Hong Kong Med J 2017 Jun;23(3):222–30 | Epub 5 May 2017
DOI: 10.12809/hkmj166023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
A descriptive study of Lewy body dementia
with functional imaging support in a Chinese
population: a preliminary study
YF Shea, MRCP(UK), FHKAM (Medicine);
LW Chu, MD, FRCP;
SC Lee, BHS(Nursing)
Geriatrics Division, Department of Medicine, Queen Mary Hospital,
Pokfulam, Hong Kong
Corresponding author: Dr YF Shea (elphashea@gmail.com)
Abstract
Introduction: Lewy body dementia includes
dementia with Lewy bodies and Parkinson’s disease
dementia. There have been limited clinical studies
among Chinese patients with Lewy body dementia.
This study aimed to review the presenting clinical
features and identify risk factors for complications
including falls, dysphagia, aspiration pneumonia,
pressure sores, and mortality in Chinese patients
with Lewy body dementia. We also wished to
identify any difference in clinical features of patients
with Lewy body dementia with and without an
Alzheimer’s disease pattern of functional imaging.
Methods: We retrospectively reviewed 23 patients
with Lewy body dementia supported by functional
imaging. Baseline demographics, presenting clinical
and behavioural and psychological symptoms of
dementia, functional and cognitive assessment
scores, and complications during follow-up were
reviewed. Patients with Lewy body dementia were
further classified as having an Alzheimer’s disease
imaging pattern if functional imaging demonstrated
bilateral temporoparietal hypometabolism or
hypoperfusion with or without precuneus and
posterior cingulate gyrus hypometabolism or
hypoperfusion.
Results: The pre-imaging accuracy of clinical
diagnosis was 52%. In 83% of patients, behavioural
and psychological symptoms of dementia were
evident. Falls, dysphagia, aspiration pneumonia,
pressure sores, and death occurred in 70%, 52%,
26%, 26%, and 30% of patients, respectively with
corresponding event rates per person-years of 0.32,
0.17, 0.18, 0.08, and 0.10. Patients with aspiration
pneumonia compared with those without were more
likely to have dysphagia (100% vs 35%; P=0.01).
Deceased patients with Lewy body dementia,
compared with alive patients, had a higher (median
[interquartile range]) presenting Clinical Dementia
Rating score (1 [1-2] vs 0.5 [0.5-1.0]; P=0.01), lower
mean (± standard deviation) baseline Barthel index
(13 ± 7 vs 18 ± 4; P=0.04), and were more likely to be
prescribed levodopa (86% vs 31%; P=0.03). Patients
with Lewy body dementia with an Alzheimer’s
disease pattern of functional imaging, compared
with those without the pattern, were younger at
presentation (mean ± standard deviation, 73 ± 6 vs
80 ± 6 years; P=0.02) and had a lower Mini-Mental State
Examination score at 1 year (15 ± 8 vs 22 ± 6; P=0.05).
Conclusions: Falls, dysphagia, aspiration pneumonia,
and pressure sores were common among patients
with Lewy body dementia. Those with an Alzheimer’s
disease pattern of functional imaging had a younger
age of onset and lower 1-year Mini-Mental State
Examination score.
New knowledge added by this study
- Behavioural and psychological symptoms of dementia were present in 83% of patients with Lewy body dementia (LBD).
- Falls, dysphagia, aspiration pneumonia, and pressure sores were common complications in LBD patients.
- Chinese LBD patients with an Alzheimer’s disease pattern of functional imaging had a younger age of onset and lower 1-year Mini-Mental State Examination score.
- Such information is useful in the formulation of a management plan, including advance care planning, for Chinese LBD patients.
Introduction
Lewy body dementia (LBD) includes dementia with
Lewy bodies (DLB) and Parkinson’s disease dementia
(PDD).1 Pathological hallmarks of LBD include α-synuclein neuronal inclusions (Lewy bodies and Lewy neurites) with subsequent neuronal loss.1
The difference between DLB and PDD lies in the
sequence of onset of dementia and parkinsonism,
although syndromes and pathological changes
become similar with progression.1 2 Thus, they are regarded as a continuum instead of two separate
entities. In western studies, approximately 10% to
15% of patients with dementia had DLB.1 In contrast,
only 3% of 1532 patients with dementia followed up
at the memory clinic of Queen Mary Hospital in
Hong Kong had LBD (unpublished data). It is likely
that LBD remains under-recognised among the
Chinese population.
Compared with autopsy, sensitivity and
specificity for clinical diagnosis of DLB have been
reported to be 32% and 95%, respectively.1 In our
memory clinic, 50% of DLB patients were initially
misdiagnosed (mostly as Alzheimer’s disease [AD]
in 75% of cases).3 There has been only one case series
of 35 Chinese DLB patients and diagnosis was based
mainly on clinical criteria.4 The presence of occipital
hypometabolism on [18F]-2-fluoro-2-deoxy-D-glucose
positron emission tomography (18FDG-PET)
has a sensitivity of 90% and specificity of 71% to 80% in
differentiating AD and DLB patients.5
Pathologies of AD are common in DLB
patients; 35% of patients with Parkinson’s disease
fulfil the pathological diagnostic criteria of AD,
while deposition of amyloid plaques is present in
approximately 85% of DLB patients.6 A meta-analysis
revealed a positive amyloid scan in 57% and 35% of
patients with DLB and PDD, respectively.5 A higher
cortical amyloid burden has been associated with
greater cortical and medial temporal lobe atrophy in
LBD patients.6 Significant cortical amyloid burden
may accelerate the cognitive decline in LBD patients,
suggesting the possibility of a synergistic contribution
of AD pathologies to LBD dementia.6 On 18FDG-PET,
apart from bilateral occipital hypometabolism,
some LBD patients also demonstrate an AD pattern
of hypometabolism, ie temporoparietal, posterior
cingulate gyrus or precuneus hypometabolism
(Fig).7 In a recent study comparing 12 patients
with DLB and an AD pattern of hypometabolism
on 18FDG-PET with 11 patients with DLB and no
AD pattern of hypometabolism, the former had
a higher prevalence of visual hallucinations and
extracampine hallucination.7 As far as we are aware,
an AD pattern of functional imaging has not been
studied in Chinese patients with DLB.
In contrast with AD, the clinical features of
patients with DLB are unfamiliar to the general
public. In a recent study that involved 125 carers of
LBD patients, 82% to 96% expressed a wish to have
information and support about visual hallucinations,
changes in the brain and the body, and ways to cope
with behavioural changes.8 Unfortunately clinical
studies of LBD among the Chinese population
are limited and none has examined the long-term
outcomes of LBD, including falls, dysphagia,
aspiration pneumonia, pressure sores, mortality,
and behavioural and psychological symptoms of
dementia (BPSD).
Based on a review of the clinical records of
all LBD patients followed up in our memory clinic,
the current study aimed to review the presenting
clinical features and identify risk factors for long-term
outcomes including falls, dysphagia, aspiration
pneumonia, pressure sores, and mortality in Chinese
patients with LBD. It was hoped that this would
provide useful clinical information for carers of such
patients. We also wished to identify any difference in
clinical features of LBD patients with and without an
AD pattern of functional imaging. We hypothesised
that LBD patients with an AD pattern of functional
imaging would have a young age at presentation or
diagnosis due to concomitant AD pathologies.
Methods
This was a retrospective case series of Chinese LBD
patients. The case records of Chinese patients with
LBD who attended a memory clinic at Queen Mary
Hospital between 1 January 2007 and 31 December
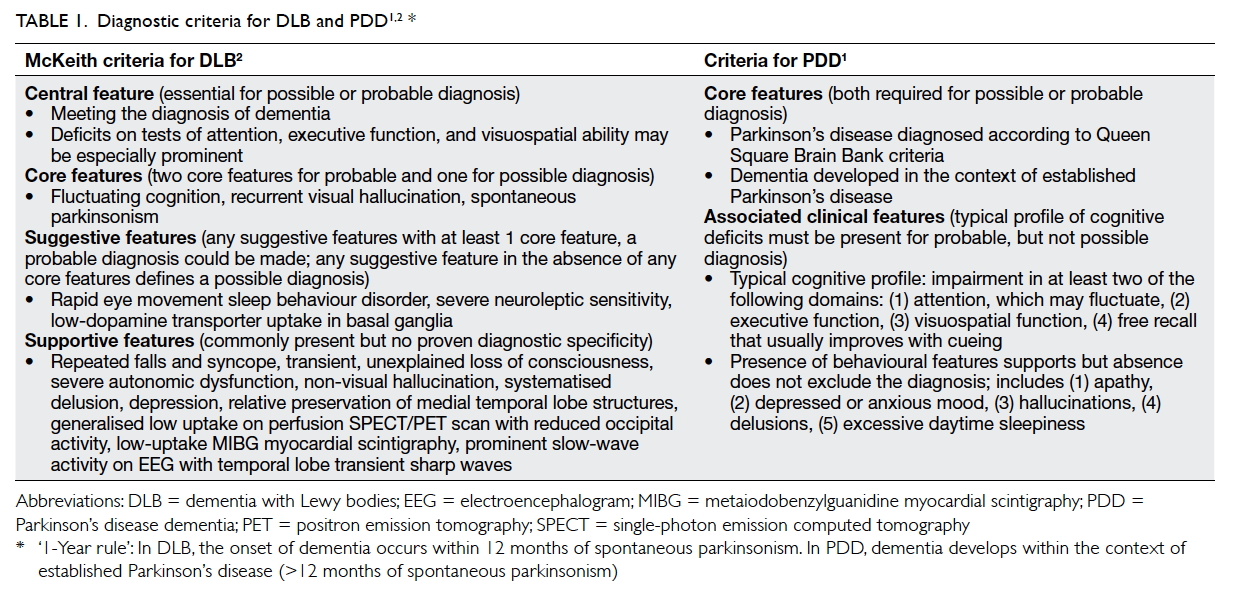
2015 were reviewed. This study was done in accordance with the principles outlined in the Declaration of Helsinki. Probable DLB was diagnosed according to McKeith criteria2 (Table 11 2). Probable PDD was diagnosed according to the following:
the patient should meet the diagnostic criteria of
Queen Square Brain Bank criteria with dementia
developing in the context of established Parkinson’s
disease with cognitive impairment in more than one
domain and severe enough to impair daily life (Table 11 2). The differentiation between DLB and PDD
was based on temporal sequence of symptoms—for
DLB, dementia developed before or within 1 year of
parkinsonism (ie the clinical syndrome characterised
by tremor, bradykinesia, rigidity, and postural
instability); for PDD, dementia developed more than
1 year after the established diagnosis of Parkinson’s
disease.1 All patients underwent functional imaging
in the form of 18FDG-PET or technetium-99m
hexamethylpropylene amine oxime single-photon
emission computed tomography (SPECT) that would
show either hypometabolism or hypoperfusion of
the occipital lobes, respectively. Data on baseline
demographics, baseline and first-year Mini-Mental
State Examination (MMSE) score,9 Clinical Dementia
Rating (CDR) score,10 age-adjusted Charlson
Comorbidity Index,11 baseline Neuropsychiatric
Inventory (NPI) score,12 baseline Barthel index–20,13 presenting cognitive symptoms, and BPSD were
derived from clinical records. ‘Time to diagnosis’
was defined as the difference between the date of
first presentation to the memory clinic and the date
of first diagnosis of LBD. Patients with DLB were
further classified as having an ‘AD imaging pattern’
if the functional imaging demonstrated bilateral
temporoparietal hypometabolism or hypoperfusion
with or without precuneus and posterior cingulate
gyrus hypometabolism or hypoperfusion (Fig).7 14
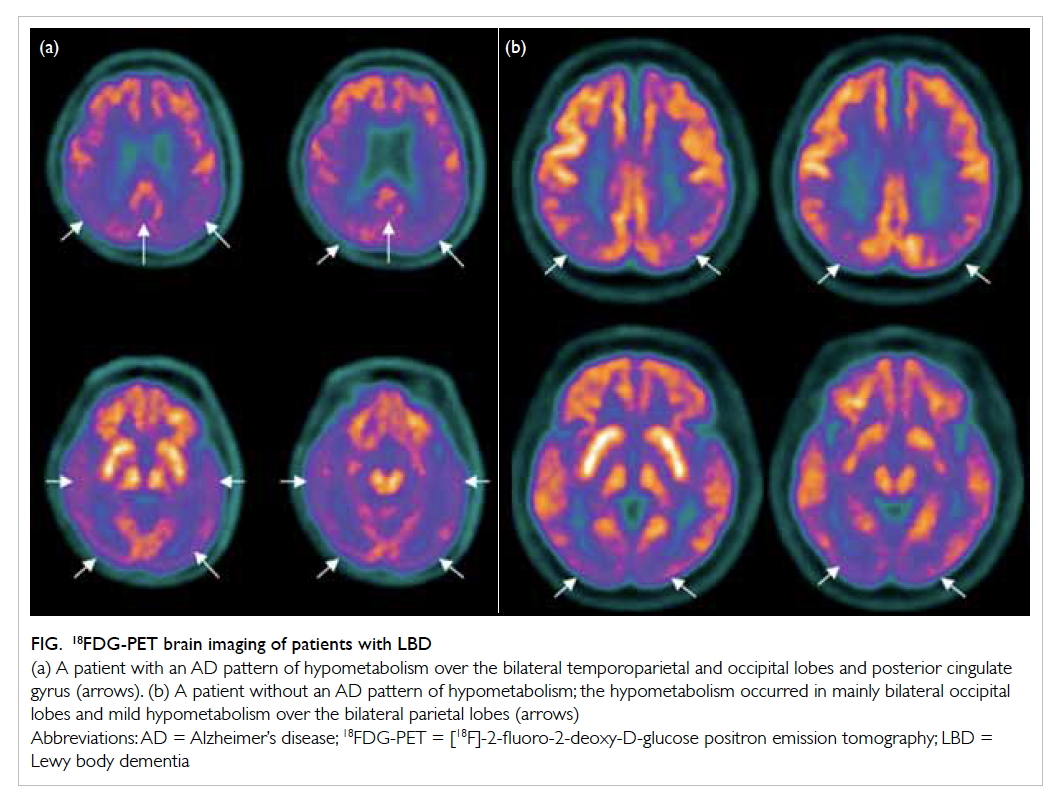
Figure. 18FDG-PET brain imaging of patients with LBD
(a) A patient with an AD pattern of hypometabolism over the bilateral temporoparietal and occipital lobes and posterior cingulate gyrus (arrows). (b) A patient without an AD pattern of hypometabolism; the hypometabolism occurred in mainly bilateral occipital lobes and mild hypometabolism over the bilateral parietal lobes (arrows)
Clinical outcomes including falls, dysphagia,
aspiration pneumonia, development of pressure
sores, and mortality were traced. For patients
with a history of falls, geriatric day hospital (GDH)
training was traced including the pre-/post-training
Elderly Mobility Scale15 and Berg Balance Scale.16 Parkinsonism medication was often titrated at the
GDH. Dysphagia was further subclassified as oral
or pharyngeal according to the clinical
assessment by a speech therapist (ST) or, if available,
a video fluoroscopic swallowing study (VFSS).17
Penetration was defined as barium material entering
the airway but not passing below the vocal cords;
aspiration was defined as barium material passing
below the level of the vocal cords.18 The locations
of pressure sores and staging, according to National
Pressure Ulcer Advisory Panel,19 were recorded.
‘Time to event’ was defined as the difference between
the date of diagnosis and first appearance of these
events.
Statistical analyses
Parametric variables were expressed as mean ± standard deviation and non-parametric variables were expressed by median (interquartile range
[IQR]). Descriptive statistics were used to express the
frequency of defining features of LBD. Chi squared
test or Fisher’s exact test was used to compare
categorical variables. Independent-samples t test
or Mann-Whitney U test were used to compare
continuous variables when appropriate. Statistical
significance was inferred by a two-tailed P value
of <0.05. All statistical analyses were carried out
using the Statistical Package for the Social Sciences
(Windows version 18.0; SPSS Inc, Chicago [IL], United States).
Results
Baseline demographics and clinical characteristics
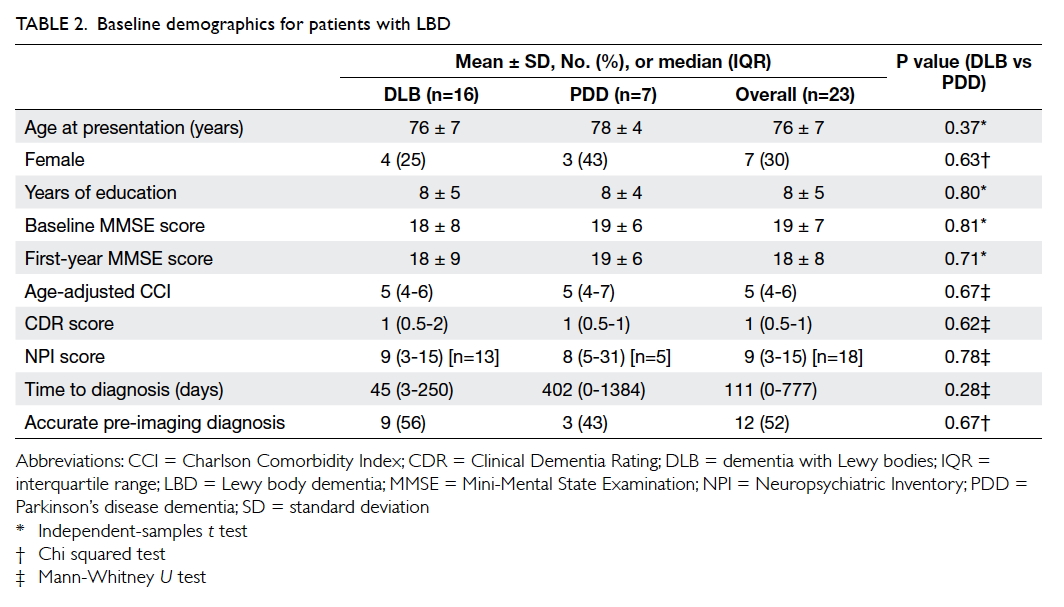
There were 23 patients with LBD (16 with DLB and
7 with PDD). The mean age at presentation was 76 ±
7 years and the mean MMSE score at presentation
was 19 ± 7 with a total duration of follow-up
of 72 patient-years (mean follow-up, 1138 ± 698
days). The baseline demographics of the patients are
summarised in Table 2. There was no statistically significant difference in baseline demographics
between DLB and PDD patients. The time to diagnosis
appeared to be longer but not statistically significant
for PDD patients, possibly due to very small
numbers in the two groups. The overall accuracy of
diagnosis was 52%. Six (38%) of the 16 DLB patients
were initially misdiagnosed as AD. The frequency of
defining clinical characteristics of LBD among DLB
and PDD patients is summarised in Table 3. There were no statistically significant differences (results
not shown). Of note, 69% of DLB patients presented
with parkinsonism and 74% of LBD patients had
vivid visual hallucinations. Information about the
content of visual hallucinations was available for
14 of 17 patients: 50% (n=7) involved persons, 7%
(n=1) involved objects, 21% (n=3) a combination
of persons and animals, 7% (n=1) a combination
of insects and animal, 7% (n=1) a combination of
insects and objects, and 7% (n=1) a combination of
animal and objects. An NPI score was available for
78% (18/23) at baseline, of whom 83% (15/18) had
a score of ≥1, and 78% (14/18) had at least one NPI
subcategory rated as severe, ie ≥4. The three most
common BPSD as indicated by a NPI score of ≥1
were visual hallucination (56%), anxiety (50%), and
apathy (50%). There was no significant difference
in BPSD in terms of NPI score for DLB and PDD
patients (results not shown).
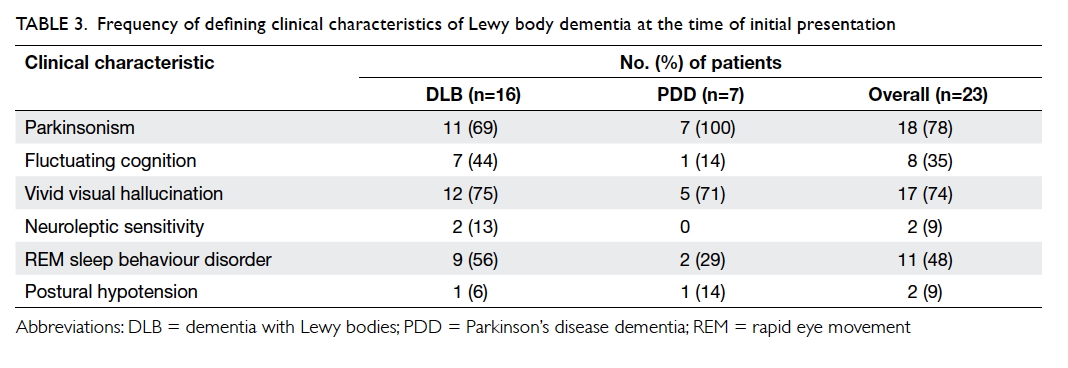
Table 3. Frequency of defining clinical characteristics of Lewy body dementia at the time of initial presentation
Falls
Of the patients, 16 (70%) had a total of 23 falls
(all non-syncopal) with four complicated by bone
fractures and two associated with intracerebral
haemorrhage. The event rate was 0.32 per person-years.
Ten patients underwent GDH training after
their fall(s). The median time to first GDH training
(from the time of diagnosis) was 56 (IQR, 56-663)
days with a mean time of 93 ± 44 days. Paired-samples
t test did not identify any significant pre-/post-training
difference in Elderly Mobility Scale15 or Berg
Balance Scale16 scores (10 ± 4 vs 11 ± 5, P=0.81 and
25 ± 13 vs 25 ± 14, P=1.0, respectively). Comparison
of LBD patients with and without a fall history
revealed no significant difference in clinical features
(including visual hallucination, parkinsonism,
and fluctuation of consciousness) or medication
(including benzodiazepine or antipsychotics)
[results not shown]. Parkinsonism was numerically
more prevalent among fallers (88% vs 57%; P=0.14).
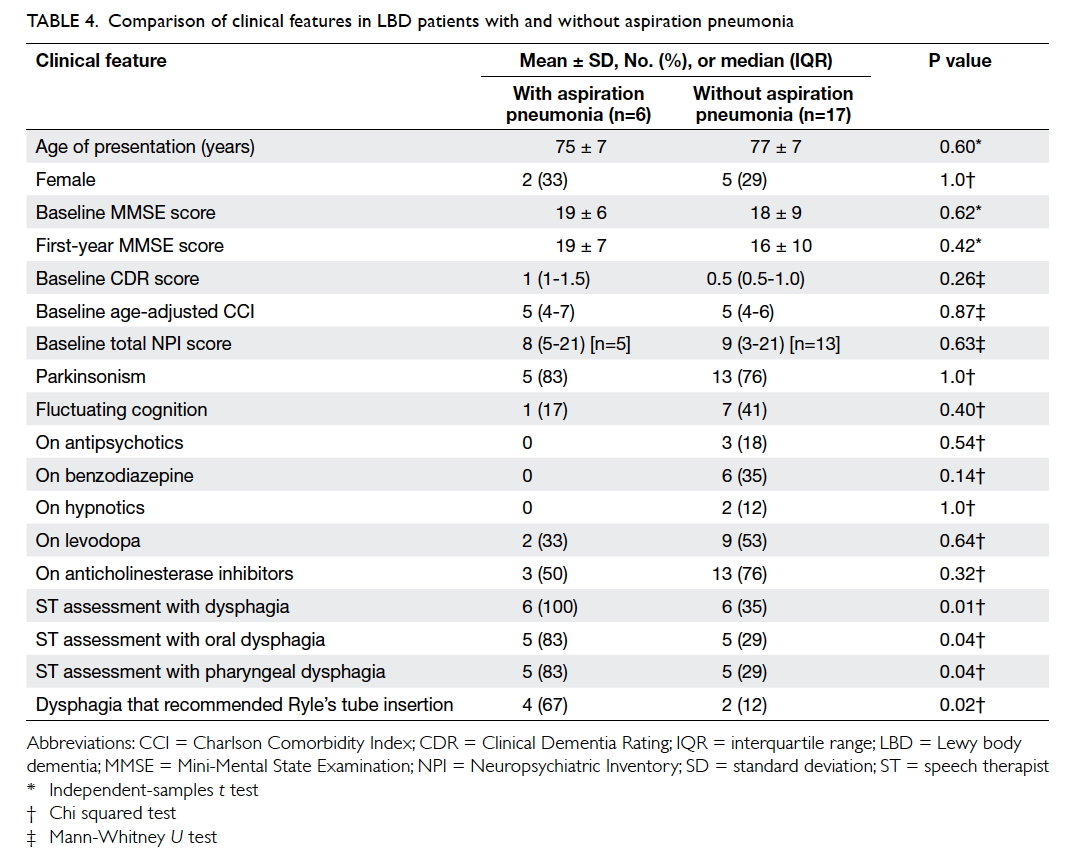
Dysphagia and aspiration pneumonia
Of the patients, six (26%) had a total of 13 episodes
of pneumonia, and 12 (52%) had dysphagia. The
mean time to first episode of aspiration pneumonia
was 866 ± 689 days (median, 634; IQR, 315-1456
days; n=6) and to first diagnosis of dysphagia 951 ± 734 days (median, 862; IQR, 311-1624 days; n=12).
Five patients underwent VFSS (time to VFSS: 831
± 728; median, 723; IQR, 154-1562 days). Three of
four patients with penetration developed aspiration
pneumonia; both patients with aspiration on VFSS
developed aspiration pneumonia. The event rates
were 0.17 and 0.18 per person-years for dysphagia
and aspiration pneumonia, respectively. Patients
with LBD with a history of aspiration pneumonia
compared with those without were more likely to
have been identified by the ST to have
dysphagia (100% vs 35%; P=0.01), oral dysphagia
(83% vs 29%; P=0.04), pharyngeal dysphagia (83% vs
29%; P=0.04), or dysphagia that required Ryle’s tube
insertion (67% vs 12%; P=0.02) [Table 4].
Pressure sores
Six (26%) patients developed pressure sores with four
over the sacrum and two over the heel (two in stage
1, two in stage 3, and two in stage 4). The mean time
to development of pressure sore was 978 ± 599 days
(median, 994; IQR, 379-1528 days). The event rate
was 0.08 per person-years. A comparison between
LBD patients with or without pressure sores did not
identify any significant difference in clinical features
(results not shown).
Mortality
Seven (30%) patients died of various diseases: three
(43%) of pneumonia, two (29%) of unexplained
cardiac arrest (UCA), and one (14%) each of pressure
sore sepsis and choking. The mean time to death was
894 ± 617 days (median, 798; IQR, 312-1597 days).
The event rate was 0.10 per person-years. Deceased
patients with LBD, compared with alive patients,
scored a higher presenting CDR (median [IQR]: 1
[1-2] vs 0.5 [0.5-1.0]; P=0.01), lower mean baseline
Barthel index (13 ± 7 vs 18 ± 4; P=0.04), and were
more likely to have been prescribed levodopa (86%
vs 31%; P=0.03).
Alzheimer’s disease pattern of functional imaging
Of the patients, 19 underwent 18FDG-PET and
four underwent perfusion SPECT imaging.
Twelve patients (9 DLB and 3 PDD) had
an AD pattern of functional imaging: all 12 had
bilateral temporoparietal lobe hypometabolism/hypoperfusion; three patients concomitantly had
hypoperfusion/hypometabolism over the posterior
cingulate gyrus and one patient had additional
hypometabolism over the precuneus. Patients with
LBD with an AD pattern of functional imaging
compared with those without were younger at
presentation (73 ± 6 vs 80 ± 6 years; P=0.02) and
had a lower MMSE score at 1 year (15 ± 8 vs 22 ± 6;
P=0.05). There was no difference in the presentation
of visual hallucination between the two groups of
patients (Table 5).
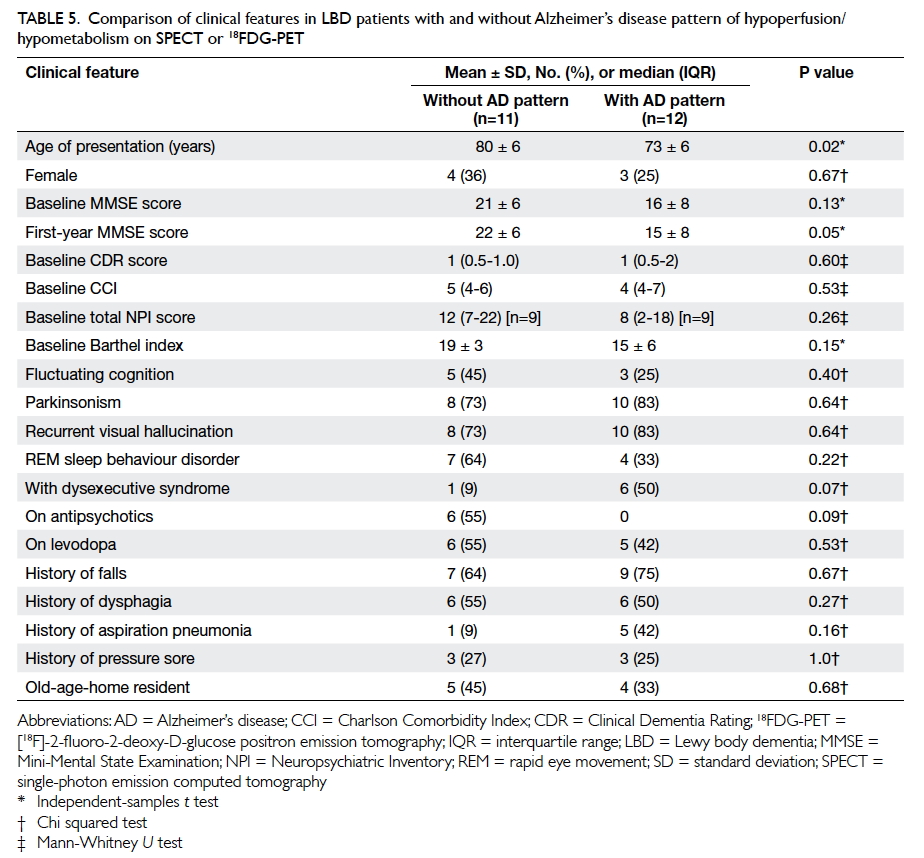
Table 5. Comparison of clinical features in LBD patients with and without Alzheimer’s disease pattern of hypoperfusion/hypometabolism on SPECT or 18FDG-PET
Discussion
An accurate diagnosis of LBD is important. It allows
prescription of acetylcholinesterase inhibitors or
avoidance of antipsychotics in view of the risk of
neuroleptic sensitivity. An overall accuracy of clinical
diagnosis of 52% in our study is similar to findings
in western studies, eg 62% by a neurologist.20 Our
results reveal that no single diagnostic criteria or
test is infallible and the diagnosis needs follow-up
review and support from functional imaging when
appropriate. In our series, 38% of DLB patients
were initially misdiagnosed as AD. This is thought
to have been related to the presence of amnesia in
all patients at initial presentation: only 69% of DLB
patients presented with parkinsonism. There was no
difference in the clinical features of DLB and PDD
patients. Braak et al21 have proposed a pathological
staging of Parkinson’s disease with Lewy body
pathology starting in the dorsal IX/X motor nucleus
or adjoining intermediate reticular zone, and
spreading rostrally in the brainstem then to the
limbic system and subsequently to the neocortex
with the underlying mechanism being the spread
of α-synuclein from cell to cell. This might explain
why DLB and PDD can progress and later overlap
clinically.
Compared with the previous literature review
of Chinese LBD case reports (1980-2012) by Han et al4 including 31 DLB and four PDD patients with a younger mean age of onset (67 ± 10 years), more
patients in our series presented with cognitive
decline (100% vs 60%), parkinsonism (78% vs 9%),
visual hallucinations (74% vs 9%), and rapid eye movement
sleep behaviour disorder (48% vs 11%).
These differences may be related to the heightened
awareness of the core features of LBD among
Chinese doctors in recent years or because patients
in our series presented at a more advanced stage of
dementia (Han et al4 did not state the severity of
dementia). Nonetheless, both case series reported
similar rates of postural hypotension (9% vs 3%) and
BPSD (83% vs 86%).4 The rate of postural hypotension
in both case series is much lower than that reported
in other literature on DLB patients, ie 50%.22 The
lower rates of postural hypotension may be related to
under-recognition. Similar to our findings, in a study
of 22 Chinese DLB patients (mean age, 74 ± 8 years;
mean MMSE score, 16 ± 7; mean NPI score, 24 ± 16),
three most commonly observed BPSDs were visual
hallucinations (86%), delusions (64%), and anxiety
(59%); total NPI score was an independent predictor
of caregiver burden (odds ratio=1.537; P=0.048).22
Clinicians should pay particular attention to BPSD,
particularly visual hallucinations and anxiety
symptoms, when managing Chinese LBD patients.
In addition, falls, dysphagia, and pressure
sores can contribute to carer stress but they were
not included in previous studies of LBD patients.23 24 Nearly 70% of our LBD patients experienced falls.
This rate is greater than the previously reported
rates of 11% to 44%.25 26 Although visuospatial impairment, cognitive fluctuation, parkinsonism,
or visual hallucinations were proposed as
possible mechanisms that contributed to falls, only
parkinsonism was identified in one study of 51 AD
and 27 DLB patients as an independent risk factor.25 26 Because of the limited sample size, no significant risk
factors could be identified in our series. With regard
to the finding of limited improvement in mobility
after GDH training, it is likely that many factors affect
the mobility of LBD patients. These factors include
dementia, postural hypotension, and poor balance
from disease. Clinicians should alert carers of the risk of falls and offer advice about general measures for
falls prevention, including addressing environmental
risk factors and use of safety alarms. Compared with
Londos et al’s finding17 that 29% of 82 LBD patients
(median age, 77 years; median MMSE score, 20) had
dysphagia on VFSS, we reported a higher
rate of 52%. When identified by STs,
LBD patients and their carers should be given
advice about diet modification (eg use of thickeners)
and postural changes (eg chin tuck). In addition,
clinicians should titrate the levodopa dose as far as
possible.27
Given that LBD is an irreversible
neurodegenerative disease, advance care planning
(ACP) forms a major part of care.28 Our data for
dysphagia, aspiration pneumonia, pressure sores,
and mortality can offer useful information during
ACP for Chinese LBD patients. Since those patients
who have died had a higher presenting CDR score,
lower Barthel Index, and greater usage of levodopa
(which probably reflects more severe parkinsonism),
ACP should be initiated earlier in LBD patients
with these features. It has been reported that LBD
patients can have UCA and in our series two (28.6%)
patients died of UCA. Unexplained cardiac arrest is
proposed to be related to pathological involvement
of the intermediolateral columns of the spinal
cord, autonomic ganglia, and sympathetic neurons,
affecting either respiration or heart rhythm.29 Such
risk of UCA should be explained during ACP.
Presence of hypometabolism/hypoperfusion over
the temporoparietal lobes/precuneus/posterior
cingulate gyrus was used as surrogate markers of
concomitant AD pathologies in our LBD patients. As
far as we are aware, this is the first study to show that
concomitant AD pathologies among Chinese LBD
patients can result in an early age of presentation
or diagnosis and lower MMSE score at 1 year. Our
findings provide further evidence of the synergistic
contribution of AD pathologies to LBD dementia.6
This study has several limitations. It was a
single-centre retrospective case series, therefore, we
considered clinical features only as present or absent
when clearly stated as such. This might have affected
our results. Pathological diagnosis was not obtained
including pathological proof of concomitant AD
pathologies. Since all subjects were recruited from
the memory clinic, LBD patients who present to a
psychiatric clinic may be different, eg with more
BPSD or visual hallucinations. The severity of
parkinsonism was not graded so the influence of
parkinsonism on long-term outcomes such as falls
or aspiration pneumonia was not fully analysed.
Although our case series comprised the largest
number of Chinese patients with LBD supported by
functional imaging, the number remained limited.
Our findings should be confirmed by a larger study
with Pittsburgh compound B imaging to delineate
the concomitant presence of amyloid plaques.
Conclusions
Falls, dysphagia, aspiration pneumonia, and pressure
sores were common among LBD patients. Lewy body dementia
patients with an AD pattern of neuroimaging had
an earlier age of diagnosis or presentation and lower
1-year MMSE scores. Such information is useful in
the formulation of a management plan for Chinese
LBD patients.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body
dementias. Lancet 2015;386:1683-97. Crossref
2. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and
management of dementia with Lewy bodies: third report
of the DLB consortium. Neurology 2005;65:1863-72. Crossref
3. Shea YF, Ha J, Lee SC, Chu LW. Impact of 18FDG PET
and 11C-PIB PET brain imaging on the diagnosis of
Alzheimer’s disease and other dementias in a regional
memory clinic in Hong Kong. Hong Kong Med J
2016;22:327-33.
4. Han D, Wang Q, Gao Z, Chen T, Wang Z. Clinical features
of dementia with lewy bodies in 35 Chinese patients.
Transl Neurodegener 2014;3:1. Crossref
5. Valkanova V, Ebmeier KP. Neuroimaging in dementia.
Maturitas 2014;79:202-8. Crossref
6. Gomperts SN. Imaging the role of amyloid in PD dementia
and dementia with Lewy bodies. Curr Neurol Neurosci
Rep 2014;14:472. Crossref
7. Chiba Y, Fujishiro H, Ota K, et al. Clinical profiles of
dementia with Lewy bodies with and without Alzheimer’s disease-like hypometabolism. Int J Geriatr Psychiatry
2015;30:316-23. Crossref
8. Killen A, Flynn D, De Brún A, et al. Support and information
needs following a diagnosis of dementia with Lewy bodies.
Int Psychogeriatr 2016;28:495-501. Crossref
9. Chiu FK, Lee HC, Chung WS, Kwong PK. Reliability and
validity of the Cantonese version of Mini-Mental State
Examination: a preliminary study. J Hong Kong Coll
Psychiatr 1994;4(2 Suppl):S25-8.
10. Morris JC. The Clinical Dementia Rating (CDR): current
version and scoring rules. Neurology 1993;43:2412-4. Crossref
11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A
new method of classifying prognostic comorbidity in
longitudinal studies: development and validation. J Chronic
Dis 1987;40:373-83. Crossref
12. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S,
Carusi DA, Gornbein J. The Neuropsychiatric Inventory:
comprehensive assessment of psychopathology in
dementia. Neurology 1994;44:2308-14. Crossref
13. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL
Index: a reliability study. Int Disabil Stud 1988;10:61-3. Crossref
14. McKhann GM, Knopman DS, Chertkow H, et al. The
diagnosis of dementia due to Alzheimer’s disease:
recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic
guidelines for Alzheimer’s disease. Alzheimers Dement
2011;7:263-9. Crossref
15. Smith R. Validation and reliability of the Elderly Mobility
Scale. Physiotherapy 1994;80:744-7. Crossref
16. Berg KO, Wood-Dauphinee SL, Williams JI, Maki
B. Measuring balance in the elderly: validation of an
instrument. Can J Public Health 1992;83 Suppl 2:S7-11.
17. Londos E, Hanxsson O, Alm Hirsch I, Janneskog A, Bülow
M, Palmqvist S. Dysphagia in Lewy body dementia—a
clinical observational study of swallowing function by
videofluoroscopic examination. BMC Neurol 2013;13:140. Crossref
18. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood
JL. A penetration-aspiration scale. Dysphagia 1996;11:93-8. Crossref
19. National Pressure Ulcer Advisory Panel. NPUAP pressure
injury stages. Available from: http://www.npuap.org/resources/educational-and-clinical-resources/npuap-pressure-injury-stages/. Accessed 7 May 2016.
20. Galvin JE. Improving the clinical detection of Lewy body
dementia with the Lewy body composite risk score.
Alzheimers Dement (Amst) 2015;1:316-24. Crossref
21. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur
EN, Braak E. Staging of brain pathology related to sporadic
Parkinson’s disease. Neurobiol Aging 2003;24:197-211. Crossref
22. Takemoto M, Sato K, Hatanaka N, et al. Different clinical
and neuroimaging characteristics in early stage Parkinson’s disease with dementia and dementia with Lewy bodies. J
Alzheimers Dis 2016;52:205-11. Crossref
23. Liu S, Jin Y, Shi Z, et al. The effects of behavioral
and psychological symptoms on caregiver burden in
frontotemporal dementia, Lewy body dementia, and
Alzheimer’s disease: clinical experience in China. Aging
Ment Health 2016 Feb 16:1-7. Epub ahead of print. Crossref
24. Leggett AN, Zarit S, Taylor A, Galvin JE. Stress and burden
among caregivers of patients with Lewy body dementia.
Gerontologist 2011;51:76-85. Crossref
25. Imamura T, Hirono N, Hashimoto M, et al. Fall-related
injuries in dementia with Lewy bodies (DLB) and
Alzheimer’s disease. Eur J Neurol 2000;7:77-9. Crossref
26. Kudo Y, Imamura T, Sato A, Endo N. Risk factors for falls in
community-dwelling patients with Alzheimer’s disease and
dementia with Lewy bodies: walking with visuocognitive
impairment may cause a fall. Dement Geriatr Cogn Disord
2009;27:139-46. Crossref
27. Alagiakrishnan K, Bhanji RA, Kurian M. Evaluation and
management of oropharyngeal dysphagia in different types
of dementia: a systematic review. Arch Gerontol Geriatr
2013;56:1-9. Crossref
28. Jethwa KD, Onalaja O. Advance care planning and
palliative medicine in advanced dementia: a literature
review. BJPsych Bull 2015;39:74-8. Crossref
29. Molenaar JP, Wilbers J, Aerts MB, et al. Sudden death: an
uncommon occurrence in dementia with Lewy bodies. J
Parkinsons Dis 2016;6:53-5. Crossref