involves the use of an advanced
medical device to alter the activity of the nervous system. Spinal cord
stimulation (SCS) is a neuromodulation technique that reduces pain by
electrical stimulation of the dorsal column of the spinal cord. Electrical
leads are placed into the epidural space either percutaneously or by
laminotomy. The electrical leads are then connected to a power source,
either an implantable pulse generator (IPG) or a radiofrequency unit. The
IPG can be surgically implanted under the skin.1
A recent study showed that 28.7% of people in Hong
Kong have chronic pain.2 This can
be a major reason for reduced psychosocial function, impaired quality of
life, and increased health care costs.2
Spinal cord stimulation mainly targets neuropathic pain and has limited
efficacy for nociceptive pain.1
Neuropathic pain is common in Hong Kong, affecting 9.03% of the total
population and 14.7% of chronic pain sufferers.2
For some patients, severe pain persists despite multidisciplinary
management. Strong opioids are often prescribed, despite their
side-effects and lack of good longterm efficacy.3
For some of these patients, SCS offers effective pain relief and
consequent improved function. The Neuromodulation Appropriateness
Consensus Committee (NACC) recommends use of neuromodulation techniques
before long-term opioids for neuropathic pain.4
Spinal cord stimulation is currently approved by the Food and Drug
Administration (FDA) for chronic pain of the trunk and limbs, low back
pain, leg pain, and failed back surgery syndrome (FBSS). European
guidelines also approve the use of SCS for refractory angina pectoris and
peripheral limb ischaemia.
More recently, newer neurostimulation modalities
have been introduced. These include high-frequency spinal cord stimulation
(HF-SCS), burst stimulation, and dorsal root ganglion (DRG) stimulation.
These techniques may improve efficacy and compensate for deficiencies of
traditional tonic SCS.
Patient selection
Careful patient assessment is required to confirm
the indication, assess suitability, and exclude contra-indications. The
NACC recommends that SCS be considered after conventional
multidisciplinary management (usually 3-6 months) has failed in patients
with neuropathic or mixed pain.4 5 Patients should have a
well-defined, non-cancer, physiological cause of pain.4 Contra-indications should be excluded. These include
systemic or local infection, coagulopathy, need for anticoagulant or
antiplatelet therapy that cannot be temporarily stopped, and uncontrolled
psychiatric/psychological problems.5
Depression, anxiety, somatisation, and poor coping are associated with
poorer outcomes following SCS implantation so psychological evaluation is
advised to ensure that there are no uncontrolled psychiatric/psychological issues.5 6 7 Unresolved
social issues, in particular those related to litigation and secondary
gain, should also be excluded. The patient should have a reasonable
cognitive ability, reasonable expectations, and be motivated to comply
with the post-implantation rehabilitation programme.
Spinal cord stimulation: technical aspects
The SCS device comprises electrode leads, an
extension cable, a pulse generator, and a programmer. Most percutaneous
leads have four to 20 electrode contacts, and these are introduced into
the epidural space via an epidural needle. In the percutaneous approach,
entry of the epidural needle into the epidural space is achieved using the
loss of resistance (LOR) technique with a LOR syringe under X-ray
guidance. After entry of the needle into the epidural space, the electrode
leads are advanced to the target level under live X-ray screening to
stimulate the dorsal column (Fig). The electrodes are then placed around the
midline of the epidural space to avoid stimulating the dorsal nerve roots
that can result in uncomfortable motor responses and dysaesthesia.1 Usually two electrode leads are placed. The target
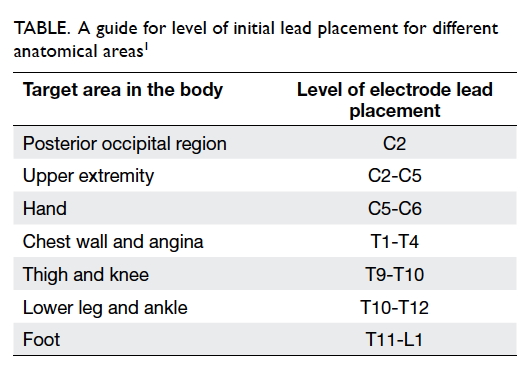
level depends on the area that needs to be stimulated (Table1). The final position of the
electrode lead is adjusted based on patient feedback during the procedure
to ensure that the area of stimulation matches the area of pain. This is
called paraesthesia mapping.
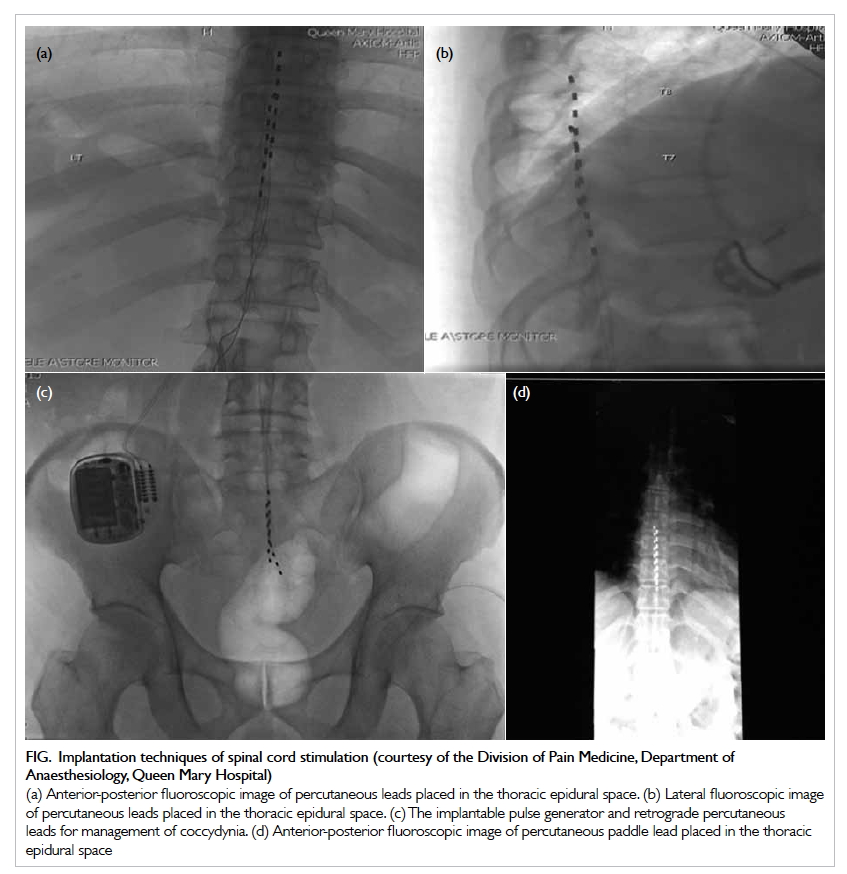
Figure. Implantation techniques of spinal cord stimulation (courtesy of the Division of Pain Medicine, Department of Anaesthesiology, Queen Mary Hospital) (a) Anterior-posterior fluoroscopic image of percutaneous leads placed in the thoracic epidural space. (b) Lateral fluoroscopic image of percutaneous leads placed in the thoracic epidural space. (c) The implantable pulse generator and retrograde percutaneous leads for management of coccydynia. (d) Anterior-posterior fluoroscopic image of percutaneous paddle lead placed in the thoracic epidural space
Three parameters are adjusted to provide
neurostimulation: frequency, amplitude, and pulse width. The frequency
determines the quality of paraesthesia: 50 Hz is most commonly used.1 The pulse width affects the size of the area of
paraesthesia and amplitude affects stimulation intensity.1 A trial period where temporary electrode leads are
inserted for approximately 5 to 7 days is needed to determine analgesic
efficacy.1 Pain relief of 50% or
greater is considered a positive trial.5
This should be accompanied by a stable level of daily activity and use of
analgesic drugs. After a successful trial, a permanent SCS implant can be
placed several weeks to 1 month later. In permanent SCS implantation, the
electrode leads are tunnelled and connected to the IPG that is implanted
under the skin in the gluteal region or lower abdominal area.
Specific pain conditions
Failed back surgery syndrome
Failed back surgery syndrome is present when
persistent pain (axial back pain and/or radicular leg pain) continues
despite back surgery. It is the most common indication for SCS with level
I-II evidence supporting the use of traditional tonic SCS for managing
FBSS.8 In the PROCESS (Prospective
Randomised Controlled Multicentre trial of the Effectiveness of Spinal
Cord Stimulation) trial, a multicentre randomised controlled trial, SCS
together with conservative medical management was superior to conservative
medical management alone in reducing leg pain, improving quality of life,
and enhancing functional capacity in patients with FBSS.9 At 12 months, 48% of patients with SCS plus medical
management obtained 50% or greater leg pain reduction versus 18% in those
with medical management only.9
A systematic review of cost-effectiveness showed
that SCS has a higher initial cost, but is more cost-effective in the long
term compared with conventional medical management.10 Such stimulation technique is also more
cost-effective than reoperation.11
Complex regional pain syndrome
Complex regional pain syndrome (CRPS) can cause
disabling pain and dysfunction of the limbs. In a randomised controlled
trial to compare SCS plus physical therapy with physical therapy alone in
the treatment of CRPS, patients with SCS had better pain control and
health-related quality of life.12
In those with an implanted SCS, 39% experienced ‘much improved’ global
perceived effect versus only 6% of control patients.12 At 2 years of follow-up, the visual analogue pain
scale decreased by 2.1 cm in the SCS–plus–physical therapy group, but did
not change in the physical therapy–only group.13
By 3 years after implantation, there was no longer any significant
difference between the groups but 95% of patients with an implant would
choose to repeat the treatment.14
A cost-effective analysis for CRPS over a 15-year period indicated that
SCS was cost-effective. 15
Refractory angina pectoris and peripheral ischaemic
limb pain
Spinal cord stimulation can cause vasodilation with
consequent improved blood flow. This is an option in the management of
patients with severe coronary artery disease and angina and for whom
revascularisation is unsuitable. It is associated with reduced angina
attacks, reduced nitrate use, and increased exercise duration compared
with conventional medical management.16
When compared with coronary artery bypass grafting, patients with SCS
achieved similar symptom relief, but required more nitrate and had lower
exercise capacity.17 It has been
shown that SCS is cost-effective for refractory angina.18
Spinal cord stimulation is also a therapeutic
option for patients with critical limb ischaemia where surgical treatment
is not possible. A meta-analysis of randomised controlled trials showed
improved analgesia and limb salvage rates.19
Other conditions
Other chronic pain conditions where SCS may be
useful include painful diabetic peripheral neuropathy, post-herpetic
neuralgia, abdominal/pelvic pain, post-amputation pain, and chest wall
pain syndromes. Results from a small randomised controlled trial indicate
better pain relief with SCS compared with medical treatment for painful
diabetic neuropathy.20 Evidence
for the other conditions is limited.
Newer neuromodulation modalities
Newer neuromodulation modalities have been
introduced in recent years, and they may further improve patient outcomes.
These include HF-SCS, burst stimulation, and DRG stimulation. The HF-SCS
and burst stimulation differ in their programming to traditional tonic
SCS. Traditional tonic SCS delivers a consistent set of pulses at a
certain amplitude, frequency, and pulse width. For tonic SCS, pulse width
is usually 300-500 µs, amplitude is 2-5 mA, and frequency is 30-100 Hz.5 21
Axial back pain, and groin and foot pain are areas that are more difficult
to target with tonic SCS.22 Tonic
SCS produces paraesthesia to surround and replace the area of pain. Some
patients, however, find this tingling sensation unpleasant. This can be
especially problematic with change in body position (especially from
sitting to standing).4 Newer
neuromodulation modalities may help tackle some of these problems.
Burst stimulation
Burst stimulation provides high-density stimulation
where groups of high-frequency impulses or ‘bursts’ are delivered
intermittently at 40 Hz. Within each ‘burst’, five pulses with a 1-ms
pulse width and 1-ms spike interval are delivered at a high frequency of
500 Hz.21 Amplitude is reduced
with the aim of providing paraesthesia-free stimulation.
Current evidence regarding burst SCS is limited. A
small randomised controlled trial showed that burst stimulation reduced
back and general pain more than that of tonic SCS.23 A systematic review, however, concluded that there
was insufficient evidence to support or discourage use of burst SCS for
chronic back and limb pain.21 Full
results of the SUNBURST (Success Using Neuromodulation with BURST) study,
a multicentre randomised controlled trial comparing burst SCS with
traditional tonic SCS in 121 patients, are awaited. The preliminary
results involving 85 patients followed up at 24 weeks show statistically
better analgesia with burst stimulation (mean difference of 6 mm visual
analogue scale points), although the clinical significance appears to be
small.24 Burst SCS was preferred
to tonic by 69% of patients.24
Burst SCS may provide improved pain control (particularly the back)
without paraesthesia.
High-frequency stimulation
The HF-SCS provides electrical stimulation at a
high frequency of 1 kHz to 10 kHz; 10 kHz is most frequently used.25 Such therapy ensures paraesthesia-free stimulation but
is not available in Hong Kong. Unlike other forms of SCS, lead
implantation for HF-SCS is based only on anatomical landmarks so
paraesthesia mapping that requires a patient to be woken up from sedation
for assessment is avoided. Implantation without paraesthesia mapping makes
the procedure simpler, and duration of surgery is more predictable.
Clinical studies support HF-SCS for back and leg
pain.26 27
This therapy has been shown to reduce back and leg pain, decrease opioid
use, and improve sleep and functional status.26
A large randomised controlled trial, SENZA, compared high-frequency 10-kHz
stimulation with traditional tonic SCS for back and leg pain in 198
patients.27 Patients with HF-SCS
reported significantly better back and leg pain relief than those who
received tonic SCS.27 At 24-month
follow-up, 76.5% and 72.9% of patients with HF-SCS had at least a 50%
reduction in back pain and leg pain, respectively.28 This reduction of pain was significantly higher than
that for patients using tonic stimulation, where only 49.3% had at least a
50% reduction in back pain and leg pain.28
Of note, HF-SCS also resulted in better outcomes in terms of disability
and patient satisfaction, and one third of the patients had reduced opioid
consumption.27 A retrospective
study showed that 68% of patients who did not receive satisfactory pain
relief with tonic SCS had a positive HF-SCS trial, suggesting that HF-SCS
may salvage patients who are not responsive to tonic SCS.29 The FDA has labelled HF-SCS at 10 kHz as superior to
traditional tonic SCS.22
Dorsal root ganglion stimulation
Stimulation of DRG involves insertion of the
electrode lead into the epidural space and then positioning of it into the
neural foramen laterally to stimulate the DRG.1
While tonic, burst, and HF-SCS act on second-order neurons in the spinal
cord, DRG exerts its effect on the primary afferent level. The DRG is
especially useful for targeting discrete focal areas of pain, such as the
groin and foot that are difficult to target using other SCS modalities.
Another advantage of DRG stimulation is a lack of change in paraesthesia
intensity with change in position, possibly due to the stable position of
the DRG.30
Clinical studies have shown encouraging results for
DRG stimulation. A non-comparative study of patients with FBSS, CRPS, and
chronic post-surgical pain reported overall pain reduction, improved mood,
and better quality of life with DRG stimulation.31
The ACCURATE trial was the largest randomised controlled trial to compare
DRG stimulation against traditional tonic SCS.32
In 152 patients who had CRPS and/or peripheral causalgia of the lower
limbs for over 6 months, DRG stimulation resulted in better pain control
at 3 and 12 months, greater improvement in quality of life, better
functional status, and better psychological well-being.32 With DRG stimulation, 74.2% of patients had over 50%
pain reduction at 12 months, compared with only 53% of those with
traditional tonic stimulation.32
The FDA has approved the use of DRG stimulation for the treatment of
lower-limb CRPS.
Complications
The overall safety profile of SCS is good and most
complications can be reversed by removal of the implant. Incidences of
complications range from 30% to 40%, but life-threatening complications
are rare.33 34
Hardware problems
Notable hardware problems include electrode lead
migration, lead fracture and malfunction, and battery failure. Lead
migration is the most common complication occurring in 2.1% to 27% of
cases, with a mean of 15.49%.33
One study showed that it was the most common reason for surgical revision
apart from battery change.35 Lead
migration usually presents as a change in area of paraesthesia and loss of
analgesia. Diagnosis can be confirmed with X-ray that should show an
unintended relocation of the lead. Minor lead migrations can be managed by
reprogramming the stimulator. If this is unsuccessful, surgical
repositioning is required.
The incidence of electrode lead fracture ranges
from 0% to 10.2%, with a mean of 6.37%.33
Lead fracture presents as loss of pain relief. X-ray may sometimes show
the site of fracture. An impedance check needs to be performed to diagnose
lead fracture, and this usually exceeds 4000 ohms.34 The fractured lead has to be removed and a new one
placed.
Other less common hardware complications include
battery failure and extension wire failures. Battery failure occurs when
the battery inside the IPG becomes exhausted and requires replacement
before the expected date; its incidence is around 1.9%.36 Rarely, extension cable breakage or disconnection can
occur, and most will require replacement.34
Biological complications
Biological complications include infection, pain,
or discomfort over device components, dural puncture, skin erosion, and
neurological injury.
Infection is a major complication of SCS
implantation, and a common reason for removal of the device. Infection
rate ranges from 2.5% to 14%, with a mean incidence of around 5%.34 Severe infection such as epidural abscess is rare.
Infection involving the subcutaneous IPG pocket is more common than
infection involving the spinal canal. In a review of over 100 cases of
infection, 48% were caused by staphylococcus and 3% by pseudomonas.37 Some risk factors included diabetes, debility,
malnutrition, obesity, a very thin body, autoimmune disorders, use of
steroids, pre-existing infection, poor hygiene, urinary or faecal
incontinence, malabsorption syndrome, and decubitus ulcers.37
Clinical symptoms and signs of infection include
fever, local pain, erythema, swelling, wound secretion, and dehiscence.
Maintaining a high index of clinical suspicion is important for early
diagnosis, and antibiotic treatment should be started without waiting for
culture results.37 38 A positive staining and/or culture of micro-organisms
from the surgical wound or implant site confirms the diagnosis.
Superficial infections may be successfully managed with antibiotics alone.
Deep infections close to the device usually necessitate device removal.
Overall, treatment of infection without device removal is associated with
lower success rates, and it is the reason most infections ultimately
result in device removal.37 After
infection is controlled, the same device can be replaced in an anatomical
location removed from the site of the infection.34
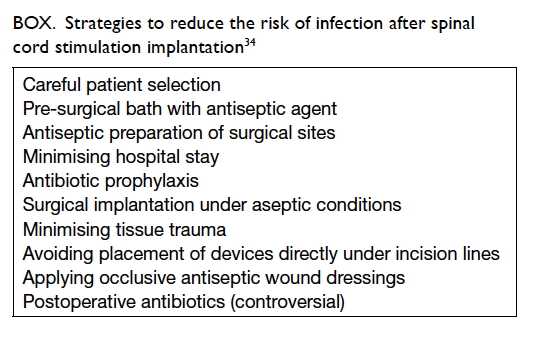
37 Some strategies to reduce risk
of infection are listed in the Box.34
Neurological injury is very rare but can occur as a
result of direct spinal cord injury from needle puncture or lead
placement. Epidural haematoma may rarely develop and lead to delayed
neurological damage. The incidence of epidural haematoma and paralysis has
been reported to be 0.3% and 0.03%, respectively.36
Early surgical consultation for exploration and decompression is required
if epidural haematoma is diagnosed.
Patients sometimes experience pain around the SCS
device such as the IPG site. The mean incidence is around 6.15%.33 Pain is usually temporary and diminishes after 7 to
14 days. Inadvertent dural puncture can occur uncommonly during Tuohy
needle insertion or electrode lead manipulation. The rate of dural
puncture has been estimated to be 0% to 0.3%, and this can result in
post-dural puncture headache.36
Subcutaneous haematoma or seroma may develop, and they most commonly occur
in the IPG pocket. The IPG pocket may subsequently become infected.
Aspiration or surgical evacuation is occasionally indicated. Skin erosions
by leads or hardware are rare, with an incidence of only 0.2%.36
Implanter training and mentorship
Appropriate training in SCS implantation is
essential to ensure optimal outcomes. The NACC recommends fellowship
training for at least 6 months, with at least 12 hours of continuing
medical education directly related to neuromodulation each year.5 Those without formal fellowship training should perform
implantation only after appropriate hands-on training with active
mentorship.5 During formal
training, the trainee should perform 10 cases under supervision as the
primary implanter.5
Practice and challenges in Hong Kong
Spinal cord stimulation is considered for
management of significant chronic pain that is refractory to conventional
management. It can be performed by pain physicians, orthopaedic surgeons,
or neurosurgeons. A percutaneous approach by pain physicians and an open
approach via laminotomy by surgeons have been performed. Patients
typically undergo multidisciplinary assessment by the SCS surgeon, pain
nurses, clinical psychologist, and physiotherapists. Suitable patients
will undergo a trial of SCS and proceed to implant if successful.
Although SCS has been available for many years in
other developed countries such as the United States, it has only started
to attract more interest in Hong Kong over the last few years. Few SCS
implants have been performed and therefore there are little local data
about its use. Despite the presence of local expertise in SCS
implantation, there is a lack of awareness and familiarity on the part of
both medical professionals and the general public. This means that
potentially suitable patients are rarely referred for an SCS trial. The
cost of an SCS implant is around HK$150 000, making it unaffordable for
many patients. While SCS may be covered by medical insurance in some other
developed countries, this is not the case in Hong Kong. There is also a
lack of government funding. Education of the general public and medical
professionals about chronic pain management and SCS, as well as financial
support from the government is imperative in order to successfully
implement SCS as an effective treatment option in Hong Kong. Competent SCS
implanters who can produce good results are crucial to generate support
from the government, other medical professionals, and the general public.
Conclusion
Spinal cord stimulation provides an effective
treatment of various chronic pain conditions such as FBSS and CRPS. It
reduces pain, improves function, increases patient satisfaction, improves
quality of life, and is also cost-effective in the long term. The option
of SCS should be considered after conservative management has failed.
Careful patient selection and assessment including placement of trial
leads are required before permanent SCS implantation. Newer
neuromodulation modalities such as burst stimulation, HF-SCS, and DRG
stimulation are producing promising results. Life-threatening or
debilitating complications are rare. Most complications can be reversed
with device removal.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Song JJ, Popescu A, Bell RL. Present and
potential use of spinal cord stimulation to control chronic pain. Pain
Physician 2014;17:235-46.
2. Cheung CW, Choi SW, Wong SS, Lee Y,
Irwin MG. Changes in prevalence, outcomes, and help-seeking behavior of
chronic pain in an aging population over the last decade. Pain Pract
2017;17:643-54. Crossref
3. Cheung CW, Chan TC, Chen PP, et al.
Opioid therapy for chronic non-cancer pain: guidelines for Hong Kong. Hong
Kong Med J 2016;22:496-505. Crossref
4. Deer TR, Mekhail N, Provenzano D, et al.
The appropriate use of neurostimulation: avoidance and treatment of
complications of neurostimulation therapies for the treatment of chronic
pain. Neuromodulation Appropriateness Consensus Committee. Neuromodulation
2014;17:571-97; discussion 597-8. Crossref
5. Deer TR, Mekhail N, Provenzano D, et al.
The appropriate use of neurostimulation of the spinal cord and peripheral
nervous system for the treatment of chronic pain and ischemic diseases:
the Neuromodulation Appropriateness Consensus Committee. Neuromodulation
2014;17:515-50; discussion 550. Crossref
6. Celestin J, Edwards RR, Jamison RN.
Pretreatment psychosocial variables as predictors of outcomes following
lumbar surgery and spinal cord stimulation: a systematic review and
literature synthesis. Pain Med 2009;10:639-53. Crossref
7. Nagel SJ, Lempka SF, Machado AG.
Percutaneous spinal cord stimulation for chronic pain: indications and
patient selection. Neurosurg Clin N Am 2014;25:723-33. Crossref
8. Grider JS, Manchikanti L,
Carayannopoulos A, et al. Effectiveness of spinal cord stimulation in
chronic spinal pain: a systematic review. Pain Physician 2016;19:E33-54.
9. Kumar K, Taylor RS, Jacques L, et al.
Spinal cord stimulation versus conventional medical management for
neuropathic pain: a multicentre randomised controlled trial in patients
with failed back surgery syndrome. Pain 2007;132:179-88. Crossref
10. Bala MM, Riemsma RP, Nixon J, Kleijnen
J. Systematic review of the cost-effectiveness of spinal cord stimulation
for people with failed back surgery syndrome. Clin J Pain
2008;24:741-56. Crossref
11. North RB, Kidd D, Shipley J, Taylor
RS. Spinal cord stimulation versus reoperation for failed back surgery
syndrome: a cost effectiveness and cost utility analysis based on a
randomized, controlled trial. Neurosurgery 2007;61:361-8; discussion
368-9. Crossref
12. Kemler MA, Barendse GA, van Kleef M,
et al. Spinal cord stimulation in patients with chronic reflex sympathetic
dystrophy. N Engl J Med 2000;343:618-24. Crossref
13. Kemler MA, De Vet HC, Barendse GA, Van
Den Wildenberg FA, Van Kleef M. The effect of spinal cord stimulation in
patients with chronic reflex sympathetic dystrophy: two years' follow-up
of the randomized controlled trial. Ann Neurol 2004;55:13-8. Crossref
14. Kemler MA, de Vet HC, Barendse GA, van
den Wildenberg FA, van Kleef M. Effect of spinal cord stimulation for
chronic complex regional pain syndrome Type I: five-year final follow-up
of patients in a randomized controlled trial. J Neurosurg
2008;108:292-8. Crossref
15. Kemler MA, Raphael JH, Bentley A,
Taylor RS. The cost-effectiveness of spinal cord stimulation for complex
regional pain syndrome. Value Health 2010;13:735-42. Crossref
16. Hautvast RW, DeJongste MJ, Staal MJ,
van Gilst WH, Lie KI. Spinal cord stimulation in chronic intractable
angina pectoris: a randomized, controlled efficacy study. Am Heart J
1998;136:1114-20. Crossref
17. Mannheimer C, Eliasson T, Augustinsson
LE, et al. Electrical stimulation versus coronary artery bypass surgery in
severe angina pectoris: the ESBY study. Circulation 1998;97:1157-63. Crossref
18. Kumar K, Rizvi S. Cost-effectiveness
of spinal cord stimulation therapy in management of chronic pain. Pain Med
2013;14:1631-49. Crossref
19. Ubbink DT, Vermeulen H. Spinal cord
stimulation for non-reconstructable chronic critical leg ischaemia.
Cochrane Database Syst Rev 2013;(2):CD004001.
20. Slangen R, Schaper NC, Faber CG, et
al. Spinal cord stimulation and pain relief in painful diabetic peripheral
neuropathy: a prospective two-center randomized controlled trial. Diabetes
Care 2014;37:3016-24. Crossref
21. Hou S, Kemp K, Grabois M. A systematic
evaluation of burst spinal cord stimulation for chronic back and limb
pain. Neuromodulation 2016;19:398-405. Crossref
22. Verrills P, Sinclair C, Barnard A. A
review of spinal cord stimulation systems for chronic pain. J Pain Res
2016;9:481-92. Crossref
23. De Ridder D, Plazier M, Kamerling N,
Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back
pain. World Neurosurg 2013;80:642-9.e1. Crossref
24. Deer TR. SUNBURST Trial Results.
Proceedings of the 19th North American Neuromodulation Society Annual Meeting; 2015 Dec 10-13; Las Vegas, USA.
25. Russo M, Van Buyten JP. 10-kHz
high-frequency SCS therapy: a clinical summary. Pain Med
2015;16:934-42. Crossref
26. AI-Kaisy A, Van Buyten JP, Smet I,
Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz
high-frequency spinal cord stimulation for patients with chronic, low back
pain: 24-month results of a prospective multicenter study. Pain Med
2014;15:347-54. Crossref
27. Kapural L, Yu C, Doust MW, et al.
Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to
traditional low-frequency spinal cord stimulation for the treatment of
chronic back and leg pain: The SENZA-RCT randomized controlled trial.
Anesthesiology 2015;123:851-60. Crossref
28. Kapural L, Yu C, Doust MW, et al.
Comparison of 10-kHz high-frequency and traditional low-frequency spinal
cord stimulation for the treatment of chronic back and leg pain: 24-month
results from a multicenter, randomized, controlled pivotal trial.
Neurosurgery 2016;79:667-77. Crossref
29. Russo M, Verrills P, Mitchell B,
Salmon J, Barnard A, Santarelli D. High frequency spinal cord stimulation
at 10 kHz for the treatment of chronic pain: 6-month Australian clinical
experience. Pain Physician 2016;19:267-80.
30. Kramer J, Liem L, Russo M, Smet I, Van
Buyten JP, Huygen F. Lack of body positional effects on paresthesias when
stimulating the dorsal root ganglion (DRG) in the treatment of chronic
pain. Neuromodulation 2015;18:50-7; discussion 57. Crossref
31. Liem L, Russo M, Huygen FJ, et al.
One-year outcomes of spinal cord stimulation of the dorsal root ganglion
in the treatment of chronic neuropathic pain. Neuromodulation
2015;18:41-8; discussion 48-9. Crossref
32. Deer TR, Levy RM, Kramer J, et al.
Dorsal root ganglion stimulation yielded higher treatment success rate for
complex regional pain syndrome and causalgia at 3 and 12 months: a
randomized comparative trial. Pain 2017;158:669-81. Crossref
33. Eldabe S, Buchser E, Duarte RV.
Complications of spinal cord stimulation and peripheral nerve stimulation
techniques: a review of the literature. Pain Med 2016;17:325-36.
34. Bendersky D, Yampolsky C. Is spinal
cord stimulation safe? A review of its complications. World Neurosurg
2014;82:1359-68. Crossref
35. Turner JA, Loeser JD, Deyo RA, Sanders
SB. Spinal cord stimulation for patients with failed back surgery syndrome
or complex regional pain syndrome: a systematic review of effectiveness
and complications. Pain 2004;108:137-47. Crossref
36. Cameron T. Safety and efficacy of
spinal cord stimulation for the treatment of chronic pain: a 20-year
literature review. J Neurosurg 2004;100(3 Suppl Spine):254-67.
37. Follett KA, Boortz-Marx RL, Drake JM,
et al. Prevention and management of intrathecal drug delivery and spinal
cord stimulation system infections. Anesthesiology 2004;100:1582-94. Crossref
38. Rudiger J, Thomson S. Infection rate
of spinal cord stimulators after a screening trial period. A 53-month
third party follow-up. Neuromodulation 2011;14:136-41; discussion
141. Crossref