DOI: 10.12809/hkmj154699
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Mondor’s disease: sclerosing thrombophlebitis
of subcutaneous veins in a patient with occult
carcinoma of the breast
SN Wong, FHKAM (Family Medicine); Loretta KP Lai, MFM (Monash), FHKAM (Family Medicine); PF Chan, MOM (CUHK), FHKAM (Family Medicine); David VK Chao, FRCGP, FHKAM (Family Medicine)
Department of Family Medicine and Primary Health Care, Kowloon East Cluster, Hospital Authority, Hong Kong
Corresponding author: Dr SN Wong (wongsn1@ha.org.hk)
Case report
In March 2011, a 47-year-old Chinese woman
who enjoyed good past health presented to a local
general out-patient clinic in Hong Kong with a
2-week history of a mildly painful cord-like structure
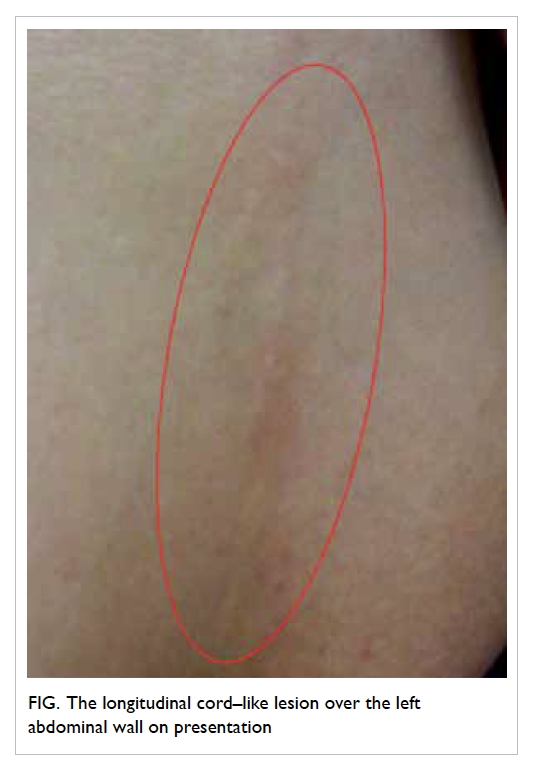
stretching from the inferior part of the left breast
to the umbilicus level (Fig). She had worked as a
dishwasher 3 months prior to the appearance of
the lesion, a job that required her to carry stacks of
heavy dishes. There was no recent trauma or surgery
of the breast and no family history of breast cancer.
Physical examination revealed a 15-cm long by
0.5-cm wide erythematous subcutaneous cord-like
lesion stretching from the inferior part of the left
breast down along the anterolateral chest wall to the
umbilicus level. The lesion was firm and mildly tender.
It was adherent to the skin, but was slightly movable
over the deeper tissues. Examination of the breasts
showed no asymmetry or skin changes. Palpation
of the right breast was normal. There was, however,
mild lumpiness over the outer upper quadrant of the
left breast although no definite breast lump found.
The nipples were normal with no discharge. There
were no palpable axillary or regional lymph nodes.
The patient was diagnosed with Mondor’s
disease with involvement of the thoracoepigastric
vein. The course of the disease was explained and
the patient was advised to avoid repetitive strain
of the chest wall. Paracetamol was prescribed
for symptomatic relief. She was referred to the
Surgical Specialist Outpatient Clinic (SOPC) for
further evaluation of left breast lumpiness. The
cord in this patient disappeared spontaneously after
approximately 5 weeks. Clinical assessment at the
SOPC in May 2011 did not identify any palpable
breast lump. Routine mammogram was later
performed in August 2012 and revealed a 2-cm ill-defined
high-density speculated lesion in the outer-upper
quadrant of the left breast. Supplementary
ultrasonography was also performed and revealed
an ill-defined irregular hypoechoic lesion measuring
0.7 x 0.6 x 1.65 cm. The patient was followed up
in the SOPC 2 weeks later and the lesion was also
clinically palpable. Core biopsy confirmed invasive
ductal carcinoma. Left modified radical mastectomy
was performed with metastatic invasive ductal
carcinoma and axillary lymph node involvement
confirmed (stage pT1cpN1). The patient was also
treated with adjuvant chemotherapy.
Discussion
Mondor’s disease is an uncommon clinical condition
of thrombophlebitis of the subcutaneous veins of
the anterolateral thoracoabdominal wall. The most
commonly affected vessels are the thoracoepigastric,
lateral thoracic, and superior epigastric veins.1 It
is characterised by sudden onset of one or more
subcutaneous palpable and visible cord(s) over the
mammary area, chest wall, or epigastrium.
Mondor’s disease occurs most commonly
in middle-aged patients, and is more common
in women than in men (ratio 3:1).2 The involved
subcutaneous veins will initially turn red and tender
and subsequently become a painless and tough
fibrous band. Pathologically, the first stage is due
to infiltration of inflammatory cells resulting in
obliterative thrombophlebitis of the affected veins,
causing redness and tenderness. In the second
stage, connective tissues proliferate in the vessels
with consequent formation of the fibrous band.1
Recanalisation of the veins occurs later and the
fibrous band will disappear within several weeks.3
It is thought to be a very uncommon disease
although the true incidence is unknown as it is self-limiting
and patients may not seek medical attention.
The diagnosis can usually be made based on clinical
history and typical physical findings. Biopsy is not
essential.
The aetiology of Mondor’s disease is still
unclear. In many cases it is idiopathic, although
it has also been linked with recent breast surgery,
local trauma, excessive physical activity with muscle
strain, an inflammatory process, infections, and
mammary pathology including mastitis or abscess.2
Association with breast cancer has also been
reported. Catana et al4 studied 63 cases of Mondor’s
disease of whom eight (12.7%) had associated breast
cancer. The authors suggested that mammography
should be performed in patients with Mondor’s
disease even when physical examination was normal.
A retrospective review in 2011 identified five cases
of Mondor’s disease of the breast; none of which
was associated with breast cancer.5 Nonetheless, the
authors advised thorough evaluation of the breast to
exclude an underlying breast cancer. In our patient,
the correlation with an occult carcinoma of the
breast could not be confirmed since there was a time
delay between the presentation of Mondor’s disease
and the diagnosis of breast carcinoma. Moreover,
the patient’s recent strenuous job with repetitive
straining of the chest wall muscle might have been a
predisposing factor.
Since Mondor’s disease is self-limiting,
symptomatic treatment is suggested. General
advice includes rest, local application of heat, and
better breast support. Oral non-steroidal anti-inflammatory
drugs provide effective symptomatic
relief.2
In summary, Mondor’s disease is a condition
that is rarely encountered in primary care.
Recognising this condition is important as it can
avoid unnecessary investigations and referral for
diagnosis. Although the association of underlying
breast cancer with Mondor’s disease has not been
confirmed, family physicians should be aware of
the possibility and conduct a thorough evaluation
including clinical examination and imaging of the
breast to exclude its presence in patients diagnosed
with Mondor’s disease.
References
1. Alvarez-Garrido H, Garrido-Ríos AA, Sanz-Muñoz C,
Miranda-Romero A. Mondor’s disease. Clin Exp Dermatol
2009;34:753-6.
Crossref
2. Mayor M, Burón I, de Mora JC, et al. Mondor’s disease. Int
J Dermatol 2000;39:922-5.
Crossref
3. Ichinose A, Fukunaga A, Terashi H, et al. Objective
recognition of vascular lesions in Mondor’s disease by
immunohistochemistry. J Eur Acad Dermatol Venereol
2008;22:168-73.
4. Catana S, Zurrida S, Veronesi P, Galimberti V, Bono A,
Pluchinotta A. Mondor’s disease and breast cancer. Cancer
1992;69:2267-70.
Crossref
5. Salemis NS, Merkouris S, Kimpouri K. Mondor’s disease of
the breast. A retrospective review. Breast Dis 2011;33:103-7.
Crossref