Hong Kong Med J 2017 Feb;23(1):54–62 | Epub 14 Dec 2016
DOI: 10.12809/hkmj164885
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Adjuvant S-1 chemotherapy after curative resection of gastric cancer in Chinese patients:
assessment of treatment tolerability and associated risk factors
Winnie Yeo, FRCP, FHKAM (Medicine)1;
KO Lam, MB, BS, FHKAM (Radiology)2;
Ada LY Law, MB, BS, FHKAM (Radiology)3;
Conrad CY Lee, FRCP, FRCR4;
CL Chiang, MB, ChB, FRCR5;
KH Au, FHKCR, FHKAM (Radiology)6;
Frankie KF Mo, MPhil, PhD7;
TH So, BChinMed, MB, BS2;
KC Lam, FHKCP, FHKAM (Medicine)8;
WT Ng, MD3;
L Li, FHKCP, FHKAM (Medicine)8
1 Department of Clinical Oncology, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Clinical Oncology, The University of Hong Kong,
Pokfulam, Hong Kong
3 Department of Clinical Oncology, Pamela Youde Nethersole Eastern
Hospital, Chai Wan, Hong Kong
4 Department of Clinical Oncology, Princess Margaret Hospital, Laichikok,
Hong Kong
5 Department of Clinical Oncology, Tuen Mun Hospital, Tuen Mun, Hong
Kong
6 Department of Clinical Oncology, United Christian Hospital, Kwun Tong,
Hong Kong
7 Comprehensive Clinical Trials Unit, Department of Clinical Oncology, The
Chinese University of Hong Kong, Shatin, Hong Kong
8 Department of Clinical Oncology, Prince of Wales Hospital, Shatin, Hong
Kong
Corresponding author: Prof Winnie Yeo (winnieyeo@cuhk.edu.hk)
Abstract
Introduction: The use of adjuvant chemotherapy
with S-1 (tegafur, gimeracil, and oteracil potassium)
has been shown to improve the outcome of patients
with gastric cancer. There are limited data on
the tolerability of S-1 in Chinese patients. In this
multicentre retrospective study, we assessed
the toxicity profile in local patients.
Methods: Patients with stage II-IIIC gastric
adenocarcinoma who had undergone curative
resection and who had received S-1 adjuvant
chemotherapy were included in the study.
Patient demographics, tumour characteristics,
chemotherapy records, as well as biochemical,
haematological, and other toxicity profiles were
extracted from medical charts. Potential factors
associated with grade 2-4 toxicities were identified.
Results: Adjuvant S-1 was administered to 30
patients. Overall, 19 (63%) patients completed eight
cycles. The most common grade 3-4 adverse events
included neutropaenia (10%), anaemia
(6.7%), septic episode (16.7%), diarrhoea (6.7%),
hyperbilirubinaemia (6.7%), and syncope (6.7%).
Dose reductions were made in 22 (73.3%) patients
and 12 (40.0%) patients had dose delays. Univariate
analyses showed that patients who underwent total
gastrectomy were more likely to experience adverse
haematological events (P=0.034). Patients with nodal
involvement were more likely to report adverse non-haematological
events (P=0.031). Patients with a
history of regular alcohol intake were more likely to
have earlier treatment withdrawal (P=0.044). Lower
body weight (P=0.007) and lower body surface area
(P=0.017) were associated with dose interruptions.
Conclusions: The tolerability of adjuvant S-1 in our
patient population was similar to that in other Asian
patient populations. The awareness of S-1–related
toxicities and increasing knowledge of potential
associated factors may enable optimisation of S-1
therapy.
New knowledge added by this study
- In line with the published data, adjuvant S-1 therapy has a tolerable toxicity profile among local patients who have undergone curative resection for gastric cancer. Total gastrectomy and nodal involvement are potential factors associated with adverse events. Lower body weight and lower body surface area are potential factors associated with dose interruptions.
- For gastric cancer patients in whom adjuvant S-1 therapy is planned, close monitoring of those who have identifiable risk factors may enable early recognition of adverse events during therapy. This may enable earlier intervention with supportive therapy and improve treatment outcome.
Introduction
Gastric cancer is the second most common cause
of cancer-related mortality worldwide, with 988 000
new cases and 736 000 deaths per year.1 Surgery is
the main treatment for operable gastric cancer but
recurrence rates are high and about 40% to 80% of
patients develop relapsed disease after surgery. The
use of adjuvant chemotherapy has been shown to
improve patient outcome.2 3 4 5 After curative resection,
common adjuvant chemotherapy regimens that have
been recently adopted in many parts of Asia include
oral administration of S-1 (tegafur, gimeracil,
and oteracil potassium) based on the Adjuvant
Chemotherapy Trial of S-1 for Gastric Cancer
(ACTS-GC) study conducted in Japan,4 as well as
oxaliplatin-capecitabine combination chemotherapy
based on the Capecitabine and Oxaliplatin Adjuvant
Study in Stomach Cancer study.5 These studies have
shown that adjuvant S-1 for 1 year or oxaliplatin-capecitabine
combination chemotherapy for 6
months following curative gastrectomy with D2
lymph node dissection increases both overall survival
(OS) and relapse-free survival in pathological stage
II or III gastric cancer.4 5
S-1 is an oral anticancer agent comprising
tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP), and
oteracil potassium (Oxo) at a molar ratio of 1:0.4:1.6
Tegafur is a prodrug of 5-fluorouracil (5-FU); CDHP
is a potent reversible inhibitor of 5-FU degradation;
and Oxo is an inhibitor of the enzyme orotate
phosphoribosyltransferase (OPRT) that catalyses
the phosphorylation of 5-FU.6 Pharmacokinetic
analyses have confirmed that S-1 has potent anti-tumour
activity, and oral S-1 administration
results in a similar serum concentration of 5-FU to
intravenous 5-FU whilst sparing patients the need
for continuous intravenous infusion of 5-FU and
consequent toxicity.7 Nonetheless early studies have
also shown that toxicity profiles may differ between
Asian and non-Asian patients. In earlier studies in
Japanese patients, the dose-limiting toxicity was
bone marrow suppression that occurred prior to
gastrointestinal adverse events. In contrast, studies
in non-Asian patients revealed that diarrhoea
associated with abdominal discomfort and cramping
was the principal dose-limiting toxicity and bone
marrow suppression was not.8 This might be due
to the varied activity of OPRT between different
populations. In fact, OPRT activates 5-FU in the
bowel mucosa; patients with higher OPRT activity
might be expected to experience a higher incidence
of gastrointestinal adverse effects prior to bone
marrow toxicity.9
In the ACTS-GC study, the adverse events of
adjuvant S-1 were reported to be generally mild,
with 65.8% of patients being able to complete the
planned 1 year of therapy.4 While it has been known
that patients in the West have a different toxicity
profile to their Japanese counterparts,10 there are
limited data on tolerability of S-1 among Chinese
patients. In this multicentre retrospective study, we assessed the toxicity and tolerability profiles
of Hong Kong Chinese patients with gastric cancer
who had received adjuvant S-1 chemotherapy.
Methods
This was a retrospective study carrying out between
June 2013 and February 2016, and involved six local
centres in Hong Kong: Pamela Youde Nethersole
Eastern Hospital, Princess Margaret Hospital, Prince
of Wales Hospital, Tuen Mun Hospital, Queen
Mary Hospital, and United Christian Hospital.
This study has been approved by the institutional
ethics committee of each participating centre with
patient consent waived. Patients with
stage II-IIIC gastric adenocarcinoma according to
American Joint Committee on Cancer,11 who had
completed curative surgical treatment and who
had undergone S-1 adjuvant chemotherapy, were
included. Patients with stage IV disease and who
had had prior therapy with S-1 in the neoadjuvant
setting were excluded.
Adjuvant S-1 was started at least 3 weeks after
curative surgery. The intended dose of S-1 was based
on that published in the ACTS-GC trial,4 and was
40 mg/m2 twice daily for 4 weeks followed by 2
weeks of rest for each cycle. Specifically, during the
treatment weeks, patients with body surface area
(BSA) of <1.25 m2 received 80 mg daily; those with
BSA of 1.25 m2 to <1.5 m2 received 100 mg daily; and
those with BSA of ≥1.5 m2 received 120 mg daily. As
clinically indicated, dose reductions were considered
one dose level at a time; in general, one dose level
reduction refers to reducing the prior daily dose by
20 mg, eg from 120 mg to 100 mg daily. As renal
impairment has been associated with increased
incidence of myelosuppression, dose reduction
by one dose level was made in patients who had a
creatinine clearance of 40-49 mL/min. A maximum
of eight 6-weekly cycles were administered. The
dose of S-1 was reduced in patients with significant
toxicities, as assessed by the respective clinician-in-charge. Complete and differential blood count
and serum chemistry were performed before each
6-week cycle. All patients had mid-cycle follow-up
with complete and differential blood counts and
serum chemistry in the first cycle.
Patient charts were reviewed by investigators
at each centre for background information. S-1
chemotherapy records, as well as biochemical and
haematological profiles, were extracted. Adverse
events were graded according to the National
Cancer Institute’s Common Terminology Criteria
for Adverse Events (version 3.0).12 Adverse events
were documented during chemotherapy and for 28
days after the last dose of S-1. Dose interruption was
defined as a need for either any dose delay and/or
dose reduction.
Clinical characteristics are summarised as
number of patients and percentage (%) for categorical
variables, and medians with ranges for continuous
variables. The frequency of adverse events was
tabulated. Factors independently associated with
adverse events, dose interruptions, or earlier
withdrawal of S-1 were identified using the Pearson’s
Chi squared (χ2) test or the Fisher’s exact test if the
expected number in any cell was less than five for
categorical data or any cell with an expected count
of less than one for categorical data, and t test or
Wilcoxon rank-sum test for continuous data. A two-sided
P value of <0.05 was considered significant.
All statistical analyses were performed with SAS,
version 9.3 (SAS Institute Inc, Cory [NC], US).
Disease-free survival (DFS) was calculated
as the period from the date of surgery to the date
of recurrence or death from any cause; OS was
calculated as the period from the date of surgery to
the date of death from any cause. Both DFS and OS
were estimated using the Kaplan-Meier method.
Results
Characteristics of patients
Thirty patients met the eligibility criteria in the six
centres during the study period and were enrolled in
this study. Their baseline demographic and clinical
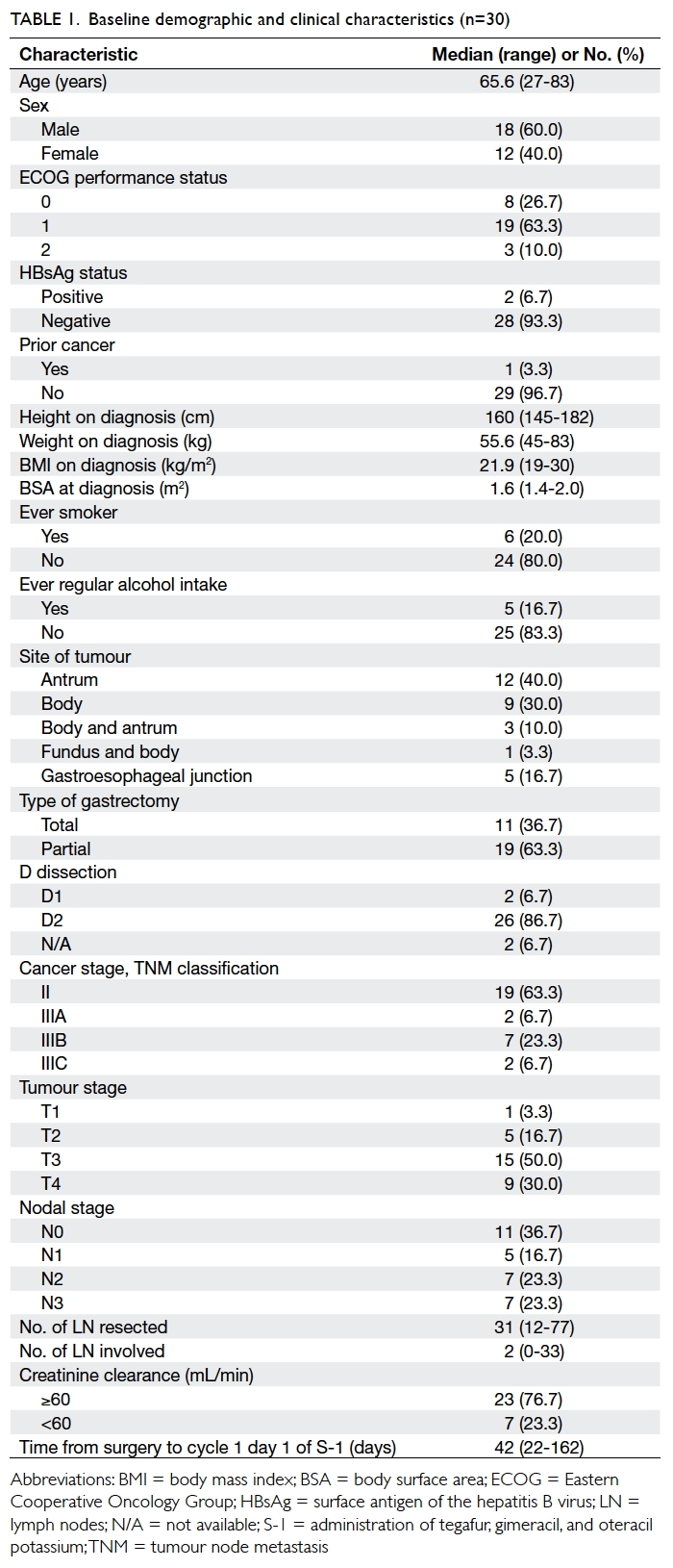
characteristics are shown in Table 1.
There were 18 males and 12 females with
a median age of 65.6 years. Of the patients, 27
(90%) had ECOG (Eastern Cooperative Oncology Group) performance status of 0 to 1. Total gastrectomy
was performed in 11 (36.7%) patients and partial
gastrectomy in 19 (63.3%). D2 dissection was
performed in 26 (86.7%) patients and two had D1
dissection; the details of two other patients were
unknown. The median number of lymph nodes
resected was 31. Cancer stage II disease was present in 19
(63.3%) patients and stage III in 11 (36.7%).
Of the 30 patients, two (6.7%), two (6.7%), one (3.3%),
two (6.7%), one (3.3%), three (10%), and 19 (63.3%) completed
one, two, three, four, five, six, and eight cycles of S-1
adjuvant chemotherapy, respectively. At the time of
data cut-off on 29 February 2016, one patient was still
on S-1, having completed six cycles of treatment. The
reasons for treatment withdrawal included toxicities
(n=5, 16.7%), patient refusal (3, 10%), recurrence
(2, 6.7%), and worsening of pre-existing Parkinson’s
disease (1, 3.3%).
Patient survival
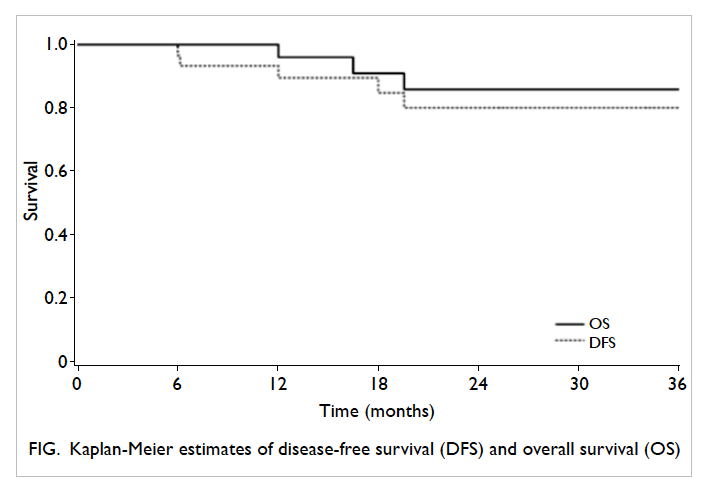
The median follow-up period was 25.3 months
(range, 16.3-29.2 months). Three patients died and
two experienced recurrence (lung and peritoneum).
The 3-year DFS and OS rates were 80.2% and 85.9%,
respectively (Fig).
Tolerability data
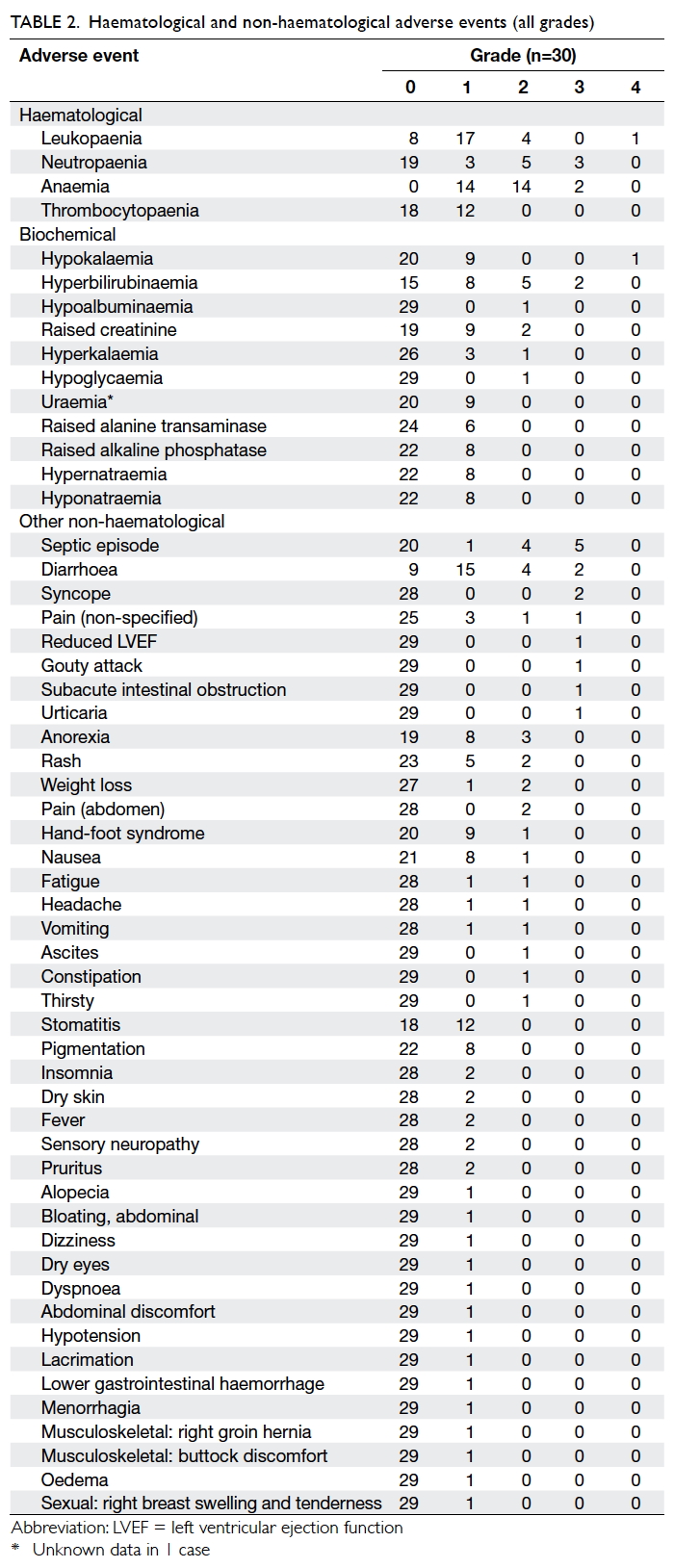
Table 2 presents the haematological and non-haematological
adverse events experienced during
treatment. Grade 3-4 haematological adverse events
included neutropaenia (n=3, 10%), leukopaenia
(1, 3.3%), and anaemia (2, 6.7%). Grade 3-4 non-haematological
adverse events included non-neutropaenic
septic episode (16.7%), diarrhoea
(6.7%), hyperbilirubinaemia (6.7%), syncope (6.7%),
reduced left ventricular ejection function (3.3%),
gouty attack (3.3%), hypokalaemia (3.3%), subacute
intestinal obstruction (3.3%), and urticaria (3.3%).
Of note, 10 patients developed a septic episode;
apart from one patient with grade 3 neutropaenic
fever, the others were non-neutropaenic. Of the
latter, four had grade 3 toxicity, four had grade 2,
and one had grade 1 toxicity; in one patient with
grade 2 toxicity, the infection was due to pulmonary
tuberculosis. This latter patient had a history of
ischaemic heart disease and he also developed grade
3 reduced left ventricular ejection function whilst on
S-1, as noted above.
Two out of 30 patients were found to be
positive for hepatitis B surface antigen. Of these two,
one was prescribed prophylactic antiviral therapy
and liver function remained normal apart from one
isolated episode of grade 2 hyperbilirubinaemia
that resolved spontaneously without other hepatic
dysfunction; the other patient did not receive
prophylactic antiviral therapy but his liver function
remained normal throughout S-1 therapy.
There were no treatment-related deaths.
Dose interruptions
Of the 30 patients, 17 (56.7%) were commenced on
a lower-than-intended dose of S-1. The reason for
reducing the first dose was: impaired renal function
(n=6; with creatinine clearance ranging from 41-48
mL/min), concern of toxicity (6), aged over 70
years (4), and borderline performance status (1);
four of these patients had further dose reduction in
subsequent cycles. Of the other 13 patients who had
the full S-1 dose in cycle 1, five had a dose reduction
from cycle 2 onwards.
Dose delays occurred in 12 (40%) patients;
these were due to delayed bone marrow recovery
(n=3), hypokalaemia (2, including 1 who also had delayed bone marrow recovery), diarrhoea (2), sepsis (2),
hypoglycaemia (1), impaired renal function (1),
reduced weight (1), and abdominal pain (1).
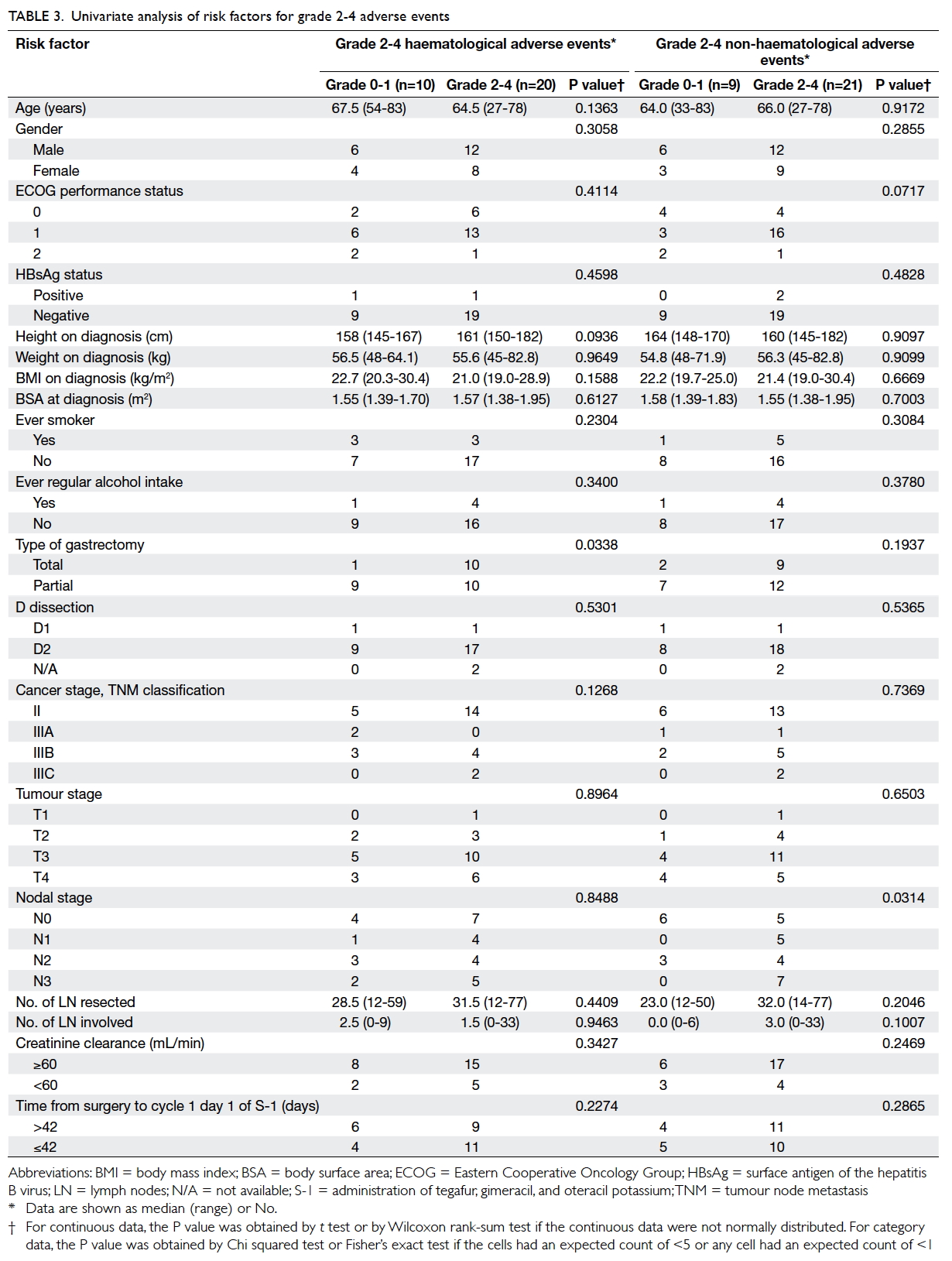
Risk factor analysis
Potential risk factors for adverse events were
assessed (Table 3). Univariate analysis showed that total gastrectomy was significantly associated with
haematological adverse events; 90.9% of patients
who had total gastrectomy in contrast to 52.6% of the
patients who had partial gastrectomy experienced
grade 2-4 adverse events (P=0.034). On univariate
analysis, nodal status was significantly associated
with non-haematological adverse events; 76.2% of
patients who experienced grade 2-4 adverse events
had nodal disease, while only 33% of those who
had grade 0-1 adverse events had nodal disease
(P=0.031).
Potential risk factors for earlier withdrawal
of S-1 and dose interruptions (dose reductions
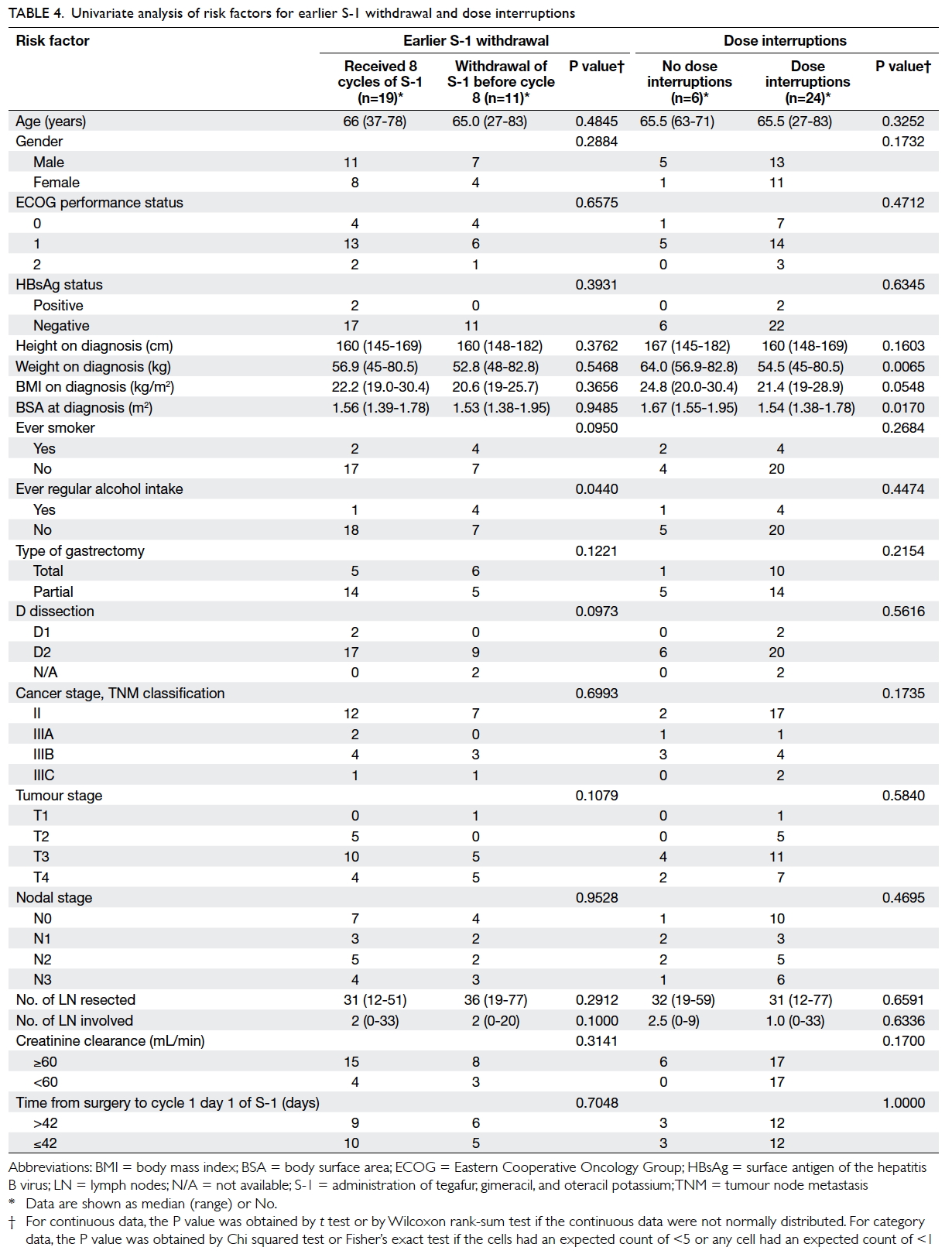
and/or dose delays) were assessed (Table 4). For earlier S-1 withdrawal, patients who had a history
of regular alcohol intake were significantly more
likely to have earlier treatment withdrawal than
non-drinkers (80% vs 28%; P=0.044), while ever-smokers
also had a tendency, though insignificant, for earlier withdrawal
than never-smokers (67% vs 29%; P=0.095). For dose
interruptions, univariate analysis showed lower
body weight (P=0.007) and lower BSA (P=0.017)
were significant associated factors, while lower body
mass index (BMI) also had an increased tendency, though insignificant,
for dose interruptions (P=0.055). The median body
weight, BMI, and BSA of those patients who had dose
interruptions were 54.5 kg, 21.4 kg/m2, and 1.54 m2,
respectively; the corresponding data for those
who did not require dose interruptions were 64.0 kg,
24.8 kg/m2, and 1.67 m2, respectively.
Discussion
Our study results indicate that adjuvant S-1
chemotherapy is feasible for our local patients after
curative resection of gastric cancer. With increased
awareness of the associated toxicity, S-1 can be
offered safely as standard adjuvant therapy. The
toxicities experienced by the studied patients were
in line with previous findings in Asian patients.4 12 13
Grade 3-4 haematological adverse events included
thrombocytopaenia and anaemia. Grade 3-4 non-haematological
adverse events that occurred in 5%
or more of the patients included non-neutropaenic
septic episode, diarrhoea, hyperbilirubinaemia, and
syncope.
Previous studies have investigated factors
associated with adverse events during S-1 therapy.
In a Korean study of 305 patients given adjuvant S-1
therapy,13 total gastrectomy was reported to be an
independent risk factor for grade 3-4 haematological
toxicities and age >65 years was an independent risk
factor for grade 3 non-haematological toxicities.
Independent risk factors for withdrawal and dose
reductions included age >65 years and male gender.
Total gastrectomy has also been reported to be
associated with a significantly greater risk of serious
adverse events in another study of Taiwanese gastric
cancer patients receiving adjuvant S-1.14
The reason for the higher incidence of serious
adverse events in patients who underwent total
gastrectomy whilst receiving S-1 treatment is
unknown. In an earlier study, a higher incidence of
adverse reactions was observed among patients who
received S-1 as adjuvant treatment after gastrectomy,
compared with those who had unresectable or
recurrent gastric cancer. The investigators suggested
the limitation in food intake soon after extensive
surgery as a possible cause of exacerbation of adverse
reactions such as anorexia and nausea, and proposed
that a delay in the start of drug administration after
gastrectomy may prevent such adverse events.15 In
another study, Taiwanese patients received palliative
S-1 for advanced gastric cancer at a median initial
dose of 37.0 mg/m2. Twelve patients had single-dosing
pharmacokinetic study on day 1, and seven
took part in a multiple-dosing pharmacokinetic
study on day 28. The results indicated that the
steady-state pharmacokinetics of 5-FU, CDHP
and Oxo could be predicted from single-dose
pharmacokinetic study. Six patients who underwent
gastrectomy had a similar pharmacokinetic profile
to another six patients who did not undergo
gastrectomy.16 Nonetheless, definitive data regarding
the pharmacokinetic profile of S-1 components
in patients who underwent different degrees of
gastrectomy are lacking.
Other factors that have been reported to be
associated with treatment-related adverse events
include low body mass and impaired renal function.16
These were supported by the present study in which
lower body weight, BMI, and BSA were associated
with an increased likelihood of dose interruptions.
Earlier reports have shown that impaired renal
function will reduce CDHP clearance and result in a
prolonged high concentration of 5-FU in plasma, and
thereby lead to more severe myelosuppression.8 17
Although impaired renal function was not identified
as a risk factor for adverse events in this study, it
has to be noted that all patients who had subnormal
creatinine clearance were offered S-1 at lower doses
at treatment initiation; this could have prevented the
occurrence of severe adverse events.
The present study was limited by its
retrospective nature and the limited number
of patients accrued. Although there is a lack of
information about patient co-morbidities, the
current data suggest that patients who had a history
of regular alcohol intake had an increased likelihood
of earlier treatment withdrawal. The survival data
are immature due to short follow-up. The findings,
however, lend support to a published report on the
acceptable toxicity profile and tolerability of S-1 as
adjuvant therapy after curative gastric surgery for
gastric cancer.4 13 14 Potential risk factors for severe
adverse events are suggested. Due to the small
sample size and retrospective nature of this study, a
prospective study with a larger patient population is
needed to confirm these findings. An awareness of
treatment-related adverse events as well as potential
associated factors may aid clinicians in managing
patients in whom S-1 therapy is planned, and
thereby improve treatment compliance and clinical
outcome.
Conclusions
Adjuvant S-1 therapy has a tolerable toxicity profile
among local patients who have undergone curative
resection for gastric cancer. For gastric cancer
patients in whom adjuvant S-1 therapy is planned,
those with identifiable risk factors should be closely
monitored for adverse events during treatment. This
may enable earlier intervention with supportive
therapy and optimise treatment outcome.
Acknowledgements
The authors gratefully acknowledge Mr Edward Choi
for his valuable statistical advice. We thank Drs Chi-ching
Law, Hoi-leung Leung, and Chung-kong Kwan
of the Department of Clinical Oncology, United
Christian Hospital for their support in this study.
Declaration
This study has been supported by an educational
grant from Taiho Pharma Singapore Pte. Ltd. W
Yeo has received honorarium for advisory role for
Eli Lilly, Novartis, Pfizer, and Bristol Myers Squibb
and has received research grant from Novartis over
the past 12 months. KO Lam has been an advisor
for Amgen, Eli Lilly, Roche, Sanofi-Aventis, and has
received honorarium from Amgen, Bayer, Eli Lilly,
Merck, Sanofi-Aventis, Taiho and research grant
from Bayer, Roche, and Taiho. Authors not named
here have disclosed no conflicts of interest.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin
DM. GLOBOCAN 2008: Cancer incidence and mortality
worldwide: IARC CancerBase No. 10. Lyon, France:
International Agency for Research on Cancer; 2010.
Available from: http://globocan.iarc.fr. Accessed 12 Oct
2011.
2. Gallo A, Cha C. Updates on esophageal and gastric cancers.
World J Gastroenterol 2006;12:3237-42. Crossref
3. GASTRIC (Global Advanced/Adjuvant Stomach Tumor
Research International Collaboration) Group, Paoletti
X, Oba K, et al. Benefit of adjuvant chemotherapy
for resectable gastric cancer: a meta-analysis. JAMA
2010;303:1729-37. Crossref
4. Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant
chemotherapy for gastric cancer with S-1, an oral
fluoropyrimidine. N Engl J Med 2007;357:1810-20. Crossref
5. Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine
and oxaliplatin for gastric cancer after D2 gastrectomy
(CLASSIC): a phase 3 open-label, randomised controlled
trial. Lancet 2012;379:315-21. Crossref
6. Shirasaka T, Shimamato Y, Ohshimo H, et al. Development
of a novel form of an oral 5-fluorouracil derivative (S-1)
directed to the potentiation of the tumor selective
cytotoxicity of 5-fluorouracil by two biochemical
modulators. Anticancer Drugs 1996;7:548-57. Crossref
7. Kubota T. The role of S-1 in the treatment of gastric cancer.
Br J Cancer 2008;98:1301-4. Crossref
8. Hirata K, Horikoshi N, Aiba K, et al. Pharmacokinetic
study of S-1, a novel oral fluorouracil antitumor drug. Clin
Cancer Res 1999;5:2000-5.
9. Chu QS, Hammond LA, Schwartz G, et al. Phase I and
pharmacokinetic study of the oral fluoropyrimidine S-1 on
a once-daily-for-28-day schedule in patients with advanced
malignancies. Clin Cancer Res 2004;10:4913-21. Crossref
10. Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic
study of S-1 plus cisplatin in patients with advanced gastric
carcinoma. J Clin Oncol 2005;23:6957-65. Crossref
11. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL,
Trotti A. American Joint Committee on Cancer. Cancer
Staging Manual. 7th ed. Chicago: Springer; 2010.
12. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0:
development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol
2003;13:176-81. Crossref
13. Jeong JH, Ryu MH, Ryoo BY, et al. Safety and feasibility
of adjuvant chemotherapy with S-1 for Korean patients
with curatively resected advanced gastric cancer. Cancer
Chemother Pharmacol 2012;70:523-9. Crossref
14. Chou WC, Chang CL, Liu KH, et al. Total gastrectomy
increases the incidence of grade III and IV toxicities in
patients with gastric cancer receiving adjuvant TS-1
treatment. World J Surg Oncol 2013;11:287. Crossref
15. Kinoshita T, Nashimoto A, Yamamura Y, et al. Feasibility
study of adjuvant chemotherapy with S-1 (TS-1; tegafur,
gimeracil, oteracil potassium) for gastric cancer. Gastric
Cancer 2004;7:104-9. Crossref
16. Chen JS, Chao Y, Hsieh RK, et al. A phase II and
pharmacokinetic study of first line S-1 for advanced
gastric cancer in Taiwan. Cancer Chemother Pharmacol
2011;67:1281-9. Crossref
17. Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase
II study of S-1, a novel oral derivative of 5-fluorouracil, in
advanced gastric cancer. For the S-1 Cooperative Gastric
Cancer Study Group. Oncology 2000;58:191-7. Crossref