Hong Kong Med J 2017 Apr;23(2):129–33 | Epub 17 Feb 2017
DOI: 10.12809/hkmj164883
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Preimplantation genetic diagnosis and screening
by array comparative genomic hybridisation: experience of more than 100 cases in a single centre
Judy FC Chow, MPhil1;
William SB Yeung, PhD1;
Vivian CY Lee, FHKAM (Obstetrics and Gynaecology)2;
Estella YL Lau, PhD2;
PC Ho, FRCOG, FHKAM (Obstetrics and Gynaecology)1;
Ernest HY Ng, FRCOG, FHKAM (Obstetrics and Gynaecology)1;
1 Department of Obstetrics and Gynaecology, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
2 Department of Obstetrics and Gynaecology, Queen Mary Hospital, Hong Kong
 Full
paper in PDF
Full
paper in PDF
Corresponding author: Dr William SB Yeung (wsbyeung@hku.hk)
Abstract
Introduction: Preimplantation genetic screening has
been proposed to improve the in-vitro fertilisation
outcome by screening for aneuploid embryos or
blastocysts. This study aimed to report the outcome
of 133 cycles of preimplantation genetic diagnosis
and screening by array comparative genomic
hybridisation.
Methods: This study of case series was conducted
in a tertiary assisted reproductive centre in Hong
Kong. Patients who underwent preimplantation
genetic diagnosis for chromosomal abnormalities
or preimplantation genetic screening between 1
April 2012 and 30 June 2015 were included. They
underwent in-vitro fertilisation and intracytoplasmic
sperm injection. An embryo biopsy was performed
on day-3 embryos and the blastomere was subject to
array comparative genomic hybridisation. Embryos
with normal copy numbers were replaced. The
ongoing pregnancy rate, implantation rate, and
miscarriage rate were studied.
Results: During the study period, 133 cycles of
preimplantation genetic diagnosis for chromosomal
abnormalities or preimplantation genetic screening
were initiated in 94 patients. Overall, 112 cycles
proceeded to embryo biopsy and 65 cycles had
embryo transfer. The ongoing pregnancy rate
per transfer cycle after preimplantation genetic
screening was 50.0% and that after preimplantation
genetic diagnosis was 34.9%. The implantation
rates after preimplantation genetic screening and
diagnosis were 45.7% and 41.1%, respectively and the
miscarriage rates were 8.3% and 28.6%, respectively.
There were 26 frozen-thawed embryo transfer
cycles, in which vitrified and biopsied genetically
transferrable embryos were replaced, resulting in
an ongoing pregnancy rate of 36.4% in the screening
group and 60.0% in the diagnosis group.
Conclusions: The clinical outcomes of
preimplantation genetic diagnosis and screening
using comparative genomic hybridisation in our unit
were comparable to those reported internationally.
Genetically transferrable embryos replaced in a
natural cycle may improve the ongoing pregnancy
rate and implantation rate when compared with
transfer in a stimulated cycle.
New knowledge added by this study
- Array comparative genomic hybridisation is a reliable method for preimplantation genetic diagnosis for translocation/inversion carriers, and for patients with mosaic sex chromosome aneuploidy. Replacement of vitrified embryos after warming in a natural cycle may improve the ongoing pregnancy rate and implantation rate.
- Preimplantation genetic diagnosis by array comparative genomic hybridisation shall be offered as an alternative to prenatal diagnosis for translocation/inversion carriers, and for patients with mosaic sex chromosome aneuploidy. The results of this local case series provide information, such as the anticipated percentage of genetically transferrable embryos and the expected ongoing pregnancy rate, which is useful for patient counselling before preimplantation genetic diagnosis or screening.
Introduction
Preimplantation genetic diagnosis (PGD) is an
alternative to prenatal diagnosis for detection of
chromosomal abnormalities in translocation or
inversion carrier couples. In the past 13 years,
more than 6000 cycles of PGD for chromosomal
abnormalities have been performed.1 Fluorescence
in-situ hybridisation (FISH) was first used in PGD
for translocation carriers.2 Due to its technical
limitations however,3 4 5 it has been replaced by array
comparative genomic hybridisation (aCGH) in many
centres. In our centre, we have previously shown that
the use of aCGH for PGD in translocation carriers
results in a significantly higher rate of ongoing
pregnancy than PGD by FISH.6
Aneuploidy is the most common abnormality
found in embryos derived from in-vitro fertilisation
(IVF), and leads to poor outcomes.7 8 9 10 11 12 13 Morphological
assessment of embryos or blastocysts alone, however,
cannot negate the potential risk of replacing
aneuploid embryos or blastocysts.14 Preimplantation
genetic screening (PGS) has been proposed to
improve the IVF outcomes by screening for aneuploid
embryos or blastocysts. More than 26 000 PGS
cycles have been performed worldwide.1 The aCGH
technique enables us to screen all 24 chromosomes
within 24 hours and makes fresh transfer possible
after blastomere biopsy or trophectoderm biopsy.15
A randomised study has shown that PGS by aCGH
plus selection by morphology of blastocysts can
significantly improve the ongoing pregnancy rate
in patients with good prognosis when compared
with selection of blastocysts by morphology
alone.16 Another randomised study also showed an
improvement in the implantation rate after PGS by
aCGH in addition to morphological assessment of
embryos.17 We report here the clinical outcome of
133 cycles of PGD/PGS by aCGH in a local unit.
Methods
Study population
Data from all treatment cycles performed for PGD
and PGS in the Department of Obstetrics and
Gynaecology, Queen Mary Hospital/The University
of Hong Kong from 1 April 2012 to 30 June 2015
were retrieved. This study was done in accordance
with the principles outlined in the Declaration of
Helsinki. Patient consent has been obtained. Data
were stored in a database and coded for indication.
Indications for PGS were defined as: (1) advanced
maternal age (AMA) group for patients aged >38
years; (2) recurrent miscarriage (RM) group with
patients having at least two clinical miscarriages
and negative investigations for RM; (3) repeated
implantation failure group (RIF) with those who
failed to get pregnant after three embryo transfer
cycles with at least six good-quality embryos
replaced; and (4) optional PGS group included those
with normal karyotype but who had experienced a
previous pregnancy with abnormal karyotype, and
those who opted for PGS when performing PGD for
monogenetic disease.
Indications for PGD by aCGH were divided
as follows: (1) mosaic were those with mosaic
sex chromosome abnormalities on karyotyping,
including mosaic Klinefelter’s or mosaic Turner’s
syndromes; (2) Robertsonian translocation; (3)
reciprocal translocation; (4) inversion; and (5)
double translocations.
Treatment regimen
The details of the ovarian stimulation regimen,
gamete handling, and frozen-thawed embryo
transfer (FET) have been previously described.18
Surplus good-quality blastocysts with no aneuploidy/unbalanced chromosome detected were vitrified by
the CVM Vitrification System (CryoLogic, Victoria,
Australia). If the patient did not get pregnant in
the stimulated cycle, the vitrified blastocysts were
warmed and replaced in subsequent FET cycles. The
details of biopsy and PGD/ PGS by aCGH have been
described elsewhere.6 19 In brief, a single blastomere was removed from good-quality day-3 embryos
(6-to-8 cell stage) and the blastomere underwent
whole-genome amplification (SurePlex; BlueGnome,
Cambridge, United Kingdom). Array CGH was performed using
24sure+ (BlueGnome) on reciprocal translocation
and inversion cases while other cases were tested
by 24sure V3 (BlueGnome) according to the
manufacturer’s protocol. All results were interpreted
independently by two laboratory staff, usually with
a high concordant rate (>95%). Discrepancies were
resolved through consensus.
Results
Between 1 April 2012 and 30 June 2015, 94 couples
underwent 133 cycles of ovarian stimulation
for PGD for chromosomal abnormalities, or
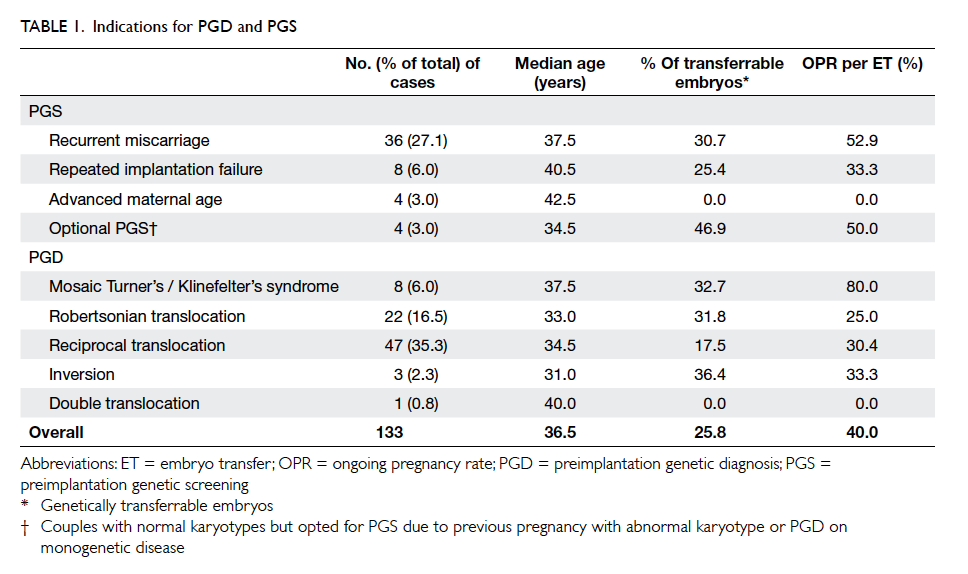
PGS with indications listed in Table 1. The most
frequent indication for PGD/PGS was reciprocal
translocation (35.3%) followed by RM (27.1%) and
Robertsonian translocation (16.5%). The median age
of the women was 36.5 (range, 25-44) years. Embryo
biopsy was performed in 112 cycles. The mean
number of embryos biopsied per retrieval cycle
was 5.6 (740/133), with 99.2% of biopsies resulting
in a conclusive diagnosis, of which only 25.8%
(191/740) were genetically transferrable. The whole-genome
amplification failed in all the samples with
inconclusive diagnosis.
Overall, PGD/PGS was cancelled in 21
(15.8%) cycles after ovarian stimulation due to poor
response (19 cycles), failed fertilisation (1 cycle), or
no sperm found in the testicular biopsy (1 cycle). In
case of poor response (<4 good-quality embryos on
day 3), cleavage-stage embryos were frozen/vitrified,
subsequently thawed/warmed, and pooled with
fresh embryos from the following stimulation cycle
for diagnosis. Fresh embryo transfer was cancelled
in 47 (42.0%) cycles after biopsy due to unavailability
of genetically transferrable embryo (31 cycles),
high serum progesterone level on the day of human
chorionic gonadotropin (>5 nmol/L; 10 cycles), risk
of ovarian hyperstimulation (2 cycles), delayed assay
(3 cycles), or patient request (1 cycle). Overall, 65
PGD/PGS cycles proceeded to embryo transfer in
the stimulated cycles with one or two blastocysts
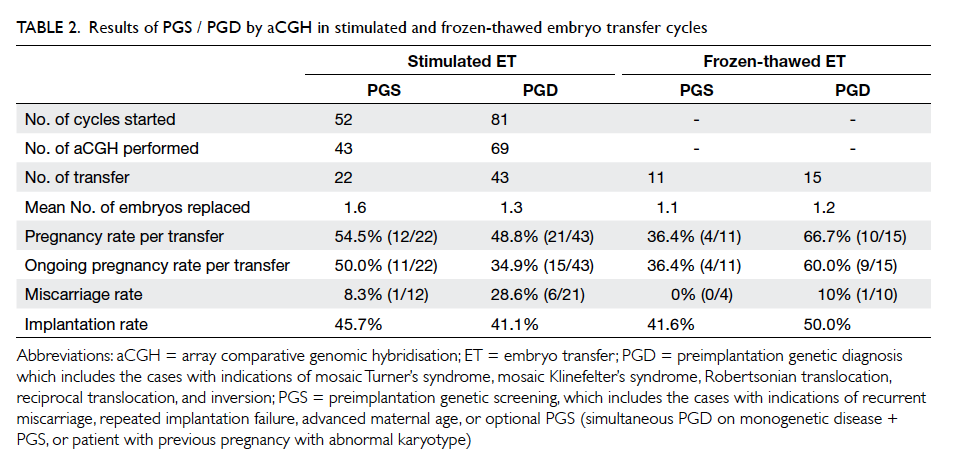
replaced on day 5 (mean, 1.4). As shown in Table 2, the result of aCGH was further subdivided into two categories (PGS and PGD) based on indications.
The ongoing pregnancy rates (pregnancy beyond
8-10 weeks of gestation) of PGS and PGD were 50.0%
(11/22) and 34.9% (15/43), respectively.
There were 26 cycles of FET in a natural cycle
in which one or two biopsied and vitrified blastocysts
were replaced (mean, 1.2), resulting in a pregnancy
rate of 36.4% (4/11) in the PGS group and 66.7%
(10/15) in the PGD group. Ongoing pregnancy rates
in the PGS and PGD group were 36.4% (4/11) and
60.0% (9/15), respectively (Table 2). The miscarriage
rates in the stimulated embryo transfer cycles and
FET cycles were 21.2% (7/33) and 7.1% (1/14),
respectively. The differences in ongoing pregnancy
rate and miscarriage rate between stimulated
embryo transfer and FET cycle were not statistically
significant. All pregnant women following PGD for
chromosomal abnormalities were referred to the
Prenatal Counselling and Diagnosis team at Tsan
Yuk Hospital for counselling and confirmation of the
PGD result by prenatal diagnosis or postnatal cord
blood karyotyping. Based on the available results of
the confirmation tests, no misdiagnosis was found in
this small series.
Discussion
The 13th data report of the ESHRE PGD Consortium
includes a total of 1071 oocyte retrieval cycles for
chromosomal abnormalities and 2979 oocyte
retrieval cycles for PGS, resulting in a delivery
rate of 21%-25% per transfer and an implantation
rate of 22%-26%.1 The ongoing pregnancy rate and
implantation rate of the present series are 34.9%-50.0% and 41.1%-45.7%, respectively.
As shown in Table 1, the percentage of
transferrable embryos varies among different
indications for PGD/PGS. In cases of PGD for
chromosomal abnormalities, as expected, the lowest
percentage of genetically transferrable embryos
was found in the reciprocal translocation group
(17.5%), followed by the Robertsonian translocation
group (31.8%) and the mosaic Turner’s / Klinefelter’s
syndrome group (32.7%). These data are in line
with those of the ESHRE PGD consortium,1 of
which the corresponding percentages are 16.6%,
33.5%, and 36.8%, respectively. The high proportion
of unbalanced gametes can be explained by the
segregation modes and behaviour of the translocated
chromosomes during meiosis.20
In the PGS group (RM, RIF, AMA, and
optional PGS), the overall percentage of genetically
transferrable embryos was 27.5% (69/251), similar
to that of the ESHRE PGD consortium (30%). It is
noteworthy that there were no transferrable embryos
in all four cases of AMA (median age, 42.5 years). It is
well known that chromosomal aneuploidy increases
exponentially with increasing maternal age.21 22
Therefore, patients with advanced age should be
counselled accordingly before the initiation of PGS
cycles.
The cancellation rate for PGD/PGS after
initiation of stimulation was 15.8% (21/133) and the
reason for cancellation in the great majority of cases
was poor ovarian response (19/21). Furthermore,
for those cases proceeding to biopsy, 42.0% (31/47)
did not have an embryo transfer, mainly due to no
normal/balanced embryos available. When a low
percentage of normal/balanced embryos is expected,
patients can consider pooling embryos from several
stimulation cycles and perform PGD/PGD in a
single batch. Such ‘batching’ can increase the chance
of having normal/balanced embryos and allow
selection of the best-quality genetically transferrable
embryos for replacement in the PGD/PGS cycle,
instead of having multiple cycles with no embryo
transfer.
There were 26 cycles of vitrified-warmed
blastocyst transfer (11 cycles after PGS and 15
cycles after PGD) performed during a natural cycle.
The ongoing pregnancy rate per transfer in these
natural cycles after PGD appeared to be higher than
those with transfer in a stimulated cycle, while the
miscarriage rate of transfer in the natural cycle was
lower than that of transfer in a stimulated cycle.
Such findings did not reach statistical significance
due to the small number of cases, however. Some
reports have suggested that transfer of embryos in
a natural cycle may result in a higher pregnancy and
implantation rate than in a stimulated cycle due to
the better receptivity of the endometrium without
gonadotropin stimulation.23 24 25 26
The limitation of the present study was the
small number of cases for each indication of PGS.
Moreover, it was not a randomised controlled trial.
The usefulness of PGS by aCGH in these cases needs
to be confirmed in a large randomised controlled
trial. It is noteworthy that aCGH cannot detect
mutation and/or small chromosomal aberrations
(<10 Mb for Robertsonian translocation, mosaic
sex chromosome aneuploidy and PGS; <5 Mb for
reciprocal translocation and inversion). False results
can be attributed to mosaicism of embryos, although
no misdiagnosis was found in the present study.
Conclusions
The clinical outcomes of PGD and PGS in our unit
were comparable to those reported internationally.
A genetically transferrable embryo after PGD that
is replaced during a natural cycle may improve the
ongoing pregnancy rate and implantation rate when
compared with transfer during a stimulated cycle.
Acknowledgements
We would like to thank the patients, nurses,
clinicians, technicians, and embryologists at the
Centre of Assisted Reproduction and Embryology,
Queen Mary Hospital–The University of Hong Kong
for their contribution in the PGD programme.
Declaration
All authors have disclosed no conflicts of interest.
References
1. De Rycke M, Belva F, Goossens V, et al. ESHRE PGD
Consortium data collection XIII: cycles from January to
December 2010 with pregnancy follow-up to October
2011. Hum Reprod 2015;30:1763-89. Crossref
2. DeUgarte CM, Li M, Surrey M, Danzer H, Hill D, DeCherney
AH. Accuracy of FISH analysis in predicting chromosomal
status in patients undergoing preimplantation genetic
diagnosis. Fertil Steril 2008;90:1049-54. Crossref
3. Li M, DeUgarte CM, Surrey M, Danzer H, DeCherney A,
Hill DL. Fluorescence in situ hybridization reanalysis of
day-6 human blastocysts diagnosed with aneuploidy on
day 3. Fertil Steril 2005;84:1395-400. Crossref
4. Velilla E, Escudero T, Munné S. Blastomere fixation
techniques and risk of misdiagnosis for preimplantation
genetic diagnosis of aneuploidy. Reprod Biomed Online
2002;4:210-7. Crossref
5. Wells D, Alfarawati S, Fragouli E. Use of comprehensive
chromosomal screening for embryo assessment:
microarrays and CGH. Mol Hum Reprod 2008;14:703-10. Crossref
6. Lee VC, Chow JF, Lau EY, Yeung WS, Ho PC, Ng EH.
Comparison between fluorescent in-situ hybridisation
and array comparative genomic hybridisation in
preimplantation genetic diagnosis in translocation carriers.
Hong Kong Med J 2015;21:16-22.
7. Bielanska M, Tan SL, Ao A. Chromosomal mosaicism
throughout human preimplantation development in vitro:
incidence, type, and relevance to embryo outcome. Hum
Reprod 2002;17:413-9. Crossref
8. Munné S, Sandalinas M, Magli C, Gianaroli L, Cohen
J, Warburton D. Increased rate of aneuploid embryos
in young women with previous aneuploid conceptions.
Prenat Diagn 2004;24:638-43. Crossref
9. Kuliev A, Cieslak J, Verlinsky Y. Frequency and distribution
of chromosome abnormalities in human oocytes.
Cytogenet Genome Res 2005;111:193-8. Crossref
10. Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti
A, Farfalli V. Embryo morphology and development are
dependent on the chromosomal complement. Fertil Steril
2007;87:534-41. Crossref
11. Munné S, Chen S, Colls P, et al. Maternal age, morphology,
development and chromosome abnormalities in over
6000 cleavage-stage embryos. Reprod Biomed Online
2007;14:628-34. Crossref
12. Hassold T, Hunt P. Maternal age and chromosomally
abnormal pregnancies: what we know and what we wish
we knew. Curr Opin Pediatr 2009;21:703-8. Crossref
13. Vanneste E, Voet T, Le Caignec C, et al. Chromosome
instability is common in human cleavage-stage embryos.
Nat Med 2009;15:577-83. Crossref
14. Alfarawati S, Fragouli E, Colls P, et al. The relationship
between blastocyst morphology, chromosomal
abnormality, and embryo gender. Fertil Steril 2011;95:520-4. Crossref
15. Rubio C, Rodrigo L, Mir P, et al. Use of array comparative
genomic hybridization (array-CGH) for embryo
assessment: clinical results. Fertil Steril 2013;99:1044-8. Crossref
16. Yang Z, Liu J, Collins GS, et al. Selection of single
blastocysts for fresh transfer via standard morphology
assessment alone and with array CGH for good prognosis
IVF patients: results from a randomized pilot study. Mol
Cytogenet 2012;5:24. Crossref
17. Yang Z, Salem SA, Liu X, Kuang Y, Salem RD, Liu J.
Selection of euploid blastocysts for cryopreservation with
array comparative genomic hybridization (aCGH) results
in increased implantation rates in subsequent frozen and
thawed embryo transfer cycles. Mol Cytogenet 2013;6:32. Crossref
18. Ng EH, Yeung WS, Lau EY, So WW, Ho PC. High
serum oestradiol concentrations in fresh IVF cycles do not
impair implantation and pregnancy rates in subsequent
frozen-thawed embryo transfer cycles. Hum Reprod
2000;15:250-5. Crossref
19. Chow JF, Yeung WS, Lau EY, et al. Singleton birth after
preimplantation genetic diagnosis for Huntington disease
using whole genome amplification. Fertil Steril 2009;92:828.e7-10.
20. Scriven PN, Handyside AH, Ogilvie CM. Chromosome
translocations: segregation modes and strategies
for preimplantation genetic diagnosis. Prenat Diagn
1998;18:1437-49. Crossref
21. Spandorfer SD, Davis OK, Barmat LI, Chung PH,
Rosenwaks Z. Relationship between maternal age and
aneuploidy in in vitro fertilization pregnancy loss. Fertil
Steril 2004;81:1265-9. Crossref
22. Hassold T, Hall H, Hunt P. The origin of human aneuploidy:
where we have been, where we are going. Hum Mol Genet
2007;16 Spec No. 2:R203-8.
23. Evans J, Hannan NJ, Edgell TA, et al. Fresh versus frozen
embryo transfer: backing clinical decisions with scientific
and clinical evidence. Hum Reprod Update 2014;20:808-21. Crossref
24. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M,
Hudson C. Clinical rationale for cryopreservation of
entire embryo cohorts in lieu of fresh transfer. Fertil Steril
2014;102:3-9. Crossref
25. Roque M. Freeze-all policy: is it time for that? J Assist
Reprod Genet 2015;32:171-6. Crossref
26. Roque M, Valle M, Guimarães F, Sampaio M, Geber S.
Freeze-all policy: fresh vs. frozen-thawed embryo transfer.
Fertil Steril 2015;103:1190-3. Crossref