Hong Kong Med J 2017 Oct;23(5):462–9 | Epub 18 Apr 2017
DOI: 10.12809/hkmj164904
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Prevalence of kidney stones and associated risk
factors in the Shunyi District of Beijing
YG Jiang, MD1;
LH He, PhD2;
GT Luo, MB3;
XD Zhang, MD1
1 Department of Urology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing 100020, China
2 Department of Occupational & Environmental Health Sciences, School of Public Health Peking University, Beijing 100191, China
3 Department of Urology, Beijing Shun-Yi Hospital, Beijing 101300, China
Corresponding authors: Dr GT Luo, Dr XD Zhang (jiangyuguang1@126.com)
Abstract
Introduction: Kidney stone formation is a
multifactorial condition that involves interaction
of environmental and genetic factors. Presence of
kidney stones is strongly related to other diseases,
which may result in a heavy economic and social
burden. Clinical data on the prevalence and
influencing factors in kidney stone disease in the
north of China are scarce. In this study, we explored
the prevalence of kidney stone and potentially
associated risk factors in the Shunyi District of
Beijing, China.
Methods: A population-based cross-sectional study
was conducted from December 2011 to November
2012 in a northern area of China. Participants were
interviewed in randomly selected towns. Univariate
analysis of continuous and categorical variables
was first performed by calculation of Spearman’s
correlation coefficient and Pearson Chi squared value,
respectively. Variables with statistical significance
were further analysed by multivariate logistic
regression to explore the potential influencing
factors.
Results: A total of 3350 participants (1091 males
and 2259 females) completed the survey and the
response rate was 99.67%. Among the participants,
3.61% were diagnosed with kidney
stone. Univariate analysis showed that significant
differences were evident in 31 variables. Blood
and urine tests were performed in 100 randomly
selected patients with kidney stone and 100 healthy
controls. Serum creatinine, calcium, and uric acid
were significantly different between the patients
with kidney stone and healthy controls. Multivariate
logistic regression revealed that being male (odds
ratio=102.681; 95% confidence interval, 1.062-9925.797), daily intake of white spirits (6.331; 1.204-33.282), and a history of urolithiasis (1797.775;
24.228-133 396.982) were factors potentially
associated with kidney stone prevalence.
Conclusions: Male gender, drinking white spirits,
and a history of urolithiasis are potentially associated
with kidney stone formation.
New knowledge added by this study
- Serum creatinine, calcium, and uric acid levels were associated with kidney stone disease.
- Male gender, drinking white spirits, and a history of urolithiasis are associated with kidney stone disease.
Introduction
Kidney stone formation is a multifactorial condition
that involves interaction of environmental and
genetic factors.1 In western countries, the prevalence
and incidence of kidney stone formation have
been reported to be 2% to 19%, with an increasing
frequency among men.2 A previous study estimated
that the overall prevalence of kidney stones in China
was 4.0% (4.8% in men and 3.0% in women).3 The
condition causes severe pain and is highly likely to be
recurrent.4 In addition, the presence of kidney stones
is strongly related to chronic kidney disease, bone
loss and fractures, kidney cancer, coronary heart
disease, hypertension, and metabolic syndrome.5 6 7 8 9
This results in a heavy economic and social
burden.10 11 An appropriate prevention strategy is urgently needed to reduce the prevalence and health
care costs that arise from the condition.
Currently, many domestic and international
reports have focused on the risk factors for kidney
stone formation. These diverse risk factors including
age, gender, race, drugs, genetic, dietary, and
environmental factors (eg occupation and heat
exposure), insulin resistance, as well as drinking
water are all reported to be associated with
kidney stone prevalence.2 12 13 14 15 Clinical data on the
prevalence and influencing factors in kidney stone
disease in the north of China are scarce however, as
is knowledge about the relationship between kidney
stone formation and blood and urine parameters.
Our study aimed to explore the
prevalence of kidney stone disease and the underlying
causes in Shunyi District, Beijing, China. Shunyi
District is an important district in the northeast of
Beijing with a population of 953 000 in 2012. The
results of this study may provide an insight into ways
that can help prevent kidney stone formation.
Methods
Sampling and participants
All procedures were carried out in accordance with
the ethical standards of the local institute and with the
1964 Helsinki declaration and its later amendments
or comparable ethical standards. This cross-sectional
survey was conducted from December
2011 to November 2012. A total of 19 towns in the
Shunyi District of Beijing were numbered randomly.
A random number table was used to choose six
towns from these 19 towns. The six towns included
Zhangzhen, Mulin, Beiwu, Nanfaxin, Renhe, and
Nancai. Residents who visited their township
hospital for a routine physical examination were
invited to participate in this survey.
The inclusion criteria of participants were
the age of ≥18 years and as resident in the town
for more than 3 years. The exclusion criteria were
presence of kidney stones, renal failure, chronic
gastric disease, urinary tract malformation, urinary
tract obstructive disease, urinary tract infection, or
hyperparathyroidism.
Verbal consent was obtained from all study
participants following provision of information about
the study objectives, procedures, and implications.
The study was approved by the Ethics Committee of
Beijing Shun-Yi Hospital.
The questionnaire and physical examination
All participants were asked to complete a
questionnaire that covered the following: (1)
demographic characteristics, including gender,
age, body mass index, workplace, and job category.
The amount of sweat following exercise was
quantified and classified as dry, wet, and moist if
the palm, forehead, axillary, and back were dry,
wet, and dripping with sweat, respectively; (2)
daily fluid intake, including water intake, source
of drinking water, habits of drinking water; and
fluid intake including tea, soup, and milk; (3) living
and dietary habits, including outdoor activities,
smoking, frequency of staple and non-staple food
intake (sweetmeats, seafood, fruit, vegetables, bean
products, dairy products, eggs, meat, and animal’s
viscera); (4) personal and family history of urinary
calculus, urinary tract infection, or hypertension;
and (5) present diagnosis of kidney stone detected by
a physician and its characteristics. To screen for the
presence of kidney stones, all participants underwent
an ultrasound examination that was performed by
two attending physicians in each hospital.
Biochemical detection
To fully explore the potentially associated factors,
100 patients with kidney stone(s) were numbered
and then randomly selected by computer for
testing of blood and urine biochemical parameters.
Specifically, levels of creatinine, calcium, phosphorus,
potassium, and uric acid were measured in an early-morning
urine and fasting blood samples using a
biochemistry analyser, AU5400 (Beckman Coulter
Ltd, United States). Blood concentration of chlorine and
sodium was also measured. Simultaneously, 100 age- and
sex-matched healthy participants who visited
these hospitals for a routine physical examination
during the study period and were confirmed to be
free of urinary calculus or endocrine metabolic
disease were selected as controls.
Statistical analysis
Data were entered into the computer using EpiData
3.1 (EpiData Association, Odense, Denmark) and
analysed using the Statistical Package for the Social
Sciences (Windows version 19.0; IBM Corp, Armonk [NY], United States). Continuous variables are presented as
mean ± standard deviation, and categorical variables
as percentages. Univariate analysis of continuous
and categorical variables was first performed by
Spearman’s correlation coefficient (rs) and Pearson
Chi squared (χ2) value, respectively. Statistically significant
variables were further analysed by multivariate
stepwise logistic regression to explore the potential
influencing factors. The inclusion criteria was 0.05,
and the exclusion criteria was 0.1. Odds ratios (ORs)
with corresponding 95% confidence intervals (CIs)
were calculated. A P value of <0.05 was considered
statistically significant.
Results
Participant characteristics
A convenient sample of 3361 subjects was invited to
participate in this study, of whom 3350 completed
the survey with 1091 males and 2259 females. The
response rate was 99.67%. The mean age of all
participants was 48.97 ± 17.02 years (range, 20-98
years), with 54.46 ± 13.23 years for males and 51.81
± 11.24 years for females.
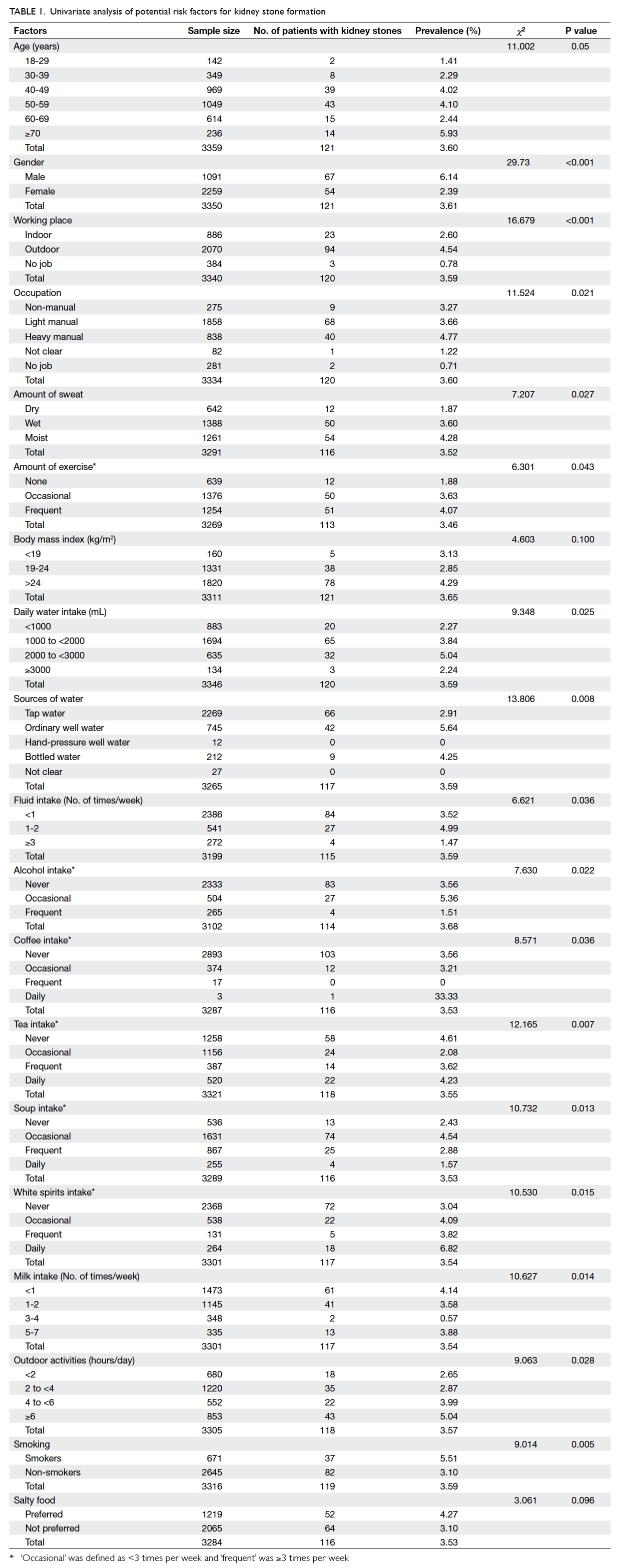
Prevalence and factors associated with presence of kidney stone
Presence of kidney stones was newly diagnosed in
121 subjects (3.61%; 95% CI, 2.88%-4.32%) including
67 (6.14%) males and 54 (2.39%) females. As shown in
Table 1, univariate analysis showed that 31 variables were significantly associated with the presence of
kidney stones—including gender; age; place of work;
occupation; amount of exercise, sweat, daily water
intake; water source; intake of fluid, alcohol, coffee,
tea, soup, white spirits, and milk; outdoor activity;
smoking; eating eggs and meat; presence of kidney
stones in relatives, parents, and siblings; as well as
personal history of hypertension, urinary stone,
urinary tract infections, chronic gastric diseases,
hyperlipidaemia, diabetes, kidney surgery, ureter
surgery; and prescription of a diuretic.
Associated biochemical variables
Biochemical parameters were measured in blood
and urine samples. As shown in Table 2, the
concentration of serum creatinine, calcium, and uric acid
in blood differed significantly in patients with and
without kidney stone(s), suggesting that the three
variables were potentially associated with this
disease. No statistical difference was observed in
the concentration of other variables. With regard to
the level of these parameters in urine, no significant
difference was found between the patients with
kidney stone and the healthy controls (Table 2).
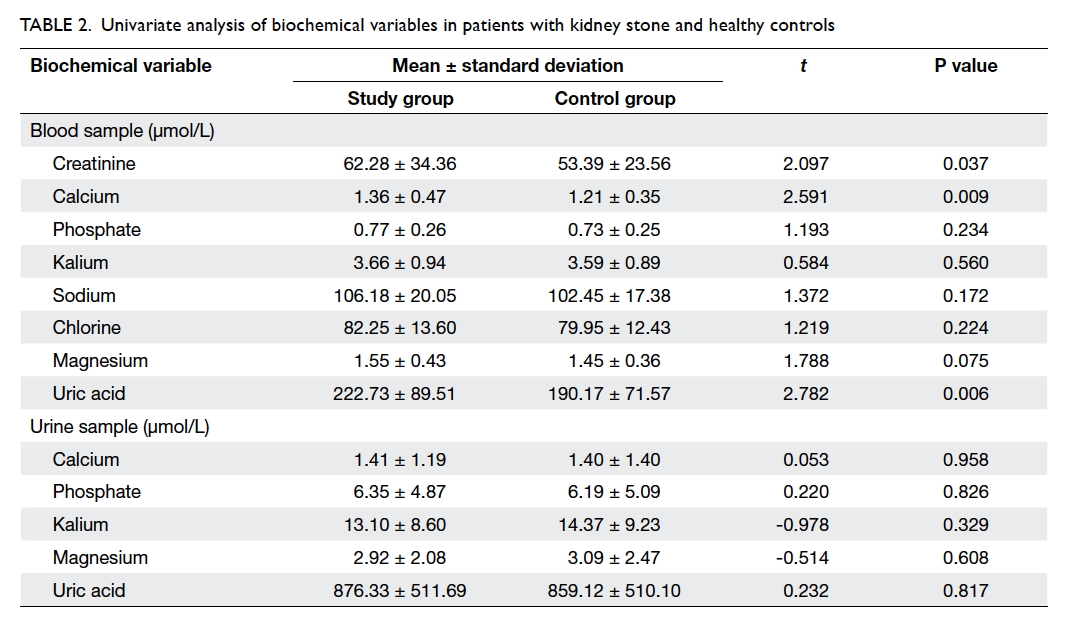
Table 2. Univariate analysis of biochemical variables in patients with kidney stone and healthy controls
Risk factors of kidney stone
Multivariate logistic regression analysis was
performed to control for the effects of confounding
factors and analyse the factors potentially associated
with kidney stone formation (Table 3). Three variables
were finally entered into the multiple logistic
regression model: male gender (OR=102.681; 95% CI, 1.062-9925.797; P=0.047), daily intake of white
spirits (OR=6.331; 95% CI, 1.204-33.282; P=0.029),
and personal history of urolithiasis (OR=1797.775;
95% CI, 24.228-133 396.982; P=0.001).
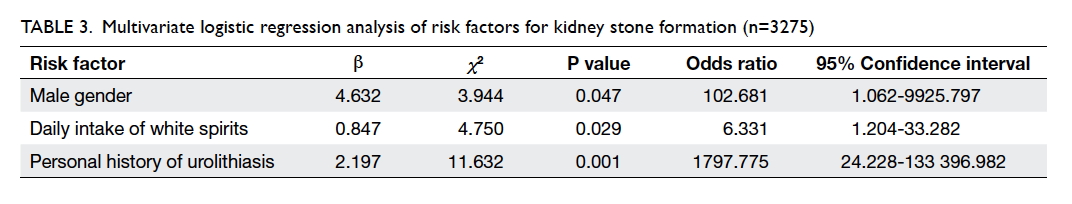
Table 3. Multivariate logistic regression analysis of risk factors for kidney stone formation (n=3275)
Discussion
In this population-based cross-sectional study, the
prevalence of kidney stones and the underlying
associated factors were investigated in the Shunyi
District of Beijing, China. A total of 1091 males
and 2259 females were enrolled in the study. The
prevalence of kidney stone was 3.61% among the
participants. The results demonstrated that male
gender, daily drinking of white spirits, and a personal
history of urolithiasis were potential risk factors for
kidney stone formation. Our results may help gain
better insight into the prevention of kidney stones.
Previous studies reported that the prevalence
of kidney stone varies with geographical location and
socio-economic conditions, and is stratified by age.16
Global epidemiological surveys demonstrated that
the mean prevalence of kidney stone was 3.25% in the
1980s and 5.64% in the 1990s.17 18 Specifically, kidney
stone affects approximately one in 20 people in Spain
and 1 in 25 in China, while the prevalence reaches up
to 9.1% in the United States and 16.9% in Northeast
Thailand.3 19 20 21 In the present study, the prevalence
was determined to be 3.61% in the six randomly
selected towns of Shunyi District, and is consistent
with the findings of the first national survey of kidney
stone in China (4.0%).3 Unfortunately, we collected
3361 subjects without collecting information about
how they were distributed in the six towns. Although
these towns were randomly selected from a total of
19 towns, self-selection bias was likely present in the
current study, as the study samples were taken by
convenient sampling from volunteers who attended
any one of the six hospitals for physical examination
for a non-specified reason. Thus, further studies
with a more representative population are needed to
verify whether the prevalence in the Shunyi District
of Beijing is in line with that in the six randomly
selected towns.
In the present study, prevalence of kidney
stones was also found to be higher in men (6.14%)
than in women (2.39%). Male gender was identified
as a risk factor in multivariate logistic regression
analysis. The reasons might be complicated. A
hormonal factor may be one of the key reasons
for the difference between men and women. For
instance, the secretion of citric acid in urine, as a
protective factor against kidney stone formation,
is promoted by oestrogen. Androgen leads to the
accumulation of some damaging factors for kidney
stone formation, such as calcium, oxalate, and uric
acid in urine.22 23 Men are also more likely to engage in heavy physical labour, to sweat more, and more often
be dehydrated. These factors are documented risk
factors for kidney stone formation.24 25 Nonetheless they were not successfully retained in the stepwise
regression in our study, implying that they did not
have an independent effect on the outcome in our
study sample, possibly due to the small sample size.
Further studies with a larger sample size are needed
to verify our findings.
A recent meta-analysis found that alcohol
intake is inversely associated with the risk of
urolithiasis.26 On the contrary, our results showed
that daily drinking of white spirits was a risk factor
for kidney stone formation. The differences might be
attributed to the varied drinking habits of different
races and countries. Curhan et al12 established
that a family history of kidney stones substantially
increased the risk of stone formation. Moreover,
increasing studies have found that patients who
have ever had urolithiasis have a higher prevalence
of kidney stone formation than those without such
a history.27 28 In concordance with these findings, our study revealed that a history of urolithiasis was
a potential risk factor for kidney stone formation.
Therefore, people who favour liquor and/or have a
history of urinary tract stones should be aware of
their higher risk for kidney stone formation and take
preventive steps.
This study has several limitations and the results
must be interpreted with caution. First, the study
sample might not be representative of the population
because of the convenient sampling of volunteers.
Also, the number of subjects excluded under each
of the exclusion criteria was not recorded. Second,
the small sample size hindered the proper control
of potential confounding factors. Third, the causal
relationship between the involved factors and kidney
stone formation could not be confirmed by a cross-sectional
survey. Fourth, recall bias and volunteer
bias could not be avoided. Finally, females were over-represented
in the sample as many males were migrant
workers and often worked in other cities. More
rigorous studies with a larger and more representative
population are needed to verify the results.
Conclusions
The prevalence of kidney stones in the current study
sample of the selected towns (Zhangzhen, Mulin,
Beiwu, Nanfaxin, Renhe, and Nancai) of Shunyi
District of Beijing, China, is 3.61%. Male gender,
daily drinking of white spirits, and a history of
urolithiasis are factors potentially associated with
kidney stone formation.
Declaration
This study was supported by Capital Medical
Development Research Fund (2009-2107). The
authors declare that they have no competing
interests.
References
1. Lu X, Gao B, Liu Z, et al. A polymorphism of matrix Gla
protein gene is associated with kidney stone in the Chinese
Han population. Gene 2012;511:127-30. Crossref
2. Romero V, Akpinar H, Assimos DG. Kidney stones: a
global picture of prevalence, incidence, and associated risk
factors. Rev Urol 2010;12:e86-96.
3. Zeng Q, He Y. Age-specific prevalence of kidney stones in
Chinese urban inhabitants. Urolithiasis 2013;41:91-3. Crossref
4. Moe OW. Kidney stones: pathophysiology and medical
management. Lancet 2006;367:333-44. Crossref
5. Ferraro PM, Taylor EN, Eisner BH, et al. History of kidney
stones and the risk of coronary heart disease. JAMA
2013;310:408-15. Crossref
6. Saucier NA, Sinha MK, Liang KV, et al. Risk factors for
CKD in persons with kidney stones: a case-control study in
Olmsted County, Minnesota. Am J Kidney Dis 2010;55:61-8. Crossref
7. Denburg MR, Leonard MB, Haynes K, et al. Risk of fracture
in urolithiasis: a population-based cohort study using the
health improvement network. Clin J Am Soc Nephrol
2014;9:2133-40. Crossref
8. Cheungpasitporn W, Thongprayoon C, O’Corragain OA, et
al. The risk of kidney cancer in patients with kidney stones:
a systematic review and meta-analysis. QJM 2015;108:205-12. Crossref
9. Jeong IG, Kang T, Bang JK, et al. Association between
metabolic syndrome and the presence of kidney stones in a
screened population. Am J Kidney Dis 2011;58:383-8. Crossref
10. Saigal CS, Joyce G, Timilsina AR; Urologic Diseases
in America Project. Direct and indirect costs of
nephrolithiasis in an employed population: opportunity
for disease management? Kidney Int 2005;68:1808-14. Crossref
11. Lotan Y. Economics and cost of care of stone disease. Adv
Chronic Kidney Dis 2009;16:5-10. Crossref
12. Curhan GC, Willett WC, Rimm EB, Stampfer MJ. Family
history and risk of kidney stones. J Am Soc Nephrol
1997;8:1568-73.
13. Asplin JR. Evaluation of the kidney stone patient. Semin
Nephrol 2008;28:99-110. Crossref
14. Basiri A, Shakhssalim N, Khoshdel AR, Pakmanesh H,
Radfar MH. Drinking water composition and incidence of
urinary calculus: introducing a new index. Iran J Kidney
Dis 2011;5:15-20.
15. Kalaitzidis RG, Damigos D, Siamopoulos KC.
Environmental and stressful factors affecting the
occurrence of kidney stones and the kidney colic. Int Urol
Nephrol 2014;46:1779-84. Crossref
16. Yasui T, Iguchi M, Suzuki S, Kohri K. Prevalence and
epidemiological characteristics of urolithiasis in Japan:
national trends between 1965 and 2005. Urology
2008;71:209-13. Crossref
17. Scott R. Prevalence of calcified upper urinary tract stone
disease in a random population survey. Report of a
combined study of general practitioners and hospital staff.
Br J Urol 1987;59:111-7. Crossref
18. Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H.
Demographic and geographic variability of kidney stones
in the United States. Kidney Int 1994;46:893-9. Crossref
19. Scales CD Jr, Smith AC, Hanley JM, Saigal CS, Urologic
Diseases in America Project. Prevalence of kidney stones
in the United States. Eur Urol 2012;62:160-5. Crossref
20. Sánchez-Martín FM, Millán Rodríguez F, Esquena Fernândez S, et al. Incidence and prevalence of published
studies about urolithiasis in Spain. A review [in Spanish].
Actas Urol Esp 2007;31:511-20. Crossref
21. Yanagawa M, Kawamura J, Onishi T, et al. Incidence of
urolithiasis in northeast Thailand. Int J Urol 1997;4:537-40. Crossref
22. Heller HJ, Sakhaee K, Moe OW, Pak CY. Etiological
role of estrogen status in renal stone formation. J Urol
2002;168:1923-7. Crossref
23. Liang L, Li L, Tian J, et al. Androgen receptor enhances
kidney stone-CaOx crystal formation via modulation of
oxalate biosynthesis & oxidative stress. Mol Endocrinol
2014;28:1291-303. Crossref
24. Zhai F, Wang H, Du S, et al. Lifespan nutrition and changing
socio-economic conditions in China. Asia Pac J Clin Nutr
2007;16 Suppl 1:374-82.
25. Galal OM. The nutrition transition in Egypt: obesity,
undernutrition and the food consumption context. Public
Health Nutr 2002;5:141-8. Crossref
26. Wang X, Xu X, Wu J, et al. Systematic review and meta-analysis
of the effect of alcohol intake on the risk of
urolithiasis including dose-response relationship. Urol Int
2015;94:194-204. Crossref
27. Indridason OS, Birgisson S, Edvardsson VO, Sigvaldason
H, Sigfusson N, Palsson R. Epidemiology of kidney stones
in Iceland: a population-based study. Scand J Urol Nephrol
2006;40:215-20. Crossref
28. Stitchantrakul W, Kochakarn W, Ruangraksa C,
Domrongkitchaiporn S. Urinary risk factors for recurrent
calcium stone formation in Thai stone formers. J Med
Assoc Thai 2007;90:688-98.