Hong Kong Med J 2017 Oct;23(5):489–96 | Epub 1 Sep 2017
DOI: 10.12809/hkmj176274
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Expanded newborn metabolic screening
programme in Hong Kong: a three-year journey
SC Chong, FHKCPaed, FHKAM (Paediatrics)1,2;
LK Law, PhD, FRCPath1,3;
Joannie Hui, FRCP (Edin), FRACP1,2;
CY Lai, MSc Nursing, FHKAN (Midwifery)4;
TY Leung, MD (CUHK), FHKAM (Obstetrics and Gynaecology)1,4;
YP Yuen, FRCPath, FHKAM (Pathology)1,3
1 Centre of Inborn Errors of Metabolism, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Paediatrics, The Chinese University of Hong Kong, Shatin, Hong Kong
3 Department of Chemical Pathology, The Chinese University of Hong Kong, Shatin, Hong Kong
4 Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Dr YP Yuen (lizyuenyp@cuhk.edu.hk)
Abstract
Introduction: No universal expanded newborn
screening service for inborn errors of metabolism
is available in Hong Kong despite its long history in
developed western countries and rapid development
in neighbouring Asian countries. To increase the
local awareness and preparedness, the Centre
of Inborn Errors of Metabolism of the Chinese
University of Hong Kong started a private inborn
errors of metabolism screening programme in July
2013. This study aimed to describe the results and
implementation of this screening programme.
Methods: We retrieved the demographics of the
screened newborns and the screening results
from July 2013 to July 2016. These data were used
to calculate quality metrics such as call-back rate
and false-positive rate. Clinical details of true-positive
and false-negative cases and their outcomes
were described. Finally, the call-back logistics for
newborns with positive screening results were
reviewed.
Results: During the study period, 30 448 newborns
referred from 13 private and public units were
screened. Of the samples, 98.3% were collected within
7 days of life. The overall call-back rate was 0.128%
(39/30 448) and the false-positive rate was 0.105%
(32/30 448). Six neonates were confirmed to have
inborn errors of metabolism, including two cases
of medium-chain acyl-coenzyme A dehydrogenase
deficiency, one case of carnitine-acylcarnitine
translocase deficiency, and three milder conditions.
One case of maternal carnitine uptake defect was
diagnosed. All patients remained asymptomatic at
their last follow-up.
Conclusion: The Centre of Inborn Errors of
Metabolism has established a comprehensive
expanded newborn screening programme for
selected inborn errors of metabolism. It sets a
standard against which the performance of other
private newborn screening tests can be compared.
Our experience can also serve as a reference for
policymakers when they contemplate establishing
a government-funded universal expanded newborn
screening programme in the future.
New knowledge added by this study
- Running an expanded newborn screening programme in public and private hospitals in Hong Kong is feasible if sufficient clinical and logistical support can be provided.
- The incidence of inborn errors of metabolism detected by expanded newborn screening is one in 4355 births in Hong Kong. The call-back rate is 0.128%.
- Our results such as call-back rate and incidence of inborn errors of metabolism will be useful for future planning for a universal expanded newborn screening programme in Hong Kong.
- Our results illustrate that expanded newborn screening is not just a laboratory test, but also a comprehensive programme with different clinical components such as pre-test counselling and post-test timely specialised management.
Introduction
The term ‘inborn errors of metabolism’ (IEM) was
coined by Archibald Garrod more than 100 years
ago.1 Such disorder is extremely heterogeneous in
clinical presentation and causes disease by either
accumulation of toxic intermediary metabolites or
lack of essential metabolites. In the 1960s, Robert
Guthrie invented a bacterial-inhibition assay based
on dried blood spots (DBS) collected on filter paper
cards to detect abnormal levels of phenylalanine in
patients with phenylketonuria (PKU).2 Newborn
screening for PKU, other IEM, and non-IEM
conditions (eg congenital hypothyroidism) became
more widespread in the subsequent two decades.
In the 1990s, tandem mass spectrometry (MS/MS) for multiplex analysis of acylcarnitines and
amino acids was applied in expanded screening of
IEM in newborns.3 4 Despite 50 years having passed
since the first PKU screening, there are still vast
differences in the practice of newborn screening in
different countries.5 6 In the United States, the first
recommended uniform newborn screening panel
was adopted in 2006.7 In the latest recommended
uniform screening panel, 20 of 34 core conditions
and 22 of 26 secondary conditions are IEM with
abnormal metabolites detectable by MS/MS.8
Screening of PKU was started in the 1960s in
Australia, New Zealand, and Japan.9 10 Other Asia
Pacific countries such as Taiwan, the Philippines, and
Korea all have adopted different expanded newborn
screening panels since then.11 Shanghai is the first
city in China to adopt expanded newborn screening,
starting in 2003.12 In Singapore, an expanded
newborn screening programme that covers more
than 25 IEMs was started in 2006.13
In Hong Kong, the only territory-wide
newborn screening programme is cord blood
screening for glucose-6-phosphate dehydrogenase
(G6PD) deficiency and congenital hypothyroidism
run by the Clinical Genetics Service, Department of
Health.14 A pilot study using an OPathPaed service
model for expanded newborn screening in a regional
public hospital in Hong Kong was conducted
between July and November 2010.15 The Centre of
Inborn Errors of Metabolism (CIEM) of the Chinese
University of Hong Kong started a private expanded
newborn metabolic screening programme in July
2013 with participants from multiple centres. This
study describes the results and screening outcome
of this newborn screening programme in the past 3
years.
Methods
The CIEM newborn screening programme offered
opt-in screening for 34 aminoacidopathies, organic
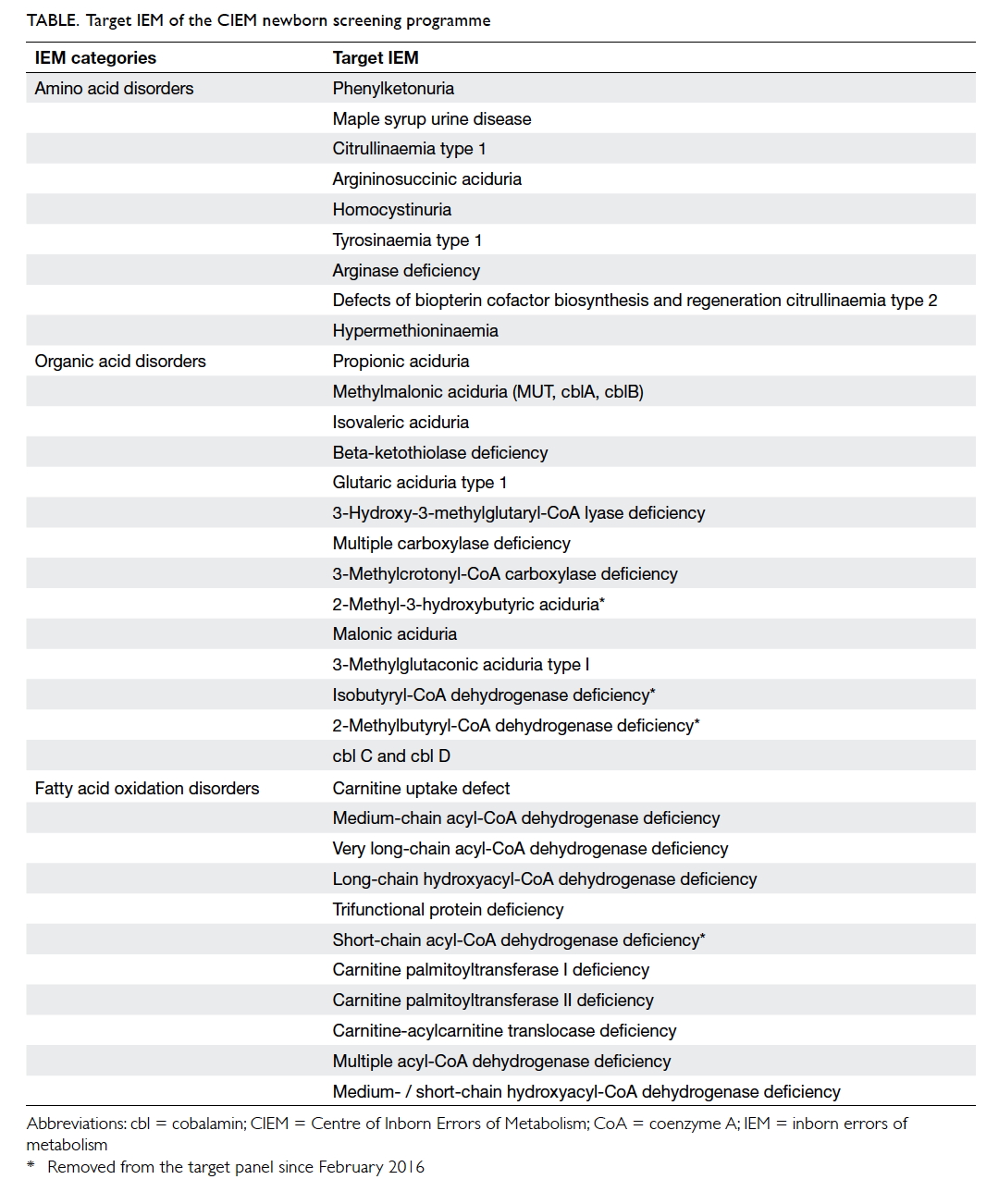
acidurias, and fatty acid oxidation disorders (Table).
Daily pre-test education and counselling were done
by doctors and nurses of the referring units. This
process was assisted by pamphlets produced by
the CIEM.16 Parents were asked to sign a consent
form after the education and counselling session.
Referring hospitals were instructed to perform a
heel prick for newborn babies between 24 hours
and 7 days after birth and spot a few drops of blood
onto a filter paper card provided by the CIEM. Apart
from basic demographic information such as date
and time of birth, the date and time of the DBS
collection, ethnicity, feeding methods, medications,
and family history of IEM were also collected. The
screening laboratory ran the MS/MS assay for IEM
screening daily from Monday to Friday. Eleven
amino acids, succinylacetone, free carnitine, and
30 acylcarnitines were analysed by the Neobase
non-derivatized MSMS kit (PerkinElmer, Waltham
[MS], US) on a Quattro Micro tandem quadrupole
mass spectrometer (Waters, Milford [MS], US). In
the initial phase, laboratory cut-offs at 1 and 99
percentiles for these 43 analytes were calculated
using results from 200 healthy newborns. These cut-offs
were then updated regularly as more normal data
were accumulated. We also compared our cut-offs
with the clinically validated cut-offs in the Region 4
Stork (R4S) MS/MS project. The R4S MS/MS project
is a web-based application for laboratory quality
improvement of newborn screening by MS/MS.17
Screen-positive results were classified as ‘uncertain’
if the abnormal analyte(s) was only mildly elevated
or ‘positive’ if the abnormal analyte(s) was markedly
elevated or the abnormal analyte pattern was highly
suggestive of a specific IEM. Post-analytical tools
in the R4S MS/MS project (https://clir.mayo.edu/)
were also used to assist result interpretation.18 The
final screening reports were authorised by a trained
chemical pathologist and a professionally qualified
scientist. The clinical team, which consisted of
metabolic paediatricians and newborn screening
nurses, was notified immediately for any positive
screening result. For each neonate with a positive
screen, a second DBS card was collected. Additional
blood and urine were usually collected at the same
time for confirmatory metabolic investigations (eg
plasma amino acid and urine organic acid analysis).
The exact course of action was determined on a
case-by-case basis. The workflow and logistics
arrangement of the CIEM screening programme are
summarised in the Figure.
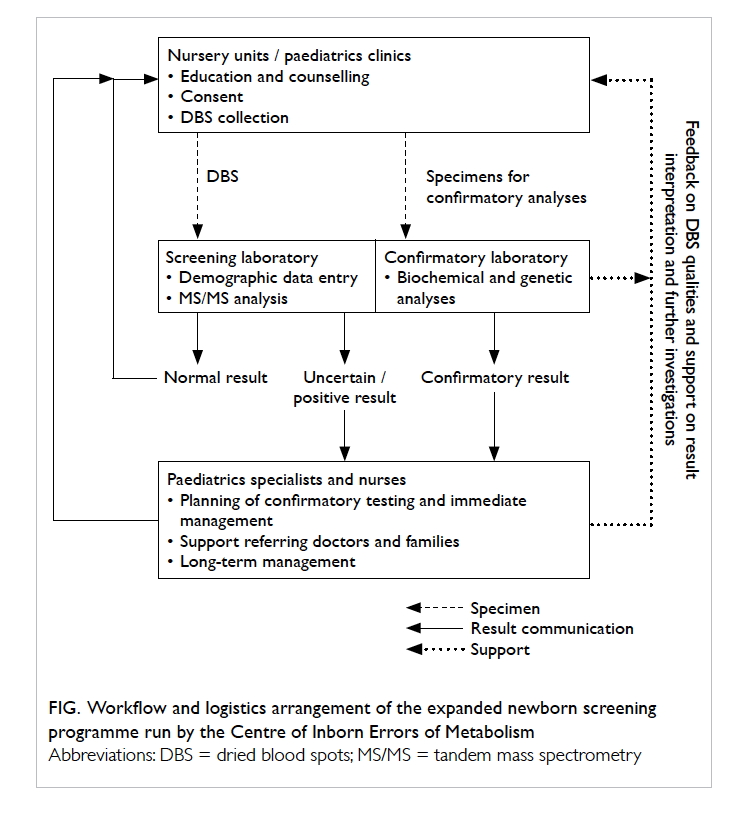
Figure. Workflow and logistics arrangement of the expanded newborn screening programme run by the Centre of Inborn Errors of Metabolism
Before and soon after launching of the CIEM
newborn screening programme, a series of seminars
and briefing sessions were organised in order to boost
the knowledge of general practitioners, nurses, and
laboratory staff on newborn screening. Continuous
support was also provided to all the referring doctors
and hospitals, especially on proper collection of DBS
and interpretation of abnormal screening results.
The CIEM also organises on-going yearly training to
midwives about newborn screening.
Data for the CIEM newborn screening
programme between July 2013 and July 2016 were
retrieved. Basic demographics of the screened
newborns, collection details for the DBS cards, call-back
rate, false-positive rate, clinical details and
outcomes of the true-positive cases, and call-back
logistics were reviewed. This study was done in accordance with the principles outlined in the Declaration of Helsinki.
Results
From July 2013 to July 2016, a total of 30 488 local
newborn babies were screened. The total number
of births during the same period was estimated to
be 186 216.19 Therefore, approximately 16% of all
newborns born between July 2013 and July 2016
were screened. The CIEM received DBS cards from
the nursery units of nine of 10 private and two of
eight public hospitals, and two paediatrics clinics
in Hong Kong. Over 95% of the screened babies
were Chinese and 2.7% were Caucasians. More than
98.3% of the DBS cards were collected within 7 days
of life. Approximately 81% of the screening results
were available in 2 calendar days and over 98% were
available in 4 calendar days. Further analysis showed
that most DBS cards with a turnaround time longer
than 4 calendar days were received just before long
holidays (eg the Lunar New Year holiday in 2014
and 2015). This is a well-known potential pitfall of
a newborn screening programme, as most screening
laboratories do not operate 7 days a week. In view
of this, the CIEM added two extra half-day services
during the Lunar New Year holiday in February 2016
to reduce the chance of delayed diagnosis.
Thirty-nine neonates had positive screening
results (four ‘positive’ and 35 ‘uncertain’) and were
called back for repeated DBS with or without
additional metabolic investigations. The call-back
rate was 0.128% (39/30 448). Six neonates (patients
1 to 6) were subsequently confirmed to have IEM
by biochemical and molecular genetic testing.
One neonate (patient 7) was confirmed to have
abnormal newborn screening results due to a defect
in maternal carnitine uptake. Details of patients 1
to 7 are described below. The false-positive rate was
0.105% (32/30 448). Among the 32 false-positive
results, 17 had low free carnitine concentrations
(range, 3.8-8.0 µmol/L) with or without low long-chain
acylcarnitines, which constituted the most
common cause of false-positive results.
Patients 1 and 2
Two siblings from the same Caucasian family were
confirmed to have medium-chain acyl-coenzyme A
dehydrogenase (MCAD) deficiency. Patient 1 was
a full-term baby girl born by vaginal delivery. The
first DBS sample showed marked elevations of C8-carnitine at 7.49
µmol/L (cut-off, <0.22 µmol/L) and
other medium-chain acylcarnitines. The diagnosis
of MCAD deficiency was confirmed by mutation
analysis of the ACADM gene. The parents were
counselled to feed their baby regularly and avoid
fasting. Patient 1 was almost 3 years old at the time
of the study and remained clinically asymptomatic.
Patient 2 was the younger sister of patient 1. Her
C8-carnitine concentration in the first DBS card
collected before 48 hours after birth was 15.2
µmol/L. She shared the same ACADM genotype as
patient 1 and also remained clinically well, and did
not require active treatment.
Patient 3
Patient 3 was a boy with carnitine-acylcarnitine
translocase (CACT) deficiency. He was born at 36
weeks and 2 days with a birth weight of 2.26 kg.
The first DBS card was collected at 31 hours of
life. He developed hypothermia, hypoglycaemia,
hyperammonaemia, and bradycardia requiring
active resuscitation with mechanical intubation at
42 hours of life. The newborn screening result was
available at 50 hours of life and showed elevated
C16-carnitine and C18:1-carnitine at 12.02 µmol/L
(cut-off, <6.66 µmol/L) and 6.32 µmol/L (cut-off,
<3.30 µmol/L), respectively. This acylcarnitine
pattern was highly suggestive of CACT deficiency
or carnitine palmitoyltransferase II deficiency.
Follow-up genetic testing confirmed the diagnosis
of CACT deficiency. Such deficiency is notorious
for its early presentation in the postnatal period,
with a high neonatal mortality rate.20 21 Although the
newborn screening result was only available after
patient 3 became symptomatic, early availability
of the screening result has greatly assisted the
neonatologists and metabolic paediatricians by
guiding the direction of clinical management. With
appropriate dietary and other management, the
clinical condition of the patient remained good and
he had normal neurodevelopment at 1.5 years of age.
Patient 4
Patient 4 was a girl with hyperphenylalaninaemia.
She was born at 40 weeks of gestation with a birth
weight of 3.45 kg. The first and second DBS showed
elevated phenylalanine at 152 µmol/L and 89
µmol/L (cut-off, <88 µmol/L), respectively. Her urinary
pterins were normal. Her plasma phenylalanine
levels were monitored for 2 years, and ranged from
210 to 434 µmol/L while the patient was receiving
an unrestricted diet. Genetic testing by sequence
analysis and multiplex ligation–dependent probe
amplification only detected a single mutation in the
PAH gene. The patient received no specific dietary
management and she had normal neurodevelopment
up to the age of 2 years.
Patient 5
Patient 5 was a full-term boy with elevated C5-carnitine at 0.52
µmol/L (cut-off, <0.48 µmol/L) in
his first newborn screen. Repeated DBS showed
persistent elevation of C5-carnitine at 0.68
µmol/L. Urinary organic acid analysis showed
elevated 2-methylbutylglycine. This result was
highly suggestive of 2-methylbutyrylglycinuria
(2-MBG) and excluded the more severe organic
acid disorder isovaleric aciduria (IVA), which
shared the same acylcarnitine marker as 2-MBG in
newborn screening. The diagnosis was subsequently
confirmed by genetic analysis of the ACADSB gene.
2-Methylbutylglycinuria is a relatively benign IEM
and the patient had normal development at the age
of 2 years without any specific treatment.
Patient 6
Patient 6 was a full-term girl whose first and second
DBS showed elevated methionine at 138 µmol/L and
309 µmol/L (cut-off, <39 µmol/L), respectively.
Following exclusion of homocystinuria, she was
diagnosed with methionine adenosyltransferase
deficiency by genetic testing. The patient was
asymptomatic during the first year of life and was
followed up at a metabolic clinic.
Incidental finding: maternal metabolic
disorder
A mother with carnitine uptake defect (CUD)
was diagnosed incidentally through the abnormal
newborn screening result for her baby. The baby of
this CUD patient had a low free carnitine level (2.1
?mol/L; cut-off, >6.4 µmol/L) in the first DBS sample,
which returned to normal on subsequent monitoring
without the need for carnitine supplementation.
Analysis of the mother showed that her serum free
carnitine level was 1.08 µmol/L only. Maternal CUD
causing low free carnitine in newborn screening
was suspected. This was later confirmed by genetic
analysis of the SLC22A5 gene. The mother had
good past health throughout her life and during the
pregnancy period. Her cardiac function was normal
at the time of diagnosis. She was subsequently
referred to a cardiologist for follow-up and was given
carnitine supplementation therapy.
False-negative case
The CIEM screening programme did not have a
mechanism to track false-negative results. Still one
false-negative result was identified. The patient was
a full-term baby girl with a body weight of 2.5 kg. She
had newborn screening done on day 3 of life and the
result was normal. In particular, her citrulline level
was 19 µmol/L (cut-off, <30 µmol/L). She presented
with prolonged jaundice at 1 month of age and was
found to have a raised plasma citrulline level at
497 µmol/L (reference range, 3-35 µmol/L). Citrin
deficiency was later confirmed by genetic analysis of
the SLC25A13 gene.
Call-back logistics
This programme involved the participation of
maternity units of 11 hospitals, including two public
and nine private hospitals, and two paediatrics
clinics. All 39 babies with positive screening results
were successfully called back within an appropriate
time-frame for follow-up investigations. Some of
the call-backs were done by the referring doctors
at their private clinics or hospitals while some were
arranged by and done at the CIEM. Our experience
showed that with proper education and professional
support, the referring paediatricians were able to
handle the call-backs of borderline abnormal results
(eg free carnitine slightly below the cut-off) at the
referring site and liaise with the CIEM for follow-up
investigations. This practice not only lowered
the workload of our metabolic paediatricians and
newborn screening nurses, but also increased
engagement of community paediatricians. For
abnormal results requiring urgent clinical attention,
the families were informed directly by our metabolic
paediatricians, who would arrange urgent admission.
Discussion
Expanded newborn screening for IEM by MS/MS
has been widely adopted by many countries in the
world for many years. Through early diagnosis
and treatment, acute metabolic decompensations
and long-term morbidity and mortality of many
IEM can be prevented. In Hong Kong, medical
practitioners and the general public are familiar
with the cord blood screening programme for G6PD
deficiency and congenital hypothyroidism, which is
a very successful screening programme with highly
satisfactory population coverage and outcome.
However, little attention has been paid to IEM
screening until recently.15 The Department of Health
and Hospital Authority have done a pilot study on
newborn screening for IEM in two public hospitals
since October 2015. The establishment of the CIEM
and its expanded newborn screening programme in
2013 has made this kind of screening service more
readily accessible to local parents who understand
the significance of IEM and opt for a private
screening service for their newborn babies. The
CIEM has successfully established a comprehensive
screening programme, which comprises education,
counselling, DBS collection, MS/MS screening,
reporting, call-back, confirmatory investigations,
and long-term follow-up and treatment. The CIEM
screening programme quickly gained acceptance
from private and public medical practitioners and
now receives DBS cards from 11 hospital nursery
units and two paediatrics clinics.
From July 2013 to July 2016, a total of 39
newborn babies were called back for abnormal
screening results. Our experience showed that
parental acceptance of abnormal screening results
was generally good and this was likely the result
of proper education and counselling before DBS
collection. During the same period, we confirmed
six cases of IEM through newborn screening and
one false-negative case was identified. The incidence
of IEM detected by this screening programme was
one in 4355. The figure is very similar to that from
previous local studies and other IEM prevalence
studies in the Chinese population.11 12 22 23
Patient 3, with CACT deficiency, presented
with hypoglycaemia and bradycardia before the
newborn screening result was available. This is not
unexpected because CACT deficiency is notorious
for its early neonatal onset and high mortality rate.20 21
For this particular case, although newborn screening
did not prevent the development of life-threatening
clinical symptoms, it did provide an early diagnosis,
which was extremely useful to the paediatricians.
This case also illustrates that screening laboratories
operating on a 5-day week may not be sufficient to
meet the clinical needs and may delay the diagnosis
of neonates born before or during weekends or
long holidays. Operating a screening laboratory on
a 7-day week, however, will increase the cost of the
screening programme. A balance between the two is
necessary.
By screening the abnormal analytes for
important IEM, some less clinically important
IEM may be revealed. For example, patient 5
had elevated C5-carnitine, which has two main
differential diagnosis, one is IVA and the other
is 2-MBG. Of note, IVA is an important organic
acid disorder. Affected patients usually present
with hyperammonaemia, metabolic acidosis, and
acute metabolic decompensation. On the other
hand, 2-MBG is a disorder of uncertain clinical
significance. Although the exact clinical course is
not yet clear, there is no case report demonstrating a
definite clinical correlation between 2-MBG and any
long-term mortality and morbidity. After conducting
a review in February 2016, the CIEM decided to
remove 2-MBG from the list of target IEM. This
can minimise the potential harm of labelling an
otherwise healthy neonate with a life-long label of an
IEM of uncertain clinical significance that does not
require any treatment.
Until a government-funded universal
expanded newborn screening service is available
in Hong Kong, private medical practitioners
are charged with the task of selecting newborn
screening service providers for their clients. Some
medical practitioners may focus on the number
of screening targets when they choose a screening
service provider and mistakenly believe the more the
better. Nonetheless, we should not ignore the harm
(eg the anxiety generated by a false-positive result)
of over-screening. Other than the appropriateness
of the screening targets, the turnaround time of the
screening tests and the availability of confirmatory
investigations are also crucial. Many screening
targets may present in the early neonatal period.
For a screening test to exert its maximum benefits,
the results should be available within a reasonable
time-frame. No newborn screening tests are
confirmatory by themselves. Therefore, whenever
there is a positive newborn screening result, further
investigations to confirm or refute the diagnosis
must be in place. Medical practitioners who use a
private newborn screening service must be aware
of this point and ensure all further investigations
generated by a positive newborn screening result
are acceptable and affordable by their patients.
The use of spot urinary specimens instead of DBS
for newborn screening is appealing to parents as
collection of urine does not require a heel prick and
thus appears to be non-invasive. Parents should be
educated that heel prick using standard devices and
done by well-trained nurses or phlebotomists are
non-traumatic and generate no harm to newborn
babies. They should also be made aware that DBS is
the standard sample of choice adopted by most, if
not all, national newborn screening programmes.
The scale and duration of this study is far from
sufficient to draw any conclusion on financial benefits
or cost-effectiveness of expanded newborn screening.
From a public service perspective, a condition
is appropriate for screening if early diagnosis
has demonstrated benefits and is cost-effective.
Both local and international studies have shown
the cost-efficiencies gained by adopting MS/MS
technology for expanded newborn screening.24 25 26
The available evidence is sufficient for policymakers
to consider implementing a universal expanded
screening programme in Hong Kong.
Conclusion
The CIEM has established a comprehensive expanded
newborn screening programme for selected IEM.
The programme involves pre-test counselling, a good
logistics arrangement for efficient incoming referral
and reporting, a readily available confirmatory
testing service and, most importantly, timely
management by medical and nursing specialists.
Each component contributes towards a successful
newborn screening programme. This screening
programme not only increases the awareness of
local health care workers and the general public of
newborn screening, but also sets a standard against
which the performance of other private newborn
screening tests can be compared. In the Hong Kong
SAR Chief Executive’s Policy Address 2017, it was
announced that the government plans to extend
its pilot newborn screening service from two to all
public hospitals with maternity wards in phases
from the second half of 2017-18.27 Our experience
could serve as a reference for policymakers when
they contemplate establishing a government-funded
universal expanded newborn screening programme
in the future.
Acknowledgement
The CIEM would like to express sincere gratitude
to the Joshua Hellmann Foundation for Orphan
Diseases for their generous donation and continuous
support. The Foundation had no role in the design
of the study; collection, analysis, or interpretation
of the data; writing, review, or approval of the
manuscript; or the decision to submit the manuscript
for publication.
Declaration
The authors have disclosed no conflicts of interest.
References
1. Garrod AE. The incidence of alkaptonuria: a study in
chemical individuality. Lancet 1902;160:1616-20. Crossref
2. Guthrie R, Susi A. A simple phenylalanine method for
detecting phenylketonuria in large populations of newborn
infants. Pediatrics 1963;32:338-43.
3. Millington DS, Norwood DL, Kodo N, Roe CR, Inoue
F. Application of fast atom bombardment with tandem
mass spectrometry and liquid chromatography/mass
spectrometry to the analysis of acylcarnitines in human
urine, blood, and tissue. Anal Biochem 1989;180:331-9. Crossref
4. Chace DH, Millington DS, Terada N, Kahler SG, Roe
CR, Hofman LF. Rapid diagnosis of phenylketonuria by
quantitative analysis for phenylalanine and tyrosine in
neonatal blood spots by tandem mass spectrometry. Clin
Chem 1993;39:66-71.
5. Boyle CA, Bocchini JA Jr, Kelly J. Reflections on 50 years of
newborn screening. Pediatrics 2014;133:961-3. Crossref
6. Therrell BL, Padilla CD, Loeber JG, et al. Current status
of newborn screening worldwide: 2015. Semin Perinatol
2015;39:171-87. Crossref
7. American College of Medical Genetics Newborn
Screening Expert Group. Newborn screening: toward a
uniform screening panel and system—executive summary.
Pediatrics 2006;117(5 Pt 2):S296-307.
8. Advisory Committee on Heritable Disorders in
Newborns and Children. Recommended uniform
screening panel. Available from: https://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendedpanel/. Accessed 17 Jan 2017.
9. Padilla CD, Therrell BL. Newborn screening in the Asia
Pacific region. J Inherit Metab Dis 2007;30:490-506. Crossref
10. Tada K, Tateda H, Arashima S, et al. Follow-up study of
a nation-wide neonatal metabolic screening program in
Japan. A collaborative study group of neonatal screening
for inborn errors of metabolism in Japan. Eur J Pediatr
1984;142:204-7. Crossref
11. Niu DM, Chien YH, Chiang CC, et al. Nationwide
survey of extended newborn screening by tandem mass
spectrometry in Taiwan. J Inherit Metab Dis 2010;33(Suppl
2):S295-305. Crossref
12. Gu X, Wang Z, Ye J, Han L, Qiu W. Newborn screening
in China: phenylketonuria, congenital hypothyroidism and
expanded screening. Ann Acad Med Singapore 2008;37(12
Suppl):107-4.
13. Lim JS, Tan ES, John CM, et al. Inborn Error of Metabolism
(IEM) screening in Singapore by electrospray ionization-tandem
mass spectrometry (ESI/MS/MS): An 8 year
journey from pilot to current program. Mol Genet Metabo
2014;113:53-61. Crossref
14. Lam ST, Cheng ML. Neonatal screening in Hong Kong and
Macau. Southeast Asian J Trop Med Public Health 2003;34
Suppl 3:73-5.
15. Mak CM, Lam C, Siu W, et al. OPathPaed service model for
expanded newborn screening in Hong Kong SAR, China.
Br J Biomed Sci 2013;70:84-8.
16. Joshua Hellmann Foundation–Newborn metabolic
screening program. Available from: http://www.obg.cuhk.edu.hk/fetal-medicine/fetal-medicine_services/iem/.
Accessed Jan 2017.
17. McHugh D, Cameron CA, Abdenur JE, et al. Clinical
validation of cutoff target ranges in newborn screening
of metabolic disorders by tandem mass spectrometry: a
worldwide collaborative project. Genet Med 2011;13:230-54. Crossref
18. Hall PL, Marquardt G, McHugh DM, et al. Postanalytical
tools improve performance of newborn screening by
tandem mass spectrometry. Genet Med 2014;16:889-95. Crossref
19. Population Estimates. Census and Statistical Department,
The Hong Kong SAR Government. Available from: https://www.censtatd.gov.hk/hkstat/sub/sp150.jsp?tableID=004&ID=0&productType=8. Accessed 28 Jun 2017.
20. Vitoria I, Martín-Hernández E, Peña-Quintana L, et al.
Carnitine-acylcarnitine translocase deficiency: experience
with four cases in Spain and review of the literature. JIMD
Rep 2015;20:11-20. Crossref
21. Lee RS, Lam CW, Lai CK, et al. Carnitine-acylcarnitine
translocase deficiency in three neonates presenting with
rapid deterioration and cardiac arrest. Hong Kong Med J
2007;13:66-8.
22. Lee HC, Mak CM, Lam CW, et al. Analysis of inborn errors
of metabolism: disease spectrum for expanded newborn
screening in Hong Kong. Chin Med J (Engl) 2011;124:983-9.
23. Hui J, Tang NL, Li CK, et al. Inherited metabolic diseases
in the Southern Chinese population: spectrum of diseases
and estimated incidence from recurrent mutations.
Pathology 2014;46:375-82. Crossref
24. Cipriano LE, Rupar CA, Zaric GS. The cost-effectiveness
of expanding newborn screening for up to 21 inherited
metabolic disorders using tandem mass spectrometry:
results from a decision-analytic model. Value Health
2007;10:83-97. Crossref
25. Ng VH, Mak CM, Johnston JM. Cost-effectiveness analysis
of newborn screening for organic acidemias in Hong Kong.
SM J Clin Pathol 2016;1:1001.
26. Grosse SD, Thompson JD, Ding Y, Glass M. The use of
economic evaluation to inform newborn screening policy
decisions: the Washington State experience. Milbank Q
2016;94:366-91. Crossref
27. The 2017 Policy Address. Available from: https://www.policyaddress.gov.hk/2017/eng/pdf/PA2017.pdf. Accessed
Aug 2017.