Hong Kong Med J 2017 Apr;23(2):158–67 | Epub 17 Mar 2017
DOI: 10.12809/hkmj165014
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Improving medication safety and diabetes management in Hong Kong: a multidisciplinary
approach
Agnes YS Chung, BPharm1;
Shweta Anand, BDS1;
Ian CK Wong, PhD2;
Kathryn CB Tan, MD, MBBCH3;
Christine FF Wong, PharmD4;
William CM Chui, MSc4;
Esther W Chan, PhD1
1 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong
2 Research Department of Practice and Policy, School of Pharmacy,
University College London, United Kingdom
3 Department of Medicine, Queen Mary Hospital, Pokfulam, Hong Kong
4 Department of Pharmacy, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr Esther W Chan (ewchan@hku.hk)
Abstract
Introduction: Patients with diabetes often require
complex medication regimens. The positive impact
of pharmacists on improving diabetes management
or its co-morbidities has been recognised worldwide.
This study aimed to characterise drug-related
problems among diabetic patients in Hong Kong
and their clinical significance, and to explore the
role of pharmacists in the multidisciplinary diabetes
management team by evaluating the outcome of
their clinical interventions.
Methods: An observational study was conducted at
the Diabetes Clinic of a public hospital in Hong Kong
from October 2012 to March 2014. Following weekly
screening, and prior to the doctor’s consultation,
selected high-risk patients were interviewed by
a pharmacist for medication reconciliation and
review. Drug-related problems were identified and
documented by the pharmacist who presented
clinical recommendations to doctors to optimise
a patient’s drug regimen and resolve or prevent
potential drug-related problems.
Results: A total of 522 patients were analysed and
417 drug-related problems were identified. The
incidence of patients with drug-related problems
was 62.8% with a mean of 0.9 (standard deviation,
0.6) drug-related problems per patient. The most
common categories of drug-related problems were
associated with dosing (43.9%), drug choice (17.3%),
and non-allergic adverse reactions (15.6%). Drugs
most frequently involved targeted the endocrine
or cardiovascular system. The majority (71.9%) of
drug-related problems were of moderate clinical
significance and 28.1% were considered minor
problems. Drug-related problems were totally solved
(50.1%) and partially solved (11.0%) by doctors’
acceptance of pharmacist recommendations, or
received acknowledgement from doctors (5.5%).
Conclusions: Pharmacists, in collaboration with
the multidisciplinary team, demonstrated a positive
impact by identifying, resolving, and preventing
drug-related problems in patients with diabetes.
Further plans for sustaining pharmacy service in
the Diabetes Clinic would enable further studies
to explore the long-term impact of pharmacists in
improving patients’ clinical outcomes in diabetes
management.
New knowledge added by this study
- Pharmacists make an important contribution to the identification, resolution, and prevention of drug-related problems by medication reconciliation and review. Most problems were related to dosing with moderate clinical significance according to Dean and Barber’s validated scale for scoring medication errors. Over half of the clinical interventions initiated by pharmacists were accepted or acknowledged by doctors to improve medication management.
- Collaboration between pharmacists and other health care professionals is valuable for the improvement of medication safety in the management of diabetes.
Introduction
Diabetes mellitus is a chronic disease that is
prevalent worldwide.1 Patients with diabetes often
require complex medication regimens and are likely
to develop multiple irreversible complications that
significantly worsen their quality of life.2 Effective
diabetes management requires collaboration among
health care professionals in a multidisciplinary
diabetes management team (DMT). Pharmacists, as
a part of the DMT, are well positioned to optimise
pharmacological treatment, educate patients about
diabetes management, and promote medication
compliance.3
The major role of a pharmacist in a DMT is
to conduct medication reconciliation (MR) and
medication review—MR is the process of comparing
a patient’s prescriptions with all their usual
medications and identifying the most complete and
updated medication history4; whereas medication
review aims to review a patient’s medical and drug
history, assess their current prescriptions, and
ascertain their drug knowledge and compliance.5
This enables pharmacists to identify drug-related
problems (DRPs) that can actually or potentially
interfere with optimum health outcomes in specific
patients.6 7 Polypharmacy (concurrent use of
multiple medications) is commonly seen in people
with chronic diseases which could lead to potential
DRPs.8 9 These DRPs might be overlooked by
prescribers and interfere with diabetes management.
In several overseas studies, pharmacists have
implemented timely interventions to resolve or
prevent DRPs by offering recommendations to
prescribers, with an acceptance rate of over 60%.10 11 12 13
The positive impact of pharmacists in improving
diabetes management or its co-morbidities has
also been recognised by interventional and controlled
observational studies worldwide.14 Greater overall
improvement in glycosylated haemoglobin, fasting
plasma glucose, blood pressure, cholesterol
levels, renal outcomes, and medication adherence
has been demonstrated in patients who received
pharmacist-led diabetes services compared with the
standard care.12 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Few studies, however, have been
conducted in Hong Kong.17 29 In view of inadequate available data and potential for expansion of local
pharmacy services, more studies are required to
investigate the future development of a sustainable
diabetes service provided by pharmacists.
This study aimed to characterise DRPs
among Chinese diabetic out-patients, and to
define the clinical significance and outcome of
pharmacist interventions; thereby highlighting
their contribution to the detection, resolution, and
prevention of DRPs to improve medication safety
and diabetes management.
Methods
Study design and setting
An observational study was conducted weekly in
the Diabetes Clinic at Queen Mary Hospital (QMH)
from October 2012 to March 2014. The study
protocol was approved by the Institutional Review
Board of the University of Hong Kong (HKU)/Hospital Authority (HA) Hong Kong West Cluster.
Informed consent was not required for the study.
Inclusion and exclusion criteria
Patients were included if they were at ‘high risk’ due
to their multiple disease state and complex drug
regimen and if they fulfilled the following criteria:
- Aged ≥65 years (elderly patients are considered having high risk for DRPs since they usually take more drugs than younger patients)
- Taking five or more medications including all routes of administration, or over-the-counter medications (regular or as needed)
- Taking medications that have a low therapeutic index or require monitoring
- Attending multiple specialist clinics
Nursing home residents were excluded due
to their relatively low risk for non-compliance,
compared with community-dwelling elderly patients.
Procedure and materials
The day before the scheduled weekly clinic
consultation, two researchers screened the medical
history, previous consultation notes, current
medications, and latest laboratory results of Chinese
elderly patients with diabetes to select high-risk
patients. Selected patient records were printed and
prepared for quick reference during the medication
interview. To facilitate data collection, a memo was
attached to the patient’s records to indicate patient
selection.
Two pharmacists from QMH and one from the
HKU attended the clinic on alternate Wednesdays to
compile a thorough medication history from selected
patients and conduct an independent medication
review prior to the medical consultation. During the
review, pharmacists also recorded medications not
shown in the Clinical Management System (CMS),
such as drugs prescribed by general practitioners
(GPs), over-the-counter products, vitamins, and
herbal supplements.
A MR form (Appendix 1) was then completed
by pharmacists, documenting the identified DRPs
and formulating an intervention proposal. The MR
forms were collected following medical consultation,
either on the same day or within the next few days.
Pharmacist intervention
For the selected high-risk patients, pharmacists
reviewed the patient’s drug regimen and made
recommendations to doctors for adjustment,
provided doctors with an updated drug list after MR,
suggested a need to further investigate a patient’s
condition, provided drug education to patients
and caregivers, reinforced the importance of drug
compliance to patients, and suggested lifestyle
modifications such as dietary control.
Drug-related problems were identified
from the completed MR forms, and pharmacist
recommendations were collected for analysis. The
CMS was checked for outcome of intervention.
Data collection
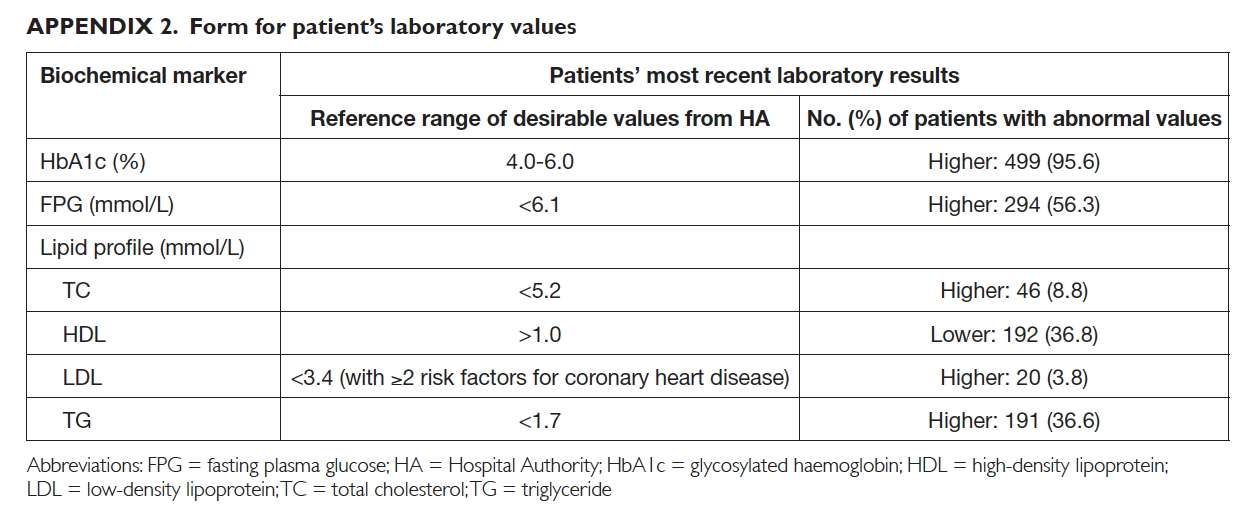
Demographic data—for example, age, gender,
drug allergy status, number of regular medications
obtained from the HA clinics, and the most
current laboratory results, including glycosylated
haemoglobin, fasting plasma glucose, and lipids
(Appendix 2)—were retrieved from the CMS.
Additional information in terms of medication,
drug storage methods, smoking status, drinking
habits, vaccination record, and latest readings from
self-monitoring of blood glucose (SMBG) was also
collected.
Data analysis
Demographic data were tabulated as frequency and
percentage using Microsoft Excel 2010. Primary
outcomes included the frequency and categories of
DRPs, drug classes involved, clinical significance of
DRPs, and outcome of pharmacist interventions.
The incidence of DRPs was also calculated as the
percentage of patients with at least one DRP.
Definition and classification of drug-related problems
Using the Pharmaceutical Care Network Europe
(PCNE) classification system for DRPs V5.01, DRPs
were categorised as ‘adverse reactions’, ‘drug choice
problem’, ‘dosing problem’, ‘drug use problem’,
‘interactions’, or ‘others’.7 This is an established system that has been revised several times with tested
validity and reproducibility11 31 and has been used in many studies.9 32 33 When a single drug was associated
with more than one possible DRP category, the one
that best described the clinical scenario was chosen.
Drugs involved in DRPs were categorised according
to their British National Formulary classification.34
The clinical significance of DRPs was assessed
to determine their actual or potential consequence
for patient health outcomes. Using a validated
scale,35 four independent reviewers (two pharmacists
and two doctors) scored the severity of each DRP
from 0 (without potential effects on the patient)
to 10 (lead to a fatal event). A mean score of <3
indicated a minor problem (very unlikely to cause
adverse effects), 3 to 7 indicated a moderate problem
(likely to cause some adverse effects or interfere
with therapeutic goals), and >7 indicated a
severe DRP that could likely cause death or lasting
impairment.
To evaluate prescribers’ acceptance level,
the outcome of pharmacist interventions was
categorised as ‘not known’, ‘solved’, ‘partially solved’,
or ‘not solved’ according to PCNE classification
V5.01.7
Results
Patient demographics and characteristics
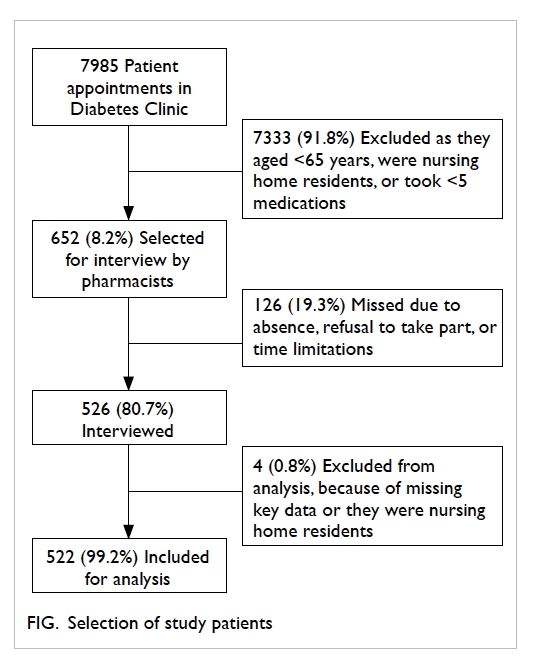
During the study period, a total of 652 patients
were included based on the selection criteria; 526
(80.7%) were interviewed, of whom 522 (99.2%) were
analysed (Fig).
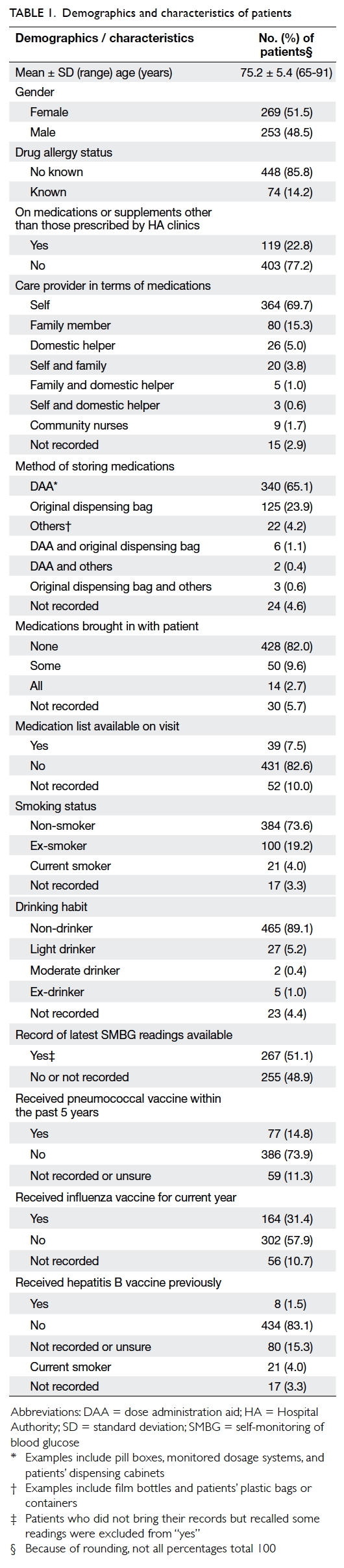
The mean (± standard deviation) age of the 522
patients was 75.2 ± 5.4 years (range, 65-91 years).
The number of prescribed regular HA medications
ranged from 5 to 17 with a mean of 9 ± 2. The
demographics and characteristics of patients are
shown in Table 1.
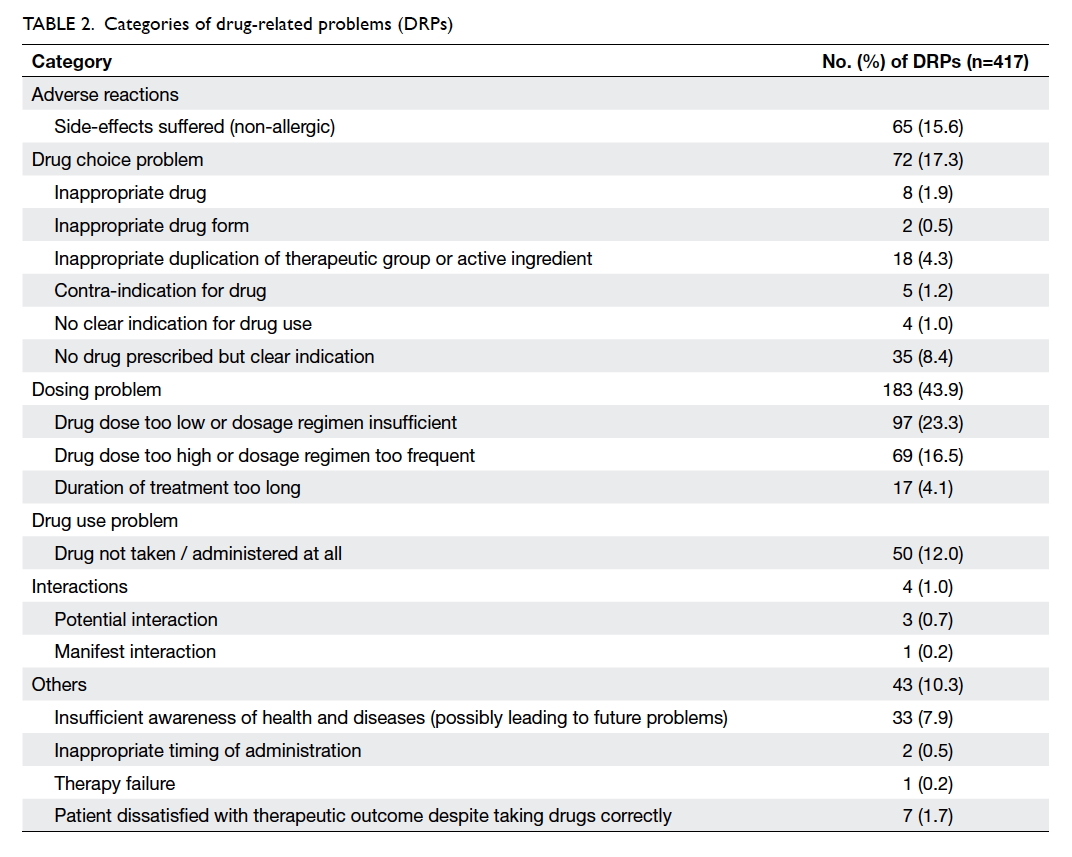
Categories of drug-related problems
A total of 417 DRPs were identified. Among the 522
patients analysed, 328 (62.8%) had at least one DRP
and the mean number of DRPs per patient was 0.9
± 0.6. The most prevalent DRP category was related
to dosing (n=183, 43.9%), followed by drug choice
(n=72, 17.3%) and non-allergic adverse reaction
(n=65, 15.6%). The subcategories of each of them
are listed in Table 2.
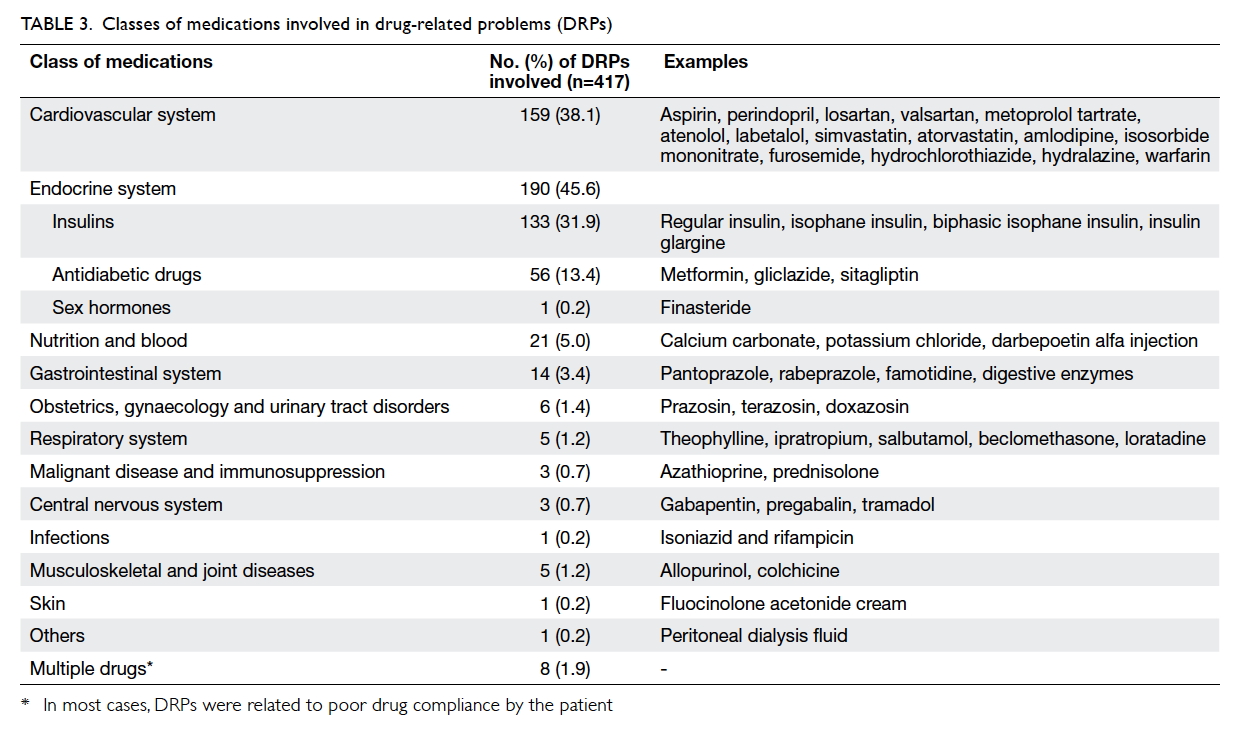
Classes of medications involved in drug-related problems
The most common classes of medication involved
were those targeting the endocrine system with 190
(45.6%) DRPs, followed by cardiovascular system
with 159 (38.1%) DRPs (Table 3).
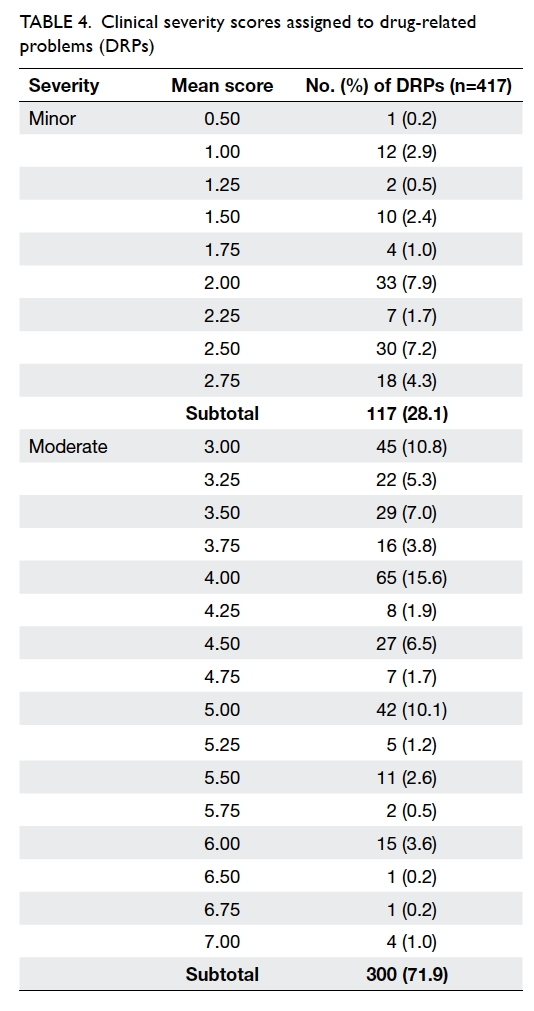
Clinical significance of drug-related problems
The mean clinical severity scores assigned to DRPs
ranged from 0.50 to 7.00. The majority of DRPs
(n=300, 71.9%) were classified as moderate with the
remainder (n=117, 28.1%) considered minor. No
clinically severe DRP was identified (Table 4).
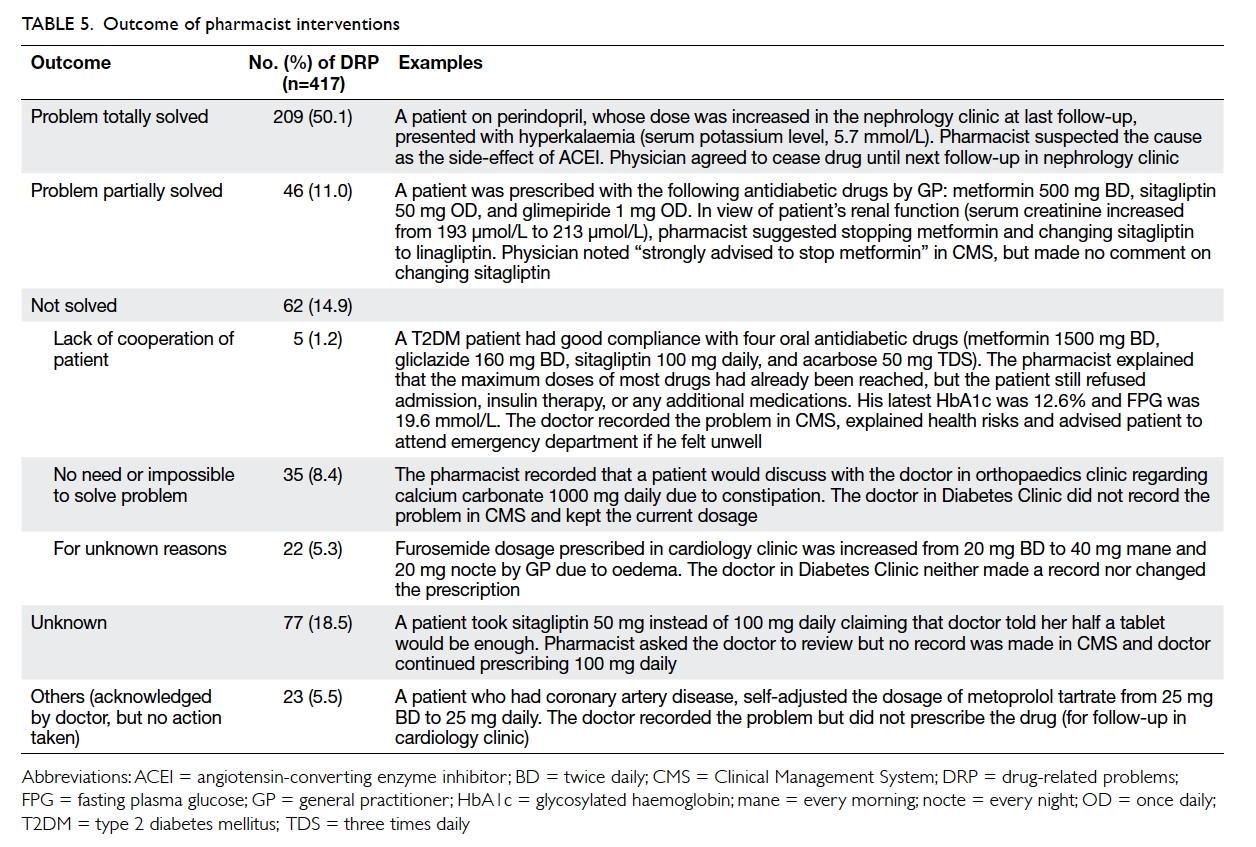
Outcome of pharmacist interventions
As Table 5 shows, modifying drug regimens or
reinforcing compliance by doctors or referral to
pharmacists solved 209 (50.1%) DRPs. On the other
hand, 46 (11.0%) DRPs were partially resolved
by doctors adjusting prescriptions, although not
according to pharmacist recommendations; 62
(14.9%) DRPs were not resolved due to patient
reluctance to change prescriptions, resolution
considered unnecessary, or for unknown reasons;
23 (5.5%) DRPs had an unknown outcome because
these were non-compliance issues not acknowledged
by doctors.
Discussion
The incidence of patients with DRPs (62.8%) and the
mean number of DRPs per patient analysed (0.9) in
this study were comparable to a Norwegian study
(59.2% and 1.2, respectively)10 but considerably
lower than those identified in four overseas studies
(incidence of 80.7%-90.5%, and mean number of
DRPs per patient between 1.9 ± 1.2 and 4.6 ± 1.7).9 11 12 36
Such discrepancies might be attributed to variations
in patient selection criteria, data collection methods,
pharmacists’ clinical experience, as well as study
duration and setting.9 36 37
The majority of DRPs were dosing problems
(43.9%), with “drug dose too low or dosage regimen
insufficient” as the largest subcategory. In contrast
to the lower percentage (5.9%-21.6%) in five overseas
studies,9 10 11 12 36 our high prevalence of dosing problems was in line with a local study of medication incidents
among hospital in-patients,38 mostly arising from
self-adjustment of dosage or frequency, confusion
about previous dose changes and dosage modification
by GPs or doctors overseas. These highlight the
pivotal role of local pharmacists in conducting MR,
reviewing drug dosages to ensure safety and efficacy,
monitoring patients’ metabolic control regularly as
well as reminding patients and/or their caregivers to
maintain an updated medication list and follow the
latest drug label instructions.
Drug choice problem was the second most
common DRP; 17.3% of DRPs related to this category,
which is comparable to the findings of two overseas
studies (9.1%, 23%)9 36 but deviating from others (31.8%-30.2%).10 11 The most common subcategory was “no drug prescribed but clear indication”, such
as the omission of angiotensin-converting enzyme
inhibitor/angiotensin-receptor blocker (ACEI/ARB) in patients with microalbuminuria or patient’s
reluctance to use insulin. Hence, pharmacists have
a role in advising doctors to adhere to the latest
treatment guidelines and educate patients about the
treatment benefits of each drug class.39 Other causes
of problems surrounding drug choice included drug
duplication and changes to drug choices by GPs to
prevent side-effects. This suggests that some DRPs
might have arisen due to the lack of a common
platform between the public and private health care
sector for sharing patient information. Pharmacists
can make a valuable contribution by establishing
a patient’s drug history by MR and by liaison with
different health care sectors.
Adverse reactions were the third most
common DRP (15.6%). The major types of “side-effects suffered (non-allergic)” were insulin-induced
hypoglycaemia, gastrointestinal disturbances, and
dizziness caused by antidiabetic drugs, for which
pharmacists recommended changes in drug choice
or dosage. Adverse reactions could lead to other
DRP categories,7 such as drug choice and drug use
problems. This reflects the pharmacist’s pivotal role
in reviewing prescribed doses, suggesting dosage
adjustments to doctors, monitoring adverse effects,
and providing information about prevention of
side-effects (such as performing SMBG regularly to
prevent hypoglycaemia).39
Drug use issues were the fourth most common
category with comparable prevalence (12.0%) with a
Malaysian study9 although this ranges widely among
other studies (3.8%-54.2%).10 11 36 Reasons for the
subcategory of “drug not taken/administered at all”
included inability to purchase a self-financed item
due to cost, ignorance of the indications, concern
about side-effects, and confusion about previous
regimen changes.40 In our study, pharmacists
mainly intervened by direct patient counselling,
recommending reinforcement of patient compliance
to doctors or suggesting changes to drug regimens.
Pharmacists could also work closely with other
DMT members to educate patients about their
disease and the most updated regimen, address
drug cost concerns or side-effects, and encourage
patients to update their medication list and use dose
administration aids such as pill boxes.41
The low prevalence of drug interactions
(1.0%) was similar to that (0.6%) in a Danish study,36
although much higher percentages were found
in three other studies (8.0%-16.3%),9 10 11 possibly
ascribed to differences in prescribing practice,
references used to define drug interactions,9 and
also because CMS could already detect a range
of clinically significant interactions when doctors
issued prescriptions. Nonetheless system checking
and prompts cannot replace clinical judgement or
recommendations of alternative regimens. Other
categories of DRPs included “insufficient awareness
of health and diseases” (such as poor dietary control)
and “inappropriate timing of administration”, but
this category could also encompass therapy failure
and inappropriate lifestyle choices, resulting in
greater variation of prevalence from overseas studies
(6.8%-46.6%).9 10 11 36 Pharmacists are ideally positioned
to advise patients about the importance of diet,
smoking cessation, regular exercise, and SMBG.22
The drug classes most implicated in DRPs
were for the endocrine system (45.6%) followed by
cardiovascular system (38.1%). These findings were
not surprising as insulins, oral antidiabetic drugs,
antihypertensive, antihyperlipidaemic, antiplatelet
agents, and ACEI/ARB are most commonly
prescribed to manage diabetes, its co-morbidities
and complications.11 39
The majority of DRPs were classified as
moderate. Among similar overseas studies, only
one analysed the clinical significance of DRPs, in
which 87% had high or medium clinical/practical
relevance.10 These findings could not be readily
compared with the present study because of
different assessment scales, potential variations
in reviewers’ clinical experience,35 and unknown
relative proportions of cases with medium and high
relevance.
Over half of the DRPs were totally solved as
doctors implemented pharmacist recommendations.
The acceptance rate was somewhat similar to that
observed in two overseas studies (60.2%-62.7%).12 13 The physicians acknowledged the provision of
service by pharmacists and were more aware of the
written recommendations provided by pharmacists.
In particular, the value of verbal communication
between different health care professionals in
resolving or preventing DRPs has been recognised
in earlier studies,10 42 43 44 45 suggesting potential improvement in the acceptance rate if pharmacists
had more time to hand over DRPs by speaking with
doctors.
The outcome of pharmacist interventions could
also be influenced by doctors’ clinical experience and
familiarity with the new service. Doctors’ acceptance
level could have been underestimated since some
of them might have neglected or missed written
information from pharmacists. This highlights the
importance of promoting the role of pharmacists to
doctors and keeping all participating doctors well-informed.
Difficulties and limitations
This pilot study allowed for an opportunity to assess
the proportion of patients who might be seen by
clinical pharmacists in a busy specialist out-patient
clinic at a teaching hospital. Approximately 10% of
patients were chosen each week and not all eligible
patients could be selected owing to time restrictions.
The number of patients interviewed was further
limited due to time constraints, patient absence or
refusal. Local figures from the QMH Diabetes Clinic
indicate that approximately 7% to 8% of all patients
who attend the clinic are deemed ‘high risk’, based
on ongoing work and prioritisation of those taking
five or more regular medications. Limited work
space was another consideration. A designated area
is required to conduct patient interviews. Further
arrangements could be made with the medical and
nursing staff in the Diabetes Clinic to access better
space.
This study only described the current situation
of DRPs. It did not assess the implementation of
interventions and their impact on patient health
outcome. As the majority of patients did not bring
their drugs to the clinic and had no medication
list available, the MR process was not always
comprehensive or effective. Only a minority of
patients could name their regular drugs. The
majority relied on pharmacist investigation and
prompts about the colour, shape, package, or
indication of each drug. Due to the potential
for misinterpretation, DRP prevalence may be
underestimated. One possible solution might be
to show patients samples of commonly prescribed
medications. Alternatively, selected patients could
be telephoned in advance and asked to bring along
their medications, although this measure may not be
sustainable. A multifaceted promotional campaign
could be introduced to encourage patients to bring
their regular medications to clinic. This has been
shown to be effective in an emergency setting.46
Although completed MR forms were
presented to doctors after the interviews, some
written information might have been missed with
a consequent lack of response to certain DRPs.
Pharmacists should ideally have informed doctors
about every DRP in person, but this was not always
possible due to time constraints and the great
volume of patients. In the long run, pharmacists
should document DRPs and their recommendations
in the CMS. This would enhance visibility and allow
doctors to input their response electronically and
facilitate organised documentation and easy data
retrieval.
Future directions
Upon completion of this study, pharmacists have
been continuing to provide MR and medication
review services in QMH Diabetes Clinic. They have
also been collecting data about DRPs to plan for a
sustainable service. Following a longer study period,
patient and staff satisfaction surveys could be
introduced and also control groups added to enable
comparison of the effectiveness of pharmacist
intervention. This would further support the
extension of hours of service and potentially the
setup of similar pharmacy services to other hospitals
and diabetes clinics in Hong Kong.
Conclusions
Approximately two thirds of patients at the Diabetes
Clinic had at least one DRP. The most frequent
categories of DRPs were related to dosing, drug
choice, and non-allergic adverse reaction. Drugs
targeting the endocrine and cardiovascular systems
were most commonly involved. The majority of DRPs
were of moderate clinical significance. Pharmacist
interventions for over half the DRPs were accepted
or acknowledged by prescribers. Through effective
communication and collaboration within the
multidisciplinary health care team, pharmacists
had a positive impact on identifying, resolving,
and preventing DRPs. Future plans to sustain the
diabetes service will enable more local research to
enhance medication safety and optimise patients’
medication regimens in diabetes management.
Acknowledgements
We would like to acknowledge Ms Cyan Chan for her
assistance in patient screening and data collection,
and pharmacists Ms Phoebe Chan (HKU); Ms Amy
Chan, Ms Dominique Yeung, Ms Katie Chan, and Mr
Ric Fung (QMH); Prof Karen Lam (QMH); nursing
and medical staff in S6 Diabetes Clinic, QMH for
their advice and contributions to service provision
in the study. We would also like to thank Mr Michael
Ling and Ms Elaine Lo (Kwong Wah Hospital);
Dr Michael Mok (Geelong Hospital, Victoria,
Australia); Dr Vickie Tse (HKU) contributing to the
independent assessment of clinical severity of DRPs;
and Dr Anthony Tam (HKU) and Sharon Law (HKU)
for proofreading the manuscript.
References
1. International Diabetes Federation International diabetes
atlas. 6th ed. Available from: http://www.diabetesatlas.org/resources/previous-editions.html. Accessed Mar 2014.
2. Fowler MJ. Microvascular and macrovascular
complications of diabetes. Clin Diabetes 2008;26:77-82. Crossref
3. Tapp H, Phillips SE, Waxman D, Alexander M, Brown R,
Hall M. Multidisciplinary team approach to improved
chronic care management for diabetic patients in an urban
safety net ambulatory care clinic. J Am Board Fam Med
2012;25:245-6. Crossref
4. Hellström LM, Bondesson Å, Höglund P, Eriksson T. Errors
in medication history at hospital admission: prevalence
and predicting factors. BMC Clin Pharmacol 2012;12:9. Crossref
5. Krska J, Cromarty JA, Arris F, et al. Pharmacist-led
medication review in patients over 65: a randomized,
controlled trial in primary care. Age Ageing 2001;30:205-11. Crossref
6. Draft statement on pharmaceutical care. ASHP Council
on Professional affairs. American Society of Hospital
Pharmacists. Am J Hosp Pharm 1993;50:126-8.
7. Pharmaceutical Care Network Europe. The PCNE
Classification V 5.01. 2006. Available from: http://www.pcne.org/upload/files/16_PCNE_classification_V5.01.pdf.
Accessed 22 Oct 2013.
8. Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy
as commonly defined is an indicator of limited value in the
assessment of drug-related problems. Br J Clin Pharmacol
2007;63:187-95. Crossref
9. Zaman Huri H, Fun Wee H. Drug related problems in type
2 diabetes patients with hypertension: a cross-sectional
retrospective study. BMC Endocr Disord 2013;13:2. Crossref
10. Granas AG, Berg C, Hjellvik V, et al. Evaluating
categorisation and clinical relevance of drug-related
problems in medication reviews. Pharm World Sci
2010;32:394-403. Crossref
11. van Roozendaal BW, Krass I. Development of an evidence-based
checklist for the detection of drug related problems
in type 2 diabetes. Pharm World Sci 2009;31:580-95. Crossref
12. Borges AP, Guidoni CM, Ferreira LD, de Freitas O, Pereira
LR. The pharmaceutical care of patients with type 2
diabetes mellitus. Pharm World Sci 2010;32:730-6. Crossref
13. DeName B, Divine H, Nicholas A, Steinke DT, Johnson CL.
Identification of medication-related problems and health
care provider acceptance of pharmacist recommendations
in the DiabetesCARE program. J Am Pharm Assoc
2008;48:731-6. Crossref
14. Kiel PJ, McCord AD. Pharmacist impact on clinical
outcomes in a diabetes disease management program via
collaborative practice. Ann Pharmacother 2005;39:1828-32. Crossref
15. Wubben DP, Vivian EM. Effects of pharmacist outpatient
interventions on adults with diabetes mellitus: a systematic
review. Pharmacotherapy 2008;28:421-36. Crossref
16. Evans CD, Watson E, Eurich DT, et al. Diabetes and
cardiovascular disease interventions by community
pharmacists: a systematic review. Ann Pharmacother
2011;45:615-28. Crossref
17. Chan CW, Siu SC, Wong CK, Lee VW. A pharmacist care
program: positive impact on cardiac risk in patients with
type 2 diabetes. J Cardiovasc Pharmacol Ther 2012;17:57-64. Crossref
18. Pepper MJ, Mallory N, Coker TN, Chaki A, Sando KR.
Pharmacists’ impact on improving outcomes in patients
with type 2 diabetes mellitus. Diabetes Educ 2012;38:409-16. Crossref
19. Jarab AS, Alqudah SG, Mukattash TL, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy
management of patients with type 2 diabetes in an
outpatient diabetes clinic in Jordan. J Manag Care Pharm
2012;18:516-26. Crossref
20. Jacobs M, Sherry PS, Taylor LM, Amato M, Tataronis GR,
Cushing G. Pharmacist Assisted Medication Program
Enhancing the Regulation of Diabetes (PAMPERED) study.
J Am Pharm Assoc 2012;52:613-21. Crossref
21. Ali M, Schifano F, Robinson P, et al. Impact of community
pharmacy diabetes monitoring and education programme
on diabetes management: a randomized controlled study.
Diabet Med 2012;29:e326-33. Crossref
22. Al Mazroui NR, Kamal MM, Ghabash NM, Yacout TA,
Kole PL, McElnay JC. Influence of pharmaceutical care on
health outcomes in patients with type 2 diabetes mellitus.
Br J Clin Pharmacol 2009;67:547-57. Crossref
23. Mehuys E, Van Bortel L, De Bolle L, et al. Effectiveness
of a community pharmacist intervention in diabetes
care: a randomized controlled trial. J Clin Pharm Ther
2011;36:602-13. Crossref
24. Shah M, Norwood CA, Farias S, Ibrahim S, Chong PH,
Fogelfeld L. Diabetes transitional care from inpatient to
outpatient setting: pharmacist discharge counseling. J
Pharm Pract 2013;26:120-4. Crossref
25. Heisler M, Hofer TP, Schmittdiel JA, et al. Improving
blood pressure control through a clinical pharmacist
outreach program in patients with diabetes mellitus
in 2 high-performing health systems: the adherence
and intensification of medications cluster randomized,
controlled pragmatic trial. Circulation 2012;125:2863-72. Crossref
26. Dobesh PP. Managing hypertension in patients with type 2
diabetes mellitus. Am J Health Syst Pharm 2006;63:1140-9. Crossref
27. Planas LG, Crosby KM, Mitchell KD, Farmer KC. Evaluation
of a hypertension medication therapy management
program in patients with diabetes. J Am Pharm Assoc
2009;49:164-70. Crossref
28. Leal S, Soto M. Chronic kidney disease risk reduction in
a Hispanic population through pharmacist-based disease-state
management. Adv Chronic Kidney Dis 2008;15:162-7. Crossref
29. Leung WY, So WY, Tong PC, Chan NN, Chan JC. Effects
of structured care by a pharmacist-diabetes specialist team
in patients with type 2 diabetic nephropathy. Am J Med
2005;118:1414. Crossref
30. American Pharmacists Association. DOTx. MED:
Pharmacist-delivered interventions to improve care for
patients with diabetes. J Am Pharm Assoc 2012;52:25-33. Crossref
31. Björkman IK, Sanner MA, Bernsten CB. Comparing 4
classification systems for drug-related problems: processes
and functions. Res Social Adm Pharm 2008;4:320-31. Crossref
32. Eichenberger PM, Lampert ML, Kahmann IV, van
Mil JW, Hersberger KE. Classification of drug-related
problems with new prescriptions using a modified PCNE
classification system. Pharm World Sci 2010;32:362-72. Crossref
33. Hohmann C, Eickhoff C, Klotz JM, Schulz M, Radziwill
R. Development of a classification system for drug-related
problems in the hospital setting (APS-Doc) and assessment
of the inter-rater reliability. J Clin Pharm Ther 2012;37:276-81. Crossref
34. British Medical Association, Royal Pharmaceutical Society
of Great Britain. British National Formulary 71. London:
British Medical Association, Royal Pharmaceutical Society;
2016.
35. Dean BS, Barber ND. A validated, reliable method of
scoring the severity of medication errors. Am J Health Syst
Pharm 1999;56:57-62.
36. Haugbølle LS, Sørensen EW. Drug-related problems
in patients with angina pectoris, type 2 diabetes and
asthma—interviewing patients at home. Pharm World Sci
2006;28:239-47. Crossref
37. Westerlund T, Almarsdottir AB, Melander A. Factors
influencing the detection rate of drug-related problems in
community pharmacy. Pharm World Sci 1999;21:245-50. Crossref
38. Song L, Chui WC, Lau CP, Cheung BM. A 3-year study of
medication incidents in an acute general hospital. J Clin
Pharm Ther 2008;33:109-14. Crossref
39. American Diabetes Association. Standards of medical care
in diabetes—2013. Diabetes Care 2013;36 Suppl 1:S11-66. Crossref
40. Odegard PS, Gray SL. Barriers to medication adherence
in poorly controlled diabetes mellitus. Diabetes Educ
2008;34:692-7. Crossref
41. Morello CM, Chynoweth M, Kim H, Singh RF, Hirsch JD.
Strategies to improve medication adherence reported by
diabetes patients and caregivers: results of a taking control
of your diabetes survey. Ann Pharmacother 2011;45:145-53. Crossref
42. Perera PN, Guy MC, Sweaney AM, Boesen KP. Evaluation
of prescriber responses to pharmacist recommendations
communicated by fax in a medication therapy management
program (MTMP). J Manag Care Pharm 2011;17:345-54. Crossref
43. Doucette WR, McDonough RP, Klepser D, McCarthy
R. Comprehensive medication therapy management:
identifying and resolving drug-related issues in a
community pharmacy. Clin Ther 2005;27:1104-11. Crossref
44. Chrischilles EA, Carter BL, Lund BC, et al. Evaluation
of the Iowa Medicaid pharmaceutical case management
program. J Am Pharm Assoc 2004;44:337-49. Crossref
45. Galt KA. Cost avoidance, acceptance, and outcomes
associated with a pharmacotherapy consult clinic in
a Veterans Affairs Medical Center. Pharmacotherapy
1998;18:1103-11.
46. Chan EW, Taylor SE, Marriott JL, Barger B. Bringing
patients’ own medications into an emergency department
by ambulance: effect on prescribing accuracy when these
patients are admitted to hospital. Med J Aust 2009;191:374-7.