Hong Kong Med J 2017 Apr;23(2):117–21 | Epub 24 Feb 2017
DOI: 10.12809/hkmj164953
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Atrial fibrillation patients who sustained
warfarin-associated intracerebral haemorrhage
have poor neurological outcomes: results from a
matched case series
MK Fong, MB, BS, FHKAM (Medicine)1;
B Sheng, MB, ChB, FHKAM (Medicine)1;
YP Chu, MB, BS, FHKAM (Medicine)1;
WT Wong, MB, ChB, MRCP1;
Patrick PK Lau, MB, ChB, FHKAM (Medicine)2;
HY Wong, MB, BS, FHKAM (Medicine)3;
KK Lau, MB, BS, FHKAM (Medicine)1
1 Department of Medicine and Geriatrics, Princess Margaret Hospital,
Laichikok, Hong Kong
2 Department of Rehabilitation, Kowloon Hospital, Argyle Street, Hong
Kong
3 Department of Medicine, Queen Elizabeth Hospital, Jordan, Hong Kong
Corresponding authors: Dr MK Fong (keison722@hotmail.com)
Abstract
Introduction: Coagulopathy-associated intracerebral
haemorrhage has become increasingly common
because of the rising demand in the ageing population
for anticoagulation for atrial fibrillation. This study
compared the clinical features and neurological
outcomes of intracerebral haemorrhage in patients
with atrial fibrillation who were prescribed warfarin
with those who were not.
Methods: This was a retrospective matched case
series of patients with intracerebral haemorrhage
from three tertiary hospitals in Hong Kong from 1
January 2006 to 31 December 2011. Patients who
developed intracerebral haemorrhage and who were
prescribed warfarin for atrial fibrillation (ICH-W
group) were compared with those with intracerebral
haemorrhage and not prescribed warfarin (ICH-C
group); they were matched for age and gender in 1:1
ratio. Clinical features and neurological outcomes
were compared, and the impact of coagulopathy on
haematoma size was also studied.
Results: We identified 114 patients in the ICH-W
group with a mean age of 75 years. Both ICH-W
and ICH-C groups had a median intracerebral
haemorrhage score of 2. There was a non–statistically
significant trend of higher intracerebral haemorrhage
volume in the ICH-W group (12.9 mL vs 10.5 mL). The
median modified Rankin Scale and the proportion
with good recovery (modified Rankin Scale score ≤3)
at 6 months were comparable. Nonetheless, ICH-W
patients had higher hospital mortality (51.8% vs
36.0%; P=0.02) and 6-month mortality (60.5% vs
43.0%; P=0.01) than ICH-C patients. Overall, 60% of
ICH-W patients had their admission international
normalised ratio within the therapeutic range
during intracerebral haemorrhage, and 14% had a
subtherapeutic admission international normalised
ratio. International normalised ratio at admission
was not associated with intracerebral haemorrhage
volume or neurological outcome.
Conclusion: Warfarin-associated intracerebral
haemorrhage in patients with atrial fibrillation
carried a higher stroke mortality than the non-warfarinised
patients.
New knowledge added by this study
- Warfarin-associated intracerebral haemorrhage (ICH) carries a high mortality.
- The reversal of coagulopathy after warfarin-associated ICH was often incomplete.
- Given the high mortality after warfarin-associated ICH, newer oral anticoagulants may be a safer alternative in patients with a high risk of cerebral bleeding. As a class of drugs, they are associated with fewer ICHs.
Introduction
Atrial fibrillation (AF) is associated with an increased
risk of stroke or systemic thromboembolism.
Approximately 5% of AF patients develop stroke or
other embolic events each year.1 Anticoagulation
with warfarin, a vitamin K antagonist, reduces
stroke risk in AF patients by 64%.2 It has been the
drug of choice for many years in both primary and
secondary stroke prevention in AF. Unfortunately,
anticoagulation increases the risk of bleeding, and
intracerebral haemorrhage (ICH) has been the most
life-threatening bleeding complication of concern.
This study aimed to compare the clinical features
and neurological outcomes of ICH in warfarinised
AF patients with those in non-warfarinised patients.
Methods
This was a retrospective matched case series of
consecutive patients with first acute ICH admitted
to the medical unit of three tertiary hospitals in
Hong Kong—Princess Margaret Hospital (PMH) and
Caritas Medical Centre (CMC) in Kowloon West,
and Queen Elizabeth Hospital (QEH) in Kowloon
Central—from 1 January 2006 to 31 December 2011.
The three hospitals cover about one quarter of the
7 million population in Hong Kong. It is a general
practice in Hong Kong that patients with acute
stroke symptoms are admitted to the medical unit (to
acute stroke unit first, and to general medical ward
if acute stroke unit is full) for further management,
with computed tomography (CT) brain scans done
within 24 hours of admission. If ICH is identified,
a neurosurgeon will be consulted for assessment.
Therefore ICH patients in the medical unit are a
good indication of the general ICH population.
We searched our electronic database for all
patients aged 18 years or above who developed first
ICH in the presence of anticoagulation with warfarin
for non-valvular AF (ICH-W group) from the three
hospitals, and matched them with a comparison
group (ICH-C group) without taking warfarin at a
1:1 ratio for age (±1 year), gender, and admission
year. The comparison group comprised patients
from the medical unit of PMH (principal study
centre) who had a first episode of ICH without
anticoagulation, regardless of any AF. Patients with
isolated subdural, subarachnoid, or intraventricular
haemorrhage were excluded. We retrieved
and compared the data regarding neurological
impairment and investigation findings, estimated
the ICH volume on CT through the ABC/2 method,
and calculated the ICH score.3 4 Hospital mortality
and 6-month modified Rankin Scale score (mRS, 0-6)
were selected as primary and secondary outcomes,
respectively. We used independent sample t test and
Mann-Whitney U test for univariate comparisons
of continuous variables (Glasgow Coma Scale score,
ICH score, ICH volume, mRS), and Chi squared
test for categorical variables. Descriptive summary
statistics, where appropriate, are presented as mean
(range) or median (interquartile range [IQR]). All
calculations were two-end conducted at a 0.05 level
of significance. The study was approved by the local
ethics committee (KW/EX-12-057[52-06]), with the
requirement of patient informed consent waived
because of its retrospective nature.
Results
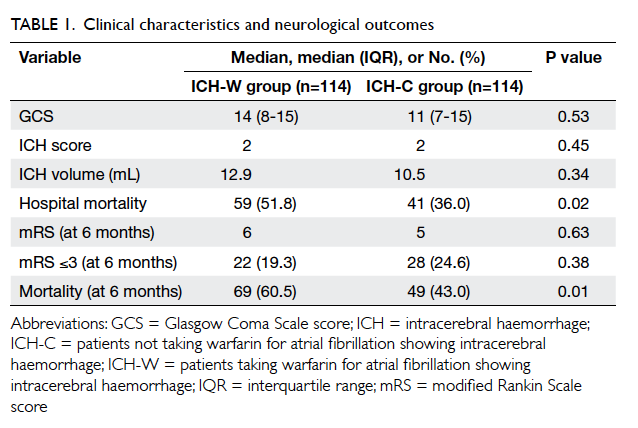
Overall, 114 patients were identified and recruited
in the ICH-W group (37 from PMH, 8 from CMC,
and 69 from QEH; Table 1) with a mean age of
75 years (range, 47-92 years) and a slight male
predominance (56.1%). The same number of patients
matched for age (±1 year) and gender were grouped
for comparison (ICH-C). Both ICH-W and ICH-C
groups had a median ICH score of 2, but there was a
trend for higher ICH volume in ICH-W patients than
in ICH-C patients (12.9 mL vs 10.5 mL) although it
did not reach statistical significance. A total of 59
patients in ICH-W died during the same admission,
and a further 10 patients had died by 6 months. The
ICH-W patients had significantly higher hospital
mortality (51.8% vs 36.0%; Chi squared test, P=0.02)
and 6-month mortality (60.5% vs 43.0%; Chi squared
test, P=0.01) than the ICH-C patients. The median
mRS and the proportion with good recovery (mRS
≤3) at 6 months were comparable for the two groups
(Table 1).
Two patients in the ICH-W group died before
determination of international normalised ratio
(INR). For the remaining 112, the admission INR
was <2.0 in 16 (14.3%) patients, 2.0-3.0 in 66 (58.9%)
patients, and >3.0 in 30 (26.8%) patients. The INR
was re-checked in 85 patients after 12 hours and
in 72 patients after 24 hours. The median INR was
corrected from 2.6 (IQR, 2.1-3.1) to 1.4 (IQR, 1.2-1.7) at 12 hours post-event, and 1.3 (IQR, 1.1-1.5) at
24 hours post-event. The corresponding percentage
of INR >1.5 was 96.4% (108/112) on admission,
35.3% (30/85) at 12 hours, and 22.2% (16/72) at 24
hours. No association was found between admission
INR and ICH volume, hospital mortality, or 6-month
mRS (Spearman’s rank correlation coefficient).
Regarding the warfarin reversal strategies,
QEH had an in-patient protocol whereas PMH and
CMC did not. Nevertheless, there was no significant
difference in mortality rate among the three
hospitals—hospital mortality/6-month mortality in
PMH 18 (48.6%)/20 (54.1%), in CMC five (62.5%)/six
(75.0%), and in QEH 36 (52.2%)/43 (62.3%).
Prothrombin complex concentrate (PCC), fresh
frozen plasma (FFP), vitamin K1 (VitK1), factor VII,
and transamin were used alone or in combination in
our ICH-W patients. Their frequency was as follows:
FFP alone (69, 60.5%), FFP + VitK1 (17, 14.9%), FFP +
PCC (9, 7.9%), PCC alone (3, 2.6%), transamin alone
(3, 2.6%), VitK1 alone (2, 1.8%), FFP + transamin (1,
0.9%), and FFP + factor VII (1, 0.9%). Nine (7.9%)
patients received no treatment—two patients died
before admission INR was available, three patients
died soon after admission INR was available,
three patients had admission INR of <1.5, and one
patient was on warfarin and aspirin for ischaemic
heart disease. The high variation in anticoagulation
reversal strategy made it impossible for any valid
comparison.
Discussion
Cardioembolic stroke related to AF carries significant
morbidity, yet anticoagulation is not without risk.
How to prevent stroke and at the same time minimise
bleeding complications has always been a dilemma
for physicians and patients. Risk assessment tools
(eg CHA2DS2-VASc and HAS-BLED score, for
assessment of thromboembolic and bleeding risk,
respectively) have been developed to assist the
decision making.5 6 It is important for clinicians to
exercise individualised medical practice, however.
In a recently published observation study on a
large hospital cohort of Chinese AF patients from
Hong Kong, the investigators found that the stroke
risk in Chinese AF patients was higher than that in
Caucasians at a given CHA2DS2-VASc score, and that
excessive risk was more prominent in the low-risk
group from CHA2DS2-VASc.7 At the same time, the
ICH incidence in Chinese AF patients taking warfarin
was also higher than that in Caucasians.8 These
observations really drive clinicians towards a better
anticoagulation strategy for Chinese AF patients.
Unfortunately ICH remains a significant threat
even with very careful patient selection and treatment
monitoring. Up to 25% of ICH can be associated with
anticoagulant usage, and the rate is still increasing
given the higher utilisation of anticoagulation in
ageing populations with rising AF prevalence.9 10
Several features of warfarin-associated ICH in our
patients deserve further elaboration, as listed below.
First, we found a large proportion (73.2%) of
warfarin-associated ICH occurred when the INR
was within or even below the therapeutic range
(INR ≤3.0). Our findings were supported by a similar
observation from another prospective study in which
68% of warfarin-associated ICH occurred at INR of
≤3.0.11 This shows that bleeding is increased even
with an INR within the therapeutic range. Moreover,
those ICH-W patients with a subtherapeutic INR
reaffirm the high intrinsic ICH risk in AF patients
who are old with multiple co-morbidities.
Second, we did not find any correlation
between initial ICH volume and admission INR.
The initial ICH volume in warfarinised and non-warfarinised
patients was not significantly different.
This is consistent with the results from some other
studies.9 12 13 In contrast, Cucchiara et al14 reported
larger haematoma volume in anticoagulant-associated
ICH than spontaneous ICH, whereas
Flaherty et al15 reported larger initial haematoma
volume if admission INR was >3.0. It is reasonable
to suppose that coagulopathy would have an impact
on haematoma volume, but is difficult to confirm in
clinical studies because it is hard to control other
covariates that affect haematoma size. The relatively
small number of subjects in the warfarin-associated
ICH group is often underpowered to reach any
solid conclusion. Nonetheless, other studies did
show that warfarin use was a known predictor of
haematoma expansion, and haematoma expansion an
independent determinant of neurological outcomes in
spontaneous ICH.12 14 16 All the consensus guidelines
agree that coagulopathy should be reversed as soon
as possible in warfarin-associated ICH.
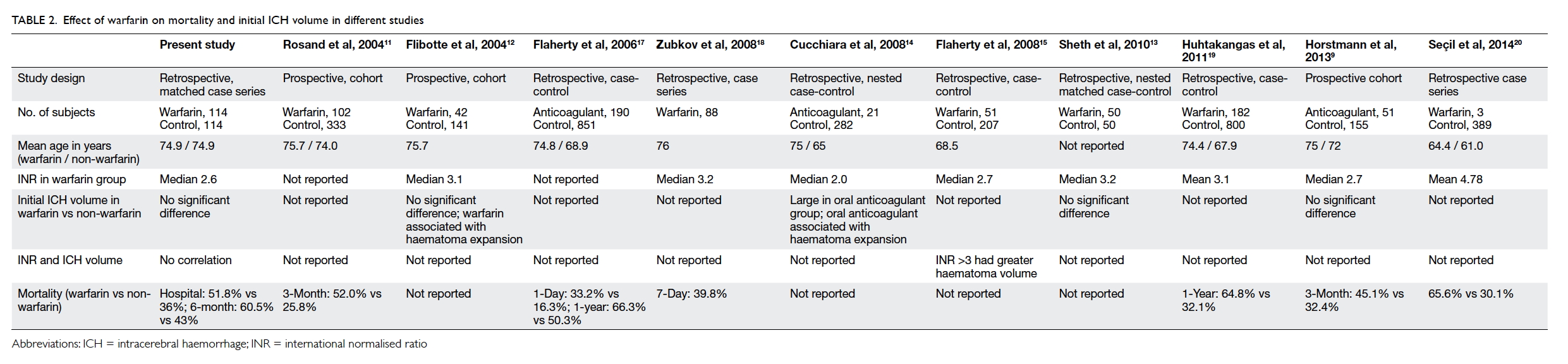
Third, both the hospital and 6-month
mortalities in our ICH-W patients were significantly
higher than those in ICH-C patients (Table 1). This
observation is highly consistent with other reports
in which warfarin-associated ICH had higher
mortality than patients without taking warfarin
(Table 2).9 11 12 13 14 15 17 18 19 20 Our study adopted a matched case
series design in order to eliminate the effect of age,
and to minimise the age-related co-morbidities that
are known predictors of poor neurological outcome
from ICH. As a result, our selected ICH-C patients
were older and probably had more co-morbidities
than the general ICH patients. This explained the
higher-than-usual mortality and poor neurological
outcomes in our ICH-C group compared with other
studies. Nonetheless, AF-related co-morbidity could
not be controlled in this design, and we believe our
observed difference in mortality and neurological
outcomes is a compound effect from AF and warfarin
use in the ICH-W patients.
Currently, the optimal therapy for
coagulopathy reversal in warfarin-associated
major bleeding is unclear and recommendations
of international guidelines are mainly derived from
consensus opinions. Treatment options include
FFP, VitK1, PCC, and recombinant factor VIIa, in
different combinations.21 One of the three study
hospitals (QEH) had an in-house protocol for
warfarin reversal. The other two hospitals provide
guidelines only. Because of the incomplete data
collection from this retrospective study design, we
were unable to analyse the haematoma growth and
warfarin reversals. The mortality, however, did not
differ among the three hospitals.
Since 2010, newer oral anticoagulants have
become available and provide an alternative to
warfarin in AF stroke prevention. The three new
oral anticoagulants—dabigatran, rivaroxaban, and
apixaban22 23 24—might differ in efficacy, but the lower
ICH rate compared with warfarin was a universal
finding from their clinical trials, and is a class effect
of these new agents. They should be considered
in Chinese AF patients at a high risk of warfarin-associated
ICH.
Limitations of study
As a retrospective study, this study has several
limitations. First, the methods and timing of warfarin
reversal were not standardised and could have
affected the neurological outcome. Second, there
was no protocol for serial CT brain and serial INR
monitoring to investigate the effect of coagulopathy
reversal on haematoma expansion, and the effect
of haematoma expansion on neurological outcome.
Third, we were uncertain about compliance with the
coagulopathy reversal protocol in QEH, and were
unable to comment on whether the standardised
protocol could improve ICH outcome. Moreover,
ICH-W patients were AF patients who had additional
vascular risk factors (to justify the use of warfarin)
that may not have been present in ICH-C patients.
This difference could potentially affect the ICH
outcome. Finally, other possible confounders such as
smoking or alcohol use were not adjusted for in this
retrospective study.
Conclusion
Warfarin-associated ICH in AF patients may be
associated with higher stroke mortality. It could be
a serious problem in Chinese AF patients who are
known to have more warfarin-associated ICH.
Declaration
The authors declared no conflicts of interest in this study.
References
1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an
independent risk factor for stroke: the Framingham Study.
Stroke 1991;22:983-8. Crossref
2. Hart RG, Pearce LA, Aguilar MI. Meta-analysis:
antithrombotic therapy to prevent stroke in patients
who have nonvalvular atrial fibrillation. Ann Intern Med
2007;146:857-67. Crossref
3. Kothari RU, Brott T, Broderick JP, et al. The ABCs of
measuring intracerebral hemorrhage volumes. Stroke
1996;27:1304-5. Crossref
4. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT,
Johnston SC. The ICH score: a simple, reliable grading scale
for intracerebral hemorrhage. Stroke 2001;32:891-7. Crossref
5. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ.
Refining clinical risk stratification for predicting stroke
and thromboembolism in atrial fibrillation using a novel
risk factor-based approach: the Euro heart survey on atrial
fibrillation. Chest 2010;137:263-72. Crossref
6. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip
GY. A novel user-friendly score (HAS-BLED) to assess 1-year
risk of major bleeding in patients with atrial fibrillation: the
Euro heart survey. Chest 2010;138:1093-100. Crossref
7. Siu CW. One more “C” for CHA2DS2-VASc score? J Am
Coll Cardiol 2015;65:1602-3. Crossref
8. Siu CW, Lip GY, Lam KF, Tse HF. Risk of stroke and
intracranial hemorrhage in 9727 Chinese with atrial
fibrillation in Hong Kong. Heart Rhythm 2014;11:1401-8. Crossref
9. Horstmann S, Rizos T, Lauseker M, et al. Intracerebral
hemorrhage during anticoagulation with vitamin K
antagonists: a consecutive observational study. J Neurol
2013;260:2046-51. Crossref
10. Flaherty ML, Kissela B, Woo D, et al. The increasing
incidence of anticoagulant-associated intracerebral
hemorrhage. Neurology 2007;68:116-21. Crossref
11. Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg
SM. The effect of warfarin and intensity of anticoagulation
on outcome of intracerebral hemorrhage. Arch Intern Med
2004;164:880-4. Crossref
12. Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand
J. Warfarin, hematoma expansion, and outcome of
intracerebral hemorrhage. Neurology 2004;63:1059-64. Crossref
13. Sheth KN, Cushing TA, Wendell L, et al. Comparison of
hematoma shape and volume estimates in warfarin versus
non-warfarin-related intracerebral hemorrhage. Neurocrit
Care 2010;12:30-4. CrossRef
14. Cucchiara B, Messe S, Sansing L, Kasner S, Lyden P, CHANT
Investigators. Hematoma growth in oral anticoagulant
related intracerebral hemorrhage. Stroke 2008;39:2993-6. Crossref
15. Flaherty ML, Tao H, Haverbusch M, et al. Warfarin use
leads to larger intracerebral hematomas. Neurology
2008;71:1084-9. Crossref
16. Davis SM, Broderick J, Hennerici M, et al. Hematoma
growth is a determinant of mortality and poor outcome
after intracerebral hemorrhage. Neurology 2006;66:1175-81. Crossref
17. Flaherty ML, Haverbusch M, Sekar P, et al. Location
and outcome of anticoagulant-associated intracerebral
hemorrhage. Neurocrit Care 2006;5:197-201. Crossref
18. Zubkov AY, Mandrekar JN, Claassen DO, Manno EM,
Wijdicks EF, Rabinstein AA. Predictors of outcome in
warfarin-related intracerebral hemorrhage. Arch Neurol
2008;65:1320-5. Crossref
19. Huhtakangas J, Tetri S, Juvela S, Saloheimo P, Bode MK,
Hillbom M. Effect of increased warfarin use on warfarin-related
cerebral hemorrhage: a longitudinal population-based
study. Stroke 2011;42:2431-5. Crossref
20. Seçil Y, Ciftçi Y, Tokuçoğlu F, Beckmann Y. Intracranial hemorrhages related with warfarin use and comparison of
warfarin and acetylsalicylic acid. J Stroke Cerebrovasc Dis
2014;23:321-6. Crossref
21. Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated
intracerebral hemorrhage: literature review and
expert opinion. Mayo Clin Proc 2007;82:82-92. Crossref
22. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran
versus warfarin in patients with atrial fibrillation. N Engl J
Med 2009;361:1139-51. Crossref
23. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus
warfarin in nonvalvular atrial fibrillation. N Engl J Med
2011;365:883-91. Crossref
24. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban
versus warfarin in patients with atrial fibrillation. N Engl J
Med 2011;365:981-92. Crossref