Hong Kong Med J 2017 Feb;23(1):19–27 | Epub 24 Oct 2016
DOI: 10.12809/hkmj154754
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Ductal carcinoma in situ of breast: detection and
treatment pattern in Hong Kong
TK Yau, FHKCR, FHKAM (Radiology);
Amy Chan, BSc, MPhil;
Polly SY Cheung, FRACS, FHKAM (Surgery)
Hong Kong Breast Cancer Foundation, 22/F, Jupiter Tower, No. 9, Jupiter
Street North Point, Hong Kong
Corresponding author: Dr TK Yau (research@hkbcf.org)
Abstract
Introduction: The treatment of ductal carcinoma
in situ has been widely reported in the western and
other Asian countries, but the relevant data in Hong
Kong are relatively limited. This study aimed to
evaluate the latest detection and treatment pattern
for ductal carcinoma in situ in Hong Kong so as to
guide planning of future service provision.
Methods: This was a retrospective case series study.
A total of 573 patients who registered with the Hong
Kong Breast Cancer Registry, and were diagnosed
and treated in Hong Kong from January 2001 to
December 2011 were reviewed.
Results: Compared with invasive breast cancer
patients, patients with ductal carcinoma in situ
were younger (median, 48.6 vs 50.3 years; P<0.001),
had a higher education level (P<0.001), had a
higher total monthly family income (P<0.001), and
more common breast-screening habits (P<0.001).
Significantly more patients with ductal carcinoma
in situ underwent breast-conserving surgery than
their invasive cancer counterparts (55.8% vs 36.7%;
P<0.001). The percentage of screen-detected ductal
carcinoma in situ was relatively lower than that
reported in other studies, but was still much higher
than that in invasive breast cancer patients (29.0% vs
4.7%; P<0.001). Screen-detected patients with ductal
carcinoma in situ tended to choose a private hospital
instead of a public hospital for treatment (P=0.05)
and to undergo breast-conserving surgery (P=0.02).
With a median follow-up of 3 years, the crude
local recurrence rate after mastectomy and breast-conserving
surgery was 0.4% and 3.3%, respectively;
44% of recurrent tumours had developed invasive
components. No regional recurrence, distant
recurrence, or cancer-related deaths were recorded.
Conclusions: In the absence of a population-based
breast screening programme in Hong Kong, ductal
carcinoma in situ is more frequently found in the
higher social classes and managed in the private
sector. The clinical outcome of ductal carcinoma in
situ is excellent and more than half of the patients can
be successfully managed with breast-conserving
surgery.
New knowledge added by this study
- This is the largest comprehensive study to evaluate the pattern of care for patients with ductal carcinoma in situ (DCIS) in Hong Kong.
- Further studies are needed to evaluate the long-term clinical outcome of DCIS in Hong Kong.
Introduction
Ductal carcinoma in situ (DCIS) of the breast
is a non-invasive, pre-cancerous lesion that
was uncommon prior to the widespread use of
mammography (MMG) screening; it is traditionally
treated by mastectomy (MTX) with cure rates
approaching 100%.1 The high incidence rate and
mortality rate of breast cancer in women2 has
led to the setting up of population-based breast
cancer screening programmes by government in 34
countries.3 4 5 6 One of the results of the popularity of
breast screening is the rise in the detected incidence
of DCIS.7 Some western studies revealed that DCIS
constituted approximately 10% to 40% of lesions
detected by MMG screening.8 In Asia, following
the pilot Singapore Breast Screening Project, the
diagnosis of DCIS also increased markedly.9 Screen-detected
DCIS showed a better clinical profile such
as smaller size and higher chance of being treated by
breast-conserving surgery (BCS).9 Early detection of
breast cancer at this stage offers the best opportunity
for curative treatment.10
The treatment of DCIS has been widely
studied and reported in the western and other
Asian countries.11 12 13 14 15 16 Although there have been no
prospective randomised trials to compare MTX
with BCS for DCIS, BCS has been widely accepted
as an alternative treatment,17 especially for small
mammographically detected lesions. In Hong
Kong, however, data on DCIS are relatively limited.
The Hong Kong Cancer Registry has only started
to release basic data of annual incidence and age
distribution of DCIS since 2009. In 2012, 3508 women
in Hong Kong were diagnosed with invasive breast
cancer and 477 women were diagnosed with DCIS18
that constituted only 12.0% of the total number of
breast cancer patients diagnosed. This incidence of
DCIS was relatively low compared with the 20.7%
in the United States.14 Since our presentation and
treatment pattern of DCIS are likely different to that
in other countries, it is necessary to examine the
particular pattern of care of DCIS in Hong Kong to
better understand this disease.
The aim of this study was to evaluate the
latest detection and treatment pattern for DCIS in
Hong Kong so as to guide planning of future service
provision.
Methods
The Hong Kong Breast Cancer Registry (HKBCR)
was first established in 2007 by the Hong Kong
Breast Cancer Foundation as a data collection and
monitoring system for breast cancer in Hong Kong.
The HKBCR aims to collect and analyse data from
all Hong Kong breast cancer patients to obtain
comprehensive information about demographics,
risk exposures, treatments, clinical outcomes,
and psychosocial impact on patients. It is the first
population-wide, cancer-specific registry for breast
cancer patients in Hong Kong and has been a member
of the International Association of Cancer Registries
since 2011, providing international standard data
management and accuracy.
Between 2008 and 2011, a total of 5393 patients
with a history of in-situ or invasive breast cancers
were registered with the HKBCR on a voluntary basis.
Of these patients, 2539 (47.1%) and 2854 (52.9%)
were recruited from private clinics or hospitals and
public hospitals, respectively. Demographics and
risk exposure data were collected from these patients
by questionnaire; clinical characteristics, detection
methods, diagnostic methods, disease stage,
histopathological profile, treatment modalities, and
clinical outcome data were extracted from their
medical records.19 Data analysis was carried out in
December 2014.
Inclusion criteria for this study were as follows:
female patient being diagnosed and treated in
Hong Kong from January 2001 to December 2011;
pure DCIS with no invasive element in ipsilateral
or contralateral breast at the time of diagnosis;
definitive surgery performed; complete pathology
details available; if axillary node sampling/dissection
was performed, the nodal status must be negative;
and no prior neoadjuvant treatment administered.
Overall, 573 patients, including 16 synchronous
patients with bilateral DCIS, from the HKBCR
fulfilled the above criteria for further study.
For comparison purposes, the records of
female patients with invasive breast cancer diagnosed
and treated in Hong Kong during the same period
were also extracted from HKBCR. Altogether,
1611 invasive breast carcinoma patients with 20
synchronous bilateral patients were retrieved for
data analysis.
In this study, local recurrence was defined as
the reappearance of cancer, invasive or non-invasive,
in the treated breast or chest wall before or at the time
of regional or distant metastases. All events were
measured from the date of the definitive surgery.
Descriptive statistics were used to describe the
patterns of demographic and pathological features.
Statistical significance was tested using Chi squared
tests for categorical variables. The Kaplan-Meier
method was applied to analyse the local recurrence
estimation. All statistical tests were two-sided and
performed at the 0.05 level of significance (P value).
The Statistical Package for the Social Sciences
(Windows version 19.0; SPSS Inc, Chicago [IL], US)
was used for all statistical analyses.
The project was approved by respective Institutional Review Board and Ethics Committee of the following hospitals: Hong Kong Sanatorium & Hospital, Hong Kong Baptist Hospital, Hong Kong Adventist Hospital, Princess Margaret Hospital, United Christian Hospital, Prince of Wales Hospital, Queen Mary Hospital, Pamela Youde Nethersole Eastern Hospital, Pok Oi Hospital, North District Hospital, Tuen Mun Hospital, and Yan Chai Hospital. All participants provided written informed consent.
Results
Of the 573 patients with DCIS of breast, the majority
(74.9%) were diagnosed and treated between 2006
and 2011. A similar distribution was found in the
1611 patients with invasive breast cancer.
Table 1 compares the demographic characteristics of DCIS and invasive breast cancer
patients. The results indicate that DCIS patients were
significantly younger (median, 48.6 vs 50.3 years;
P<0.001), had a higher education level (matriculation
or above, 34.0% vs 15.0%; P<0.001), were more likely
to be working (61.1% vs 55.8%; P<0.001), and had a
higher total monthly family income of HK$30 000
or above (32.6% vs 16.6%; P<0.001). More DCIS
patients had regular breast screening habits in the
form of self-examination (monthly: 23.6% vs 21.8%;
P<0.001), clinical breast examination (yearly: 41.5%
vs 27.3%; P<0.001), MMG screening (yearly: 24.1%
vs 10.6%; P<0.001), and breast ultrasound screening
(yearly: 20.9% vs 10.2%; P<0.001). Patients with DCIS
had a much higher chance of being asymptomatic at
diagnosis (ie screen-detected) than their invasive
breast cancer counterparts (29.0% vs 4.7%; P<0.001).
Significantly more DCIS patients underwent BCS
than their invasive cancer counterparts (55.8% vs
36.7%; P<0.001). Among those treated by BCS, DCIS
patients had a similar chance of receiving adjuvant
radiotherapy as the invasive cancer patients (94.1%
vs 93.8%). As expected, only very few (8.7%) DCIS
patients required adjuvant radiotherapy after MTX.
Although DCIS patients do not require systemic
adjuvant therapy, some may be prescribed hormone
therapy as chemoprevention. In our study, only a
small percentage of DCIS patients received hormone
therapy and the pattern was similar after BCS
or MTX (19.4% after BCS and 17.0% after MTX;
P<0.001).
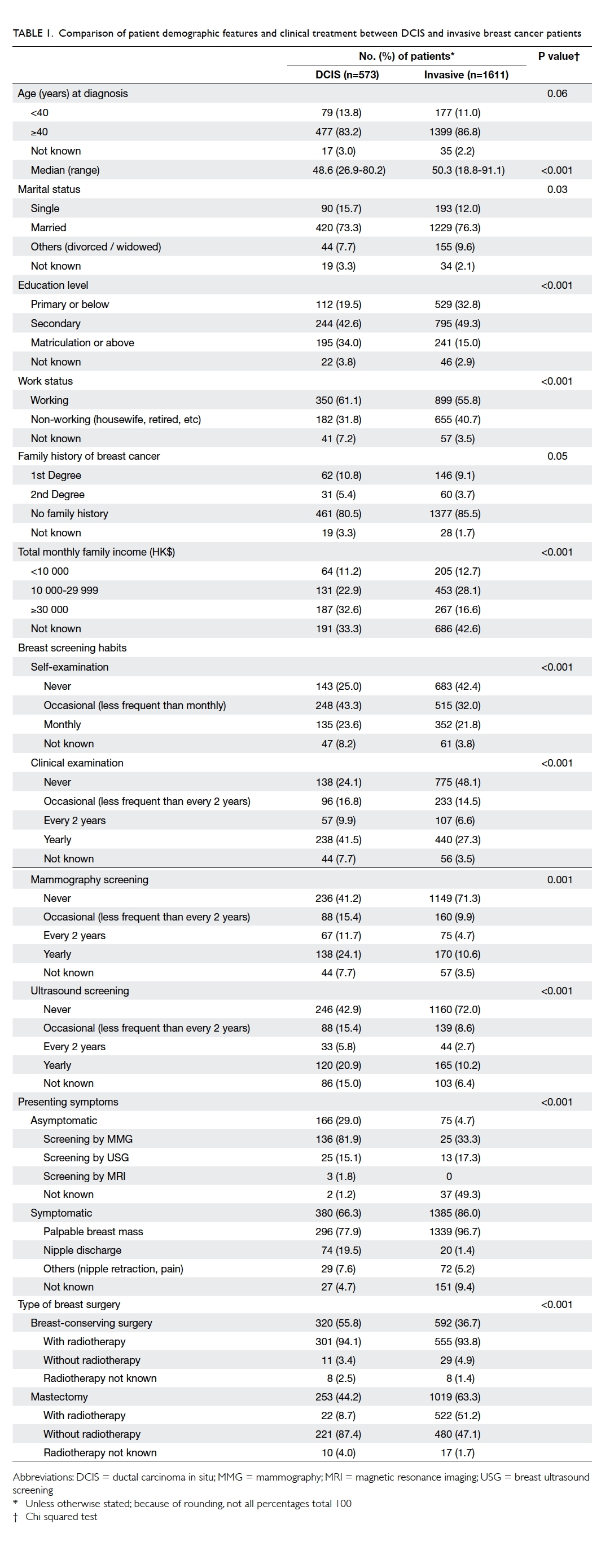
Table 1. Comparison of patient demographic features and clinical treatment between DCIS and invasive breast cancer patients
Table 2 shows the patient demographics, and clinical and pathological characteristics of
screen-detected (asymptomatic) and self-detected
(symptomatic) DCIS in Hong Kong. There was no
significant difference in the median age between
these subgroups (49.1 vs 48.5 years; P=0.23). The
screen-detected subgroup had a significantly higher
education level (matriculation or above, 42.7% vs
29.6%; P=0.01), higher total monthly family income
of HK$30 000 or above (45.7% vs 26.8%; P=0.01), and
underwent more regular clinical breast examination
(yearly: 49.4% vs 35.5%; P<0.001), MMG (every 2
years: 22.0% vs 7.0%; P<0.001), and breast ultrasound
screening (every 2 years: 11.6% vs 3.4%; P<0.001).
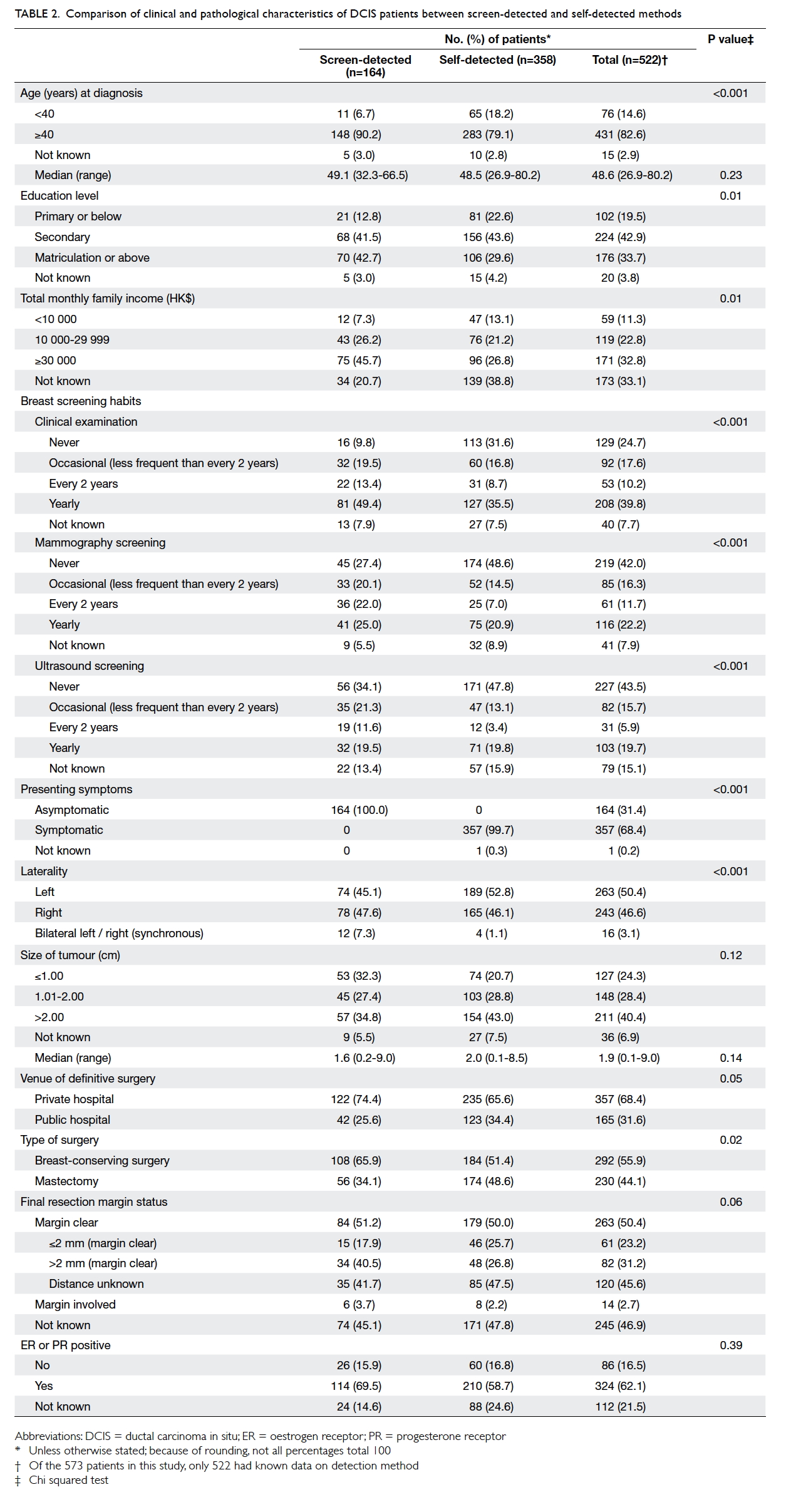
Table 2. Comparison of clinical and pathological characteristics of DCIS patients between screen-detected and self-detected methods
Among the DCIS patients, 28.6% (164/573)
were screen-detected: since MMG screening is not
usually recommended in younger women, only 14.5%
(11/76) of DCIS in patients aged below 40 years were
screen-detected compared with 34.3% (148/431) in
patients aged above 40 years. Irrespective of the type
of presentation, two thirds or more of DCIS patients
chose to have surgery at a private hospital and the
screen-detected subgroup had an even higher
tendency to do so (74.4% vs 65.6%; P=0.05). Although
there was a trend of finding smaller lesions in the
screen-detected subgroup, there was no significant
difference between the two subgroups in tumour
size (median: 1.6 cm vs 2.0 cm; P=0.14). Despite this
finding, screen-detected DCIS patients had a higher
chance of being treated by BCS than symptomatic
patients (65.9% vs 51.4%; P=0.02) [Table 2].
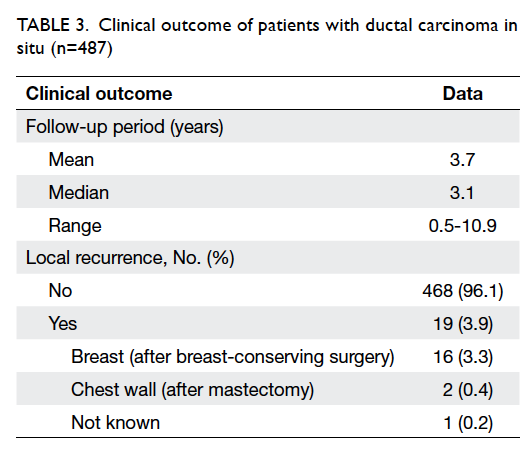
Among 573 patients with DCIS, clinical
outcome data were available for 487 patients only.
With a median follow-up of 3.1 (range, 0.5-10.9)
years, the early clinical outcome was very good and
compatible with other series. The overall crude local
recurrence rate in DCIS patients was 3.9% (19 in 487
patients) and, as expected, there was a significant
difference between MTX and BCS patients (0.4%
vs 3.3%) [Table 3]. Of the 18 patients with known pathology at recurrence, eight (44.4%) had developed
invasive components. Of the 16 BCS patients, 11
(68.8%) underwent salvage MTX at recurrence.
Overall, by 6 years, the projected local recurrence
rates after BCS were similar for DCIS patients and
invasive breast cancer patients (log rank, P=0.21; Fig). No regional recurrence, distant recurrence,
or cancer-related death were observed in the DCIS
patients.
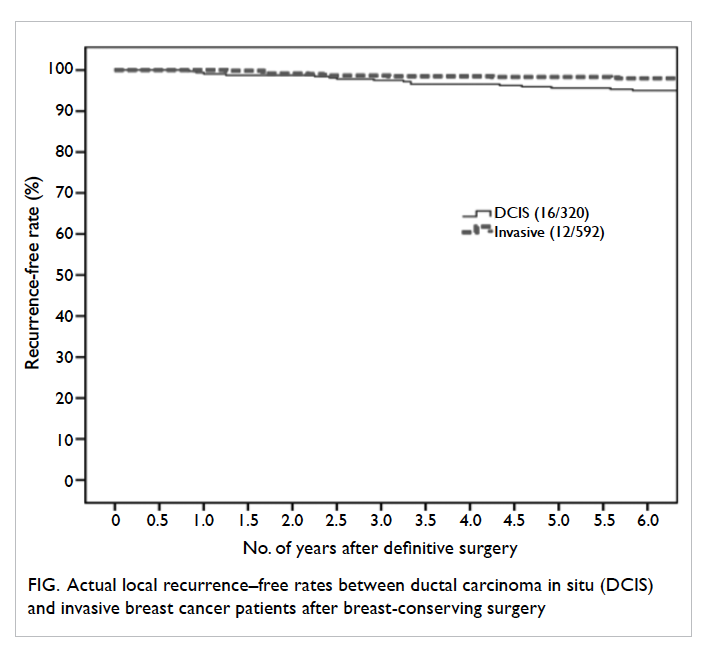
Figure. Actual local recurrence–free rates between ductal carcinoma in situ (DCIS) and invasive breast cancer patients after breast-conserving surgery
Discussion
Ductal carcinoma in situ was relatively uncommon
in western countries until the widespread use of
mass breast screening. There is strong evidence
that the recent rise in DCIS incidence is related to
the popularity of breast screening. Since there is no
government-funded breast screening programme in
Hong Kong, our study showed that only 29.0% of the
DCIS in Hong Kong was first detected by screening,
significantly lower than the 80% screen-detected rate
in DCIS of other studies.20 21 22 23 24 Our data showed that
these DCIS patients in general had a higher monthly
family income and higher level of education than
their invasive cancer counterparts and this may
contribute to their higher acceptance of self-funded
breast screening, higher breast cancer awareness,
and hence better chance of detecting breast cancer at
an early stage. Not surprisingly, the use of BCS was
also significantly more popular in the DCIS patients
compared with their invasive cancer counterparts
(55.8% vs 36.7%; P<0.001). Our results were also
consistent with a previous local study that reported
the performance of opportunistic breast screening
in local well women clinics and showed that breast
screening could achieve a higher cancer detection
rate and detect the cancer at an early stage.25
In contrast, the profile of screen-detected
and self-detected (ie symptomatic) DCIS patients
showed more similarities than differences. The overall
tumour size was not significantly different between
these subgroups, although lesions of >2 cm were less
common in the screen-detected patients (34.8%
vs 43.0%; P=0.12). Since there is no population-based
breast screening programme in Hong Kong,
these opportunistic breast screenings performed in
various laboratories may have inherent limitations.
Overseas studies have shown a considerably higher
sensitivity in organised population-based screening
than in opportunistic screening,24 although a large-scale
local self-referred breast screening centre
reported comparable performance.25
Our study showed a high acceptance of BCS for
management of DCIS in Hong Kong and nearly all
(94.1%) BCS patients also underwent postoperative
radiotherapy. Although prior randomised studies
have demonstrated the benefit of postoperative
radiotherapy in reducing both invasive and non-invasive
recurrence of DCIS after BCS, much
effort has been put into identifying a low-risk
subgroup in whom postoperative radiotherapy can
be safely omitted.26 The Van Nuys prognostic index
(VNPI)—a retrospectively derived risk classification
that combines tumour size, margin width, and
pathological classification—was developed to select
this low-risk group.27 Nonetheless, perhaps due
to contradictory findings from other studies that
reported a much higher local failure rate in the
VNPI low-risk subgroup,28 it is apparent that most
clinicians in Hong Kong had reservations when
applying the VNPI to their DCIS patients.
Although tamoxifen after local excision for
DCIS has been shown to reduce the risk of recurrent
DCIS in the ipsilateral breast (hazard ratio=0.75; 95%
confidence interval [CI], 0.61-0.92) and contralateral
breast (relative risk=0.50; 95% CI, 0.28-0.87)29 and
over 60% of our patients had positive hormonal
receptors, less than 20% DCIS patients in Hong Kong
actually received tamoxifen as chemoprevention.30
It is likely related to the concern about side-effects
(particularly the small risk of endometrial cancer)
and the lack of overall survival benefit as shown by
the Cochrane systematic review and meta-analysis.29
The lack of survival benefit is consistent with the
clinical experience that most new lesions detected
during follow-up surveillance are highly treatable.
As expected, the local recurrence rate after
MTX was very low (0.4%) in these DCIS patients;
it should be noted that 8.7% had received adjuvant
radiotherapy, probably because of close resection
margins. For DCIS patients treated by BCS, the
crude local recurrence rate in our study was 3.3%
(16 in 320 patients) and was quite similar to the
long-term experience in another regional hospital
(Pamela Youde Nethersole Eastern Hospital) in Hong
Kong. In their analysis of 155 DCIS patients treated
by BCS and radiotherapy, after a 10-year median
follow-up, the crude local recurrence rate was 5.8%
(unpublished data of Pamela Youde Nethersole Eastern Hospital). Our study did
not capture the data on the mode of detection of
local recurrences but another local study reported
that only 43% of in-breast recurrences could be
first detected by surveillance breast imaging; the
rest presented with either nipple discharge or a
palpable mass.30 Hence, patients should be advised
not to become overly dependent on breast imaging
to detect early recurrences. Although there were no
cancer-related deaths in these DCIS patients, 44%
of local recurrences in this study contained invasive
components that may still necessitate systemic
treatment in addition to further salvage surgery.
This study provides the first comprehensive
analysis of the pattern of care of DCIS in
Hong Kong. The strength of this analysis is the
comprehensiveness compared with other cancer
registries in data collection on epidemiological,
pathological, and treatment characteristics for
breast cancer. Nonetheless, data from HKBCR
might not be representative of all breast cancers in
Hong Kong since a higher proportion of patients
were recruited from private hospitals or clinics
than public hospitals in Hong Kong. Since the data
collection was done on a voluntary basis and only
started in 2008, some clinical outcome data may be
missing (approximately 10% of DCIS patients) and
the follow-up duration remains relatively short,
and may not represent the whole local population.
There was also a high proportion of missing data
for family income in the two internal comparisons.
Although the distribution of age at diagnosis in our
study did not deviate too far from that reported in
the Hong Kong Cancer Registry (a population-based
registry; Table 4), older age-groups, especially those aged 70 years and above, were under-represented
in the present study. Furthermore, we did not have
information on education, occupation, and family
income to enable comparison of socio-economic
backgrounds.
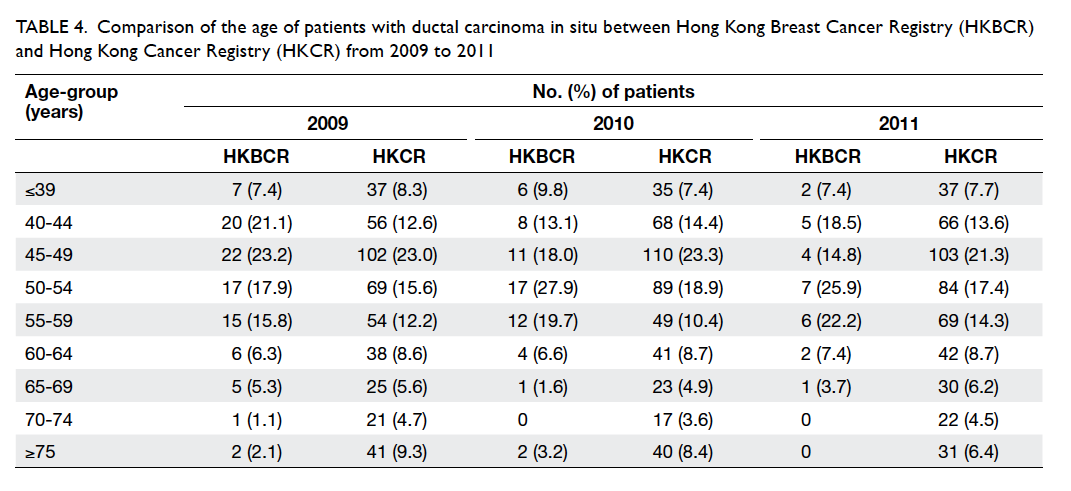
Table 4. Comparison of the age of patients with ductal carcinoma in situ between Hong Kong Breast Cancer Registry (HKBCR) and Hong Kong Cancer Registry (HKCR) from 2009 to 2011
Conclusions
Ductal carcinoma in situ in Hong Kong appears to be
a more prevalent disease in the higher social classes
with a tendency to be managed in the private sector.
More than half of DCIS patients can be successfully
treated with BCS and the early outcome is excellent
and comparable with overseas studies.9 16 Further
studies are needed to examine the long-term clinical
outcome of DCIS in Hong Kong.
Acknowledgements
The authors thank all of the patients/survivors,
doctors, and research staff who have participated
in HKBCR to facilitate data collection at clinics and
hospitals throughout the territory. The authors also
acknowledge the following steering committee members: Prof
Emily Chan, Dr John Chan, Dr Keeng-Wai Chan,
Dr Miranda Chan, Dr Sharon Chan, Dr Foon-Yiu
Cheung, Dr Peter Choi, Dr Josette Chor, Ms Yvonne
Chua, Ms Doris Kwan, Dr Wing-Hong Kwan, Dr
Stephen Law, Dr Simon Leung, Dr Lawrence Li, Dr
Janice Tsang, Dr Gary Tse, Mrs Cecilia Tseung, Dr
Tung Yuk, Ms Lorna Wong, Dr Ting-Ting Wong,
Dr CC Yau, Prof Winnie Yeo, and Prof Benny Zee
for providing guidance for the development of the
HKBCR.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Fortunato L, Poccia I, de Paula U, Santini E. Ductal
carcinoma in situ: what can we learn from clinical trials?
Int J Surg Oncol 2012;2012:296829.
2. International Agency for Research on Cancer. Globocan
2012: All cancers (excluding non-melanoma skin cancer)—estimated incidence, mortality and prevalence worldwide in 2012. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 2 Apr 2015.
3. Kwong A, Cheung PS, Wong AY, et al. The acceptance and
feasibility of breast cancer screening in the East. Breast
2008;17:42-50. Crossref
4. Biesheuvel C, Weigel S, Heindel W. Mammography
screening: evidence, history and current practice in
Germany and other European countries. Breast Care
(Basel) 2001;6:104-9. Crossref
5. Cheung PS. Global and local status of breast cancer
and screening. Paper presented at Hong Kong Breast Cancer
Foundation Seminar; 2013 Apr 14; Hong Kong.
6. Hellquist BN, Duffy SW, Abdsaleh S, et al. Effectiveness of
population-based service screening with mammography
for women ages 40 to 49 years: evaluation of the Swedish
Mammography Screening in Young Women (SCRY)
cohort. Cancer 2011;117:714-22. Crossref
7. Leonard GD, Swain SM. Ductal carcinoma in situ,
complexities and challenges. J Natl Cancer Inst
2004;96:906-20. Crossref
8. Delaney G, Ung O, Bilous M, Cahill S, Greenberg M,
Boyages J. Ductal carcinoma in situ. Part I: definition and
diagnosis. Aust N Z J Surg 1997;67:81-93. Crossref
9. Chuwa EW, Yeo AW, Koong HN, et al. Early detection of
breast cancer through population-based mammographic
screening in Asian women: a comparison study between
screen-detected and symptomatic breast cancers. Breast J
2009;15:133-9. Crossref
10. Morrow M, Strom EA, Bassett LW, et al. Standard for
the management of ductal carcinoma in situ of the breast
(DCIS). CA Cancer J Clin 2002;52:256-76. Crossref
11. Irvine T, Fentiman IS. Biology and treatment of ductal
carcinoma in situ. Expert Rev Anticancer Ther 2007;7:135-45. Crossref
12. Siziopikou KP. Ductal carcinoma in situ of the breast:
current concepts and future directions. Arch Pathol Lab
Med 2013;137:462-6. Crossref
13. Jiveliouk I, Corn B, Inbar M, Merimsky O. Ductal
carcinoma in situ of the breast in Israeli women treated by
breast-conserving surgery followed by radiation therapy.
Oncology 2009;76:30-5. Crossref
14. Virnig BA, Torchia MT, Jarosek SL, Durham S, Tuttle
TM. Data Points #14: Use of endocrine therapy following diagnosis of
ductal carcinoma in situ or early invasive breast cancer. Rockville (MD): Agency for Healthcare
Research and Quality; 2011-2012.
15. Tan KB, Lee HY, Putti TC. Ductal carcinoma in situ of the
breast in Singapore: recent trends and clinical implications.
ANZ J Surg 2002;72:793-7. Crossref
16. Chuwa EW, Tan VH, Tan PH, Yong WS, Ho GH, Wong
CY. Treatment for ductal carcinoma in situ in an Asian
population: outcome and prognostic factors. ANZ J Surg
2008;78:42-8. Crossref
17. Mouw KW, Harris JR. Irradiation in early-stage breast
cancer: conventional whole-breast, accelerated partial-breast,
and accelerated whole-breast strategies compared.
Oncology (Williston Park) 2012;26:820-30.
18. Hong Kong Cancer Registry. Leading cancer sites in Hong
Kong in 2012. Available from: http://www3.ha.org.hk/cancereg/rank_2012.pdf. Accessed 15 Jan 2015.
19. Cheung P, Hung WK, Cheung C, et al. Early data from the
first population-wide breast cancer-specific registry in
Hong Kong. World J Surg 2012;36:723-9. Crossref
20. Chua MS, Mok TS, Kwan WH, Yeo W, Zee B. Knowledge,
perceptions, and attitudes of Hong Kong Chinese women
on screening mammography and early breast cancer
management. Breast J 2005;11:52-6. Crossref
21. Schouten van der Velden AP, Peeters PH, Koot VC,
Hennipman A. Clinical presentation and surgical quality
in treatment of ductal carcinoma in situ of the breast. Acta
Oncol 2006;45:544-9. Crossref
22. Solin LJ, Fourquet A, Vicini FA, et al. Long-term outcome
after breast-conservation treatment with radiation for
mammographically detected ductal carcinoma in situ of
the breast. Cancer 2005;103:1137-46. Crossref
23. Habermann EB, Abbot A, Parsons HM, Virnig BA, Al-Refaie
WB, Tuttle TM. Are mastectomy rates really increasing in
the United States? J Clin Oncol 2010;28:3437-41. Crossref
24. Bihrmann K, Jensen A, Olsen AH, et al. Performance of
systematic and non-systematic (‘opportunistic’) screening
mammography: a comparative study from Denmark. J
Med Screen 2008;15:23-6. Crossref
25. Lui CY, Lam HS, Chan LK, et al. Opportunistic breast
cancer screening in Hong Kong; a revisit of the Kwong
Wah Hospital experience. Hong Kong Med J 2007;13:106-13.
26. Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and
radiation therapy for the treatment of intraductal breast
cancer: findings from National Surgical Adjuvant Breast
and Bowel Project B-17. J Clin Oncol 1998;16:441-52.
27. Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic
index for ductal carcinoma in situ of the breast. Cancer
1996;77:2267-74. Crossref
28. de Mascarel I, Bonichon F, MacGrogan G, et al. Application
of the Van Nuys prognostic index in a retrospective series of
367 ductal carcinomas in situ of the breast examined by
serial macroscopic sectioning: practical considerations.
Breast Cancer Res Treat 2000;61:151-9. Crossref
29. Staley H, McCallum I, Bruce J. Postoperative tamoxifen
for ductal carcinoma in situ: Cochrane systematic review
and meta-analysis. Breast 2014;23:546-51. Crossref
30. Yau TK, Sze H, Soong IS, et al. Surveillance mammography
after breast conservation therapy in Hong Kong:
effectiveness and feasibility of risk-adapted approach.
Breast 2008;17:132-7. Crossref