Hong Kong Med J 2016 Dec;22(6):556–62 | Epub 30 Sep 2016
DOI: 10.12809/hkmj154710
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Sperm retrieval rate and pregnancy rate in
infertile couples undergoing in-vitro fertilisation
and testicular sperm extraction for non-obstructive
azoospermia in Hong Kong
Jennifer KY Ko, FHKAM (Obstetrics and Gynaecology)1;
Joyce Chai, FHKAM (Obstetrics and Gynaecology)1;
Vivian CY Lee, FHKAM (Obstetrics and Gynaecology)1;
Raymond HW Li, FRCOG, FHKAM (Obstetrics and Gynaecology)1;
Estella Lau, PhD1;
KL Ho, FRCSEd (Urol), FHKAM (Surgery)2,3;
PC Tam, FRCSEd (Urol), FHKAM (Surgery)2,3;
William SB Yeung, PhD1;
PC Ho, MD1;
Ernest HY Ng, MD1
1 Department of Obstetrics and Gynaecology, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
2 Division of Urology, Department of Surgery, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
3 Private practice
Corresponding author: Dr Jennifer KY Ko (jenko@hku.hk)
Abstract
Objective: There are currently no local data on
the sperm retrieval and pregnancy rates in in-vitro
fertilisation and testicular sperm extraction cycles,
especially with regard to the presence of genetic
abnormalities. This study aimed to determine the
sperm retrieval and pregnancy rates in infertile couples
who underwent in-vitro fertilisation and testicular
sperm extraction for non-obstructive azoospermia.
Methods: This retrospective case series was
conducted at a tertiary assisted reproduction unit in
Hong Kong. Men with non-obstructive azoospermia
who underwent in-vitro fertilisation and testicular
sperm extraction between January 2001 and
December 2013 were included. The main outcome
measures were sperm retrieval and pregnancy rates.
Results: During the study period, 89 men with
non-obstructive azoospermia underwent in-vitro
fertilisation and testicular sperm extraction. Sperm
was successfully retrieved in 40 (44.9%) men.
There was no statistically significant difference in
the sperm retrieval rate of those with karyotypic
abnormalities (2/5, 40.0% vs 28/61, 45.9%; P=1.000)
and AZFc microdeletion (3/6, 50.0% vs 28/61, 45.9%;
P=1.000) compared with those without. Sperms
were successfully retrieved in patients who had
mosaic Klinefelter syndrome (2/3, 66.7%) but not in
the patient with non-mosaic Klinefelter syndrome.
No sperms were found in men with AZFa or AZFb
microdeletions. Pregnancy test was positive in 15
(16.9%) patients and the clinical pregnancy rate was 13.5% (12/89) per cycle. The clinical pregnancy rate per
transfer was 34.3% (12/35).
Conclusions: The sperm retrieval rate and clinical
pregnancy rate per initiated cycle in men undergoing
in-vitro fertilisation and testicular sperm extraction
in our unit were 44.9% and 13.5%, respectively. No
sperms could be retrieved in the presence of AZFa
and AZFb microdeletions, but karyotype and AZFc
microdeletion abnormalities otherwise did not
predict the success of sperm retrieval in couples
undergoing in-vitro fertilisation and testicular
sperm extraction. Genetic tests are important prior
to testicular sperm extraction for patient selection
and genetic counselling.
New knowledge added by this study
- Our study provides important local data for counselling of men with non-obstructive azoospermia. The sperm retrieval rate and clinical pregnancy rate per cycle in men undergoing in-vitro fertilisation and testicular sperm extraction in our unit were 44.9% and 13.5%, respectively.
- There was no statistically significant difference in the sperm retrieval and pregnancy rates in those with karyotypic abnormalities and AZFc microdeletion compared with those without. Sperms, however, were not found in men with AZFa or AZFb microdeletions.
- Although karyotype abnormalities and AZFc microdeletion did not affect the sperm retrieval and pregnancy rates in couples undergoing in-vitro fertilisation and testicular sperm extraction, karyotype and Y-microdeletion should be checked in men with non-obstructive azoospermia. The risk of vertical transmission of genetic abnormalities should be discussed and couples should be offered appropriate genetic counselling before treatment.
Introduction
Male-factor infertility is involved in about half of all infertile
couples who seek assisted reproduction treatment.1
The advent of in-vitro fertilisation (IVF) with
intracytoplasmic sperm injection (ICSI) has allowed
many men with severe male factor to have their own
genetic child.2 The development of surgical sperm
retrieval techniques by testicular sperm extraction
(TESE) has extended the possibility of fatherhood to
those with non-obstructive azoospermia (NOA).3
The reported sperm retrieval rate from TESE
varies in different studies due to inclusion of different
populations, but is generally quoted to be around
50%.4 Nevertheless TESE is invasive. In a recent
retrospective cohort study by Vloeberghs et al,5
only one (14.3%) out of seven men undergoing IVF-TESE
eventually became a biological father. Studies
have also shown a high prevalence of chromosomal
abnormalities and Y-microdeletion in infertile men
with NOA or severe oligozoospermia.6 7 8 9 These
genetic abnormalities can potentially be transmitted
vertically resulting in a child with sex aneuploidies
or boys with the same Y-microdeletion.10 Guidelines
from the American Society for Reproductive
Medicine, American Urological Association,
Canadian Urological Association, and International
Federation of Fertility Societies are unanimous in
suggesting that all men with NOA due to testicular
failure should be offered genetic testing to exclude
chromosomal abnormalities and Y chromosome
microdeletions, and that genetic counselling be
offered if an abnormality is detected.11 12 13 14
There are currently no local data on the sperm
retrieval and pregnancy rates in IVF-TESE cycles,
especially with regard to the presence of genetic
abnormalities. Such information is invaluable to
couples in pretreatment counselling and could
affect their decision about treatment options. In
this study, we determined the sperm retrieval and
pregnancy rates in infertile couples who underwent
IVF-TESE for NOA.
Methods
The study was a retrospective analysis of couples
who underwent the first IVF cycle and required
TESE for NOA at Queen Mary Hospital, a university
affiliated tertiary care hospital, from January 2001
to December 2013. They were identified from our
assisted reproductive technique database. Ethics
approval was obtained from the Institutional Review
Board of the University of Hong Kong / Hospital
Authority Hong Kong West Cluster, with the
requirement of patient informed consent waived
because of its retrospective nature.
Husbands who attended our subfertility clinic
were requested to submit one semen sample to the
andrology laboratory of our centre prior to the first
consultation. If the first semen analysis was abnormal,
they were asked to submit a second semen sample.
Semen analysis was based on the criteria of the
World Health Organization (1999, 2010).15 16 Those with azoospermia confirmed in two semen samples
following centrifugation were referred to the male
infertility clinic for further assessment by urologists.
All patients had detailed urological and reproductive
history, physical examination, and hormonal
profile including morning serum testosterone,
follicle-stimulating hormone (FSH), and luteinising
hormone.17 Men with azoospermia deemed to be due
to a non-obstructive cause, as suggested by raised
FSH and small testes, were advised to check their
karyotype and microdeletion of the Y chromosome.
The detailed techniques for chromosome analysis
and Y-microdeletion studies by polymerase chain
reaction of DNA from peripheral blood have been
previously described.6 9 18 Karyotyping was performed
by analysis of banded metaphase chromosomes
from cultured cells. At least 15 and 30 metaphases
were analysed routinely and whenever an anomaly
was suspected, respectively.6 Y-microdeletion was
analysed using six Y chromosome specific sequence
tagged site markers that corresponded to the AZFa
(sY84, sY86), AZFb (sY127, sY132), and AZFc (SY254,
sY255) regions.6 9 18 Information on karyotype and
Y-microdeletion was obtained from the medical
record of the patients, and cross-referenced with the
database of the genetic screening for male subfertility
at Tsan Yuk Hospital.
Men with NOA who wished to have their own
genetic child would be advised to undergo TESE
to retrieve sperms. The most common ovarian
stimulation protocols used in our unit were the long
gonadotropin-releasing hormone (GnRH) agonist
and GnRH antagonist protocols. Details of the
stimulation cycle have previously been described.19
Patients attended the clinic on day 2 of the treatment
cycle. Transvaginal ultrasonography was performed
to determine antral follicle count (AFC) and serum
oestradiol level was checked. Ovarian stimulation
was commenced if the serum oestradiol level was confirmed
at basal level and there was no ovarian cyst. The
gonadotropin dosage depended on the woman’s age,
AFC, and previous ovarian response. Transvaginal
ultrasound for follicular tracking was performed 7
days after the start of ovarian stimulation and every 1
to 3 days thereafter. Further dosage of gonadotropins
was titrated depending on the ovarian response
of the patient during follicular tracking. Human
chorionic gonadotropin (hCG) was given to trigger
final oocyte maturation when the mean diameter of
the leading follicle was at least 18 mm and three or
more follicles reached a mean diameter of at least
16 mm. Transvaginal ultrasound-guided oocyte
retrieval was performed 34 to 36 hours later.
The urologist was responsible for performing
TESE that was performed under general anaesthesia
on the day of oocyte retrieval. In conventional TESE,
the scrotal skin and tunica vaginalis were opened and
the testis was exposed through an incision. Bilateral
testicular biopsies from the upper, middle, and lower
poles were performed. Microdissection TESE, which
involved identification of spermatogenically active
areas under high magnification for more targeted
biopsies, was performed starting from 2010. The
biopsied testicular tissue was minced mechanically
with two microscope slides, and incubated for 1
hour to allow the sperms to swim out of the tissue.
Enzymatic digestion of the testicular tissue was
performed as described when no sperms were seen
under microscope.20 The testicular sperms were
isolated by mini-density gradient centrifugation.
Sperms with high density at the bottom of the
gradient as well as those in the interface between the
gradients were collected in two aliquots of culture
medium. Before ICSI, the embryologist searched the
sperms first in the whole aliquot containing sperms
of high density, and then in the aliquot containing
sperms at the interface if no sperm was found in
the first aliquot. Spare sperms were cryopreserved.
Fertilisation was achieved by ICSI in patients with
successful sperm retrieval. One or two embryos
were replaced 2 days after retrieval. Luteal phase
support was given with either two doses of hCG
1500 IU 5 days apart or vaginal progesterone for 2
weeks after embryo transfer. Patients were followed
up with a urinary pregnancy test 16 days after
embryo transfer and those with a positive pregnancy
test had transvaginal ultrasound scan performed 10
to 14 days later and were referred for antenatal care
at 8 to 10 weeks of gestation. Pregnancy outcome
was monitored. Pregnancy was defined as a positive
urinary pregnancy test. A clinical pregnancy was
defined as a pregnancy with the presence of one or
more intrauterine sac on transvaginal ultrasound.
An ongoing pregnancy was defined as the presence
of at least one fetal heart pulsation on ultrasound
beyond 20 weeks. When no sperms were retrieved,
the collected oocytes were either discarded, donated,
or frozen if further treatment with donor sperm was
considered.
Data analyses were performed by the Statistical
Package for the Social Sciences (Windows version
20.0; SPSS Inc, Chicago [IL], US). Comparisons
between the groups were made using the Fisher’s
exact test, and P<0.05 was considered statistically
significant.
Results
Only information from the first cycle of IVF-TESE
was included. Of 112 men who underwent
TESE during the study period, 23 were excluded
from analysis because of non-motile sperms in the
ejaculate (n=3), ejaculatory dysfunction (n=4), and
obstructive azoospermia and underwent TESE after
failed microepididymal sperm aspiration (n=16).
Therefore, 89 patients with NOA were included in
the analysis.
The mean age of the men was 37.2 (standard
deviation [SD], 6.2) years. Of the 85 patients with
smoking history available, 59 (69.4%) were non-smokers
and 26 (30.6%) were smokers. Genetic
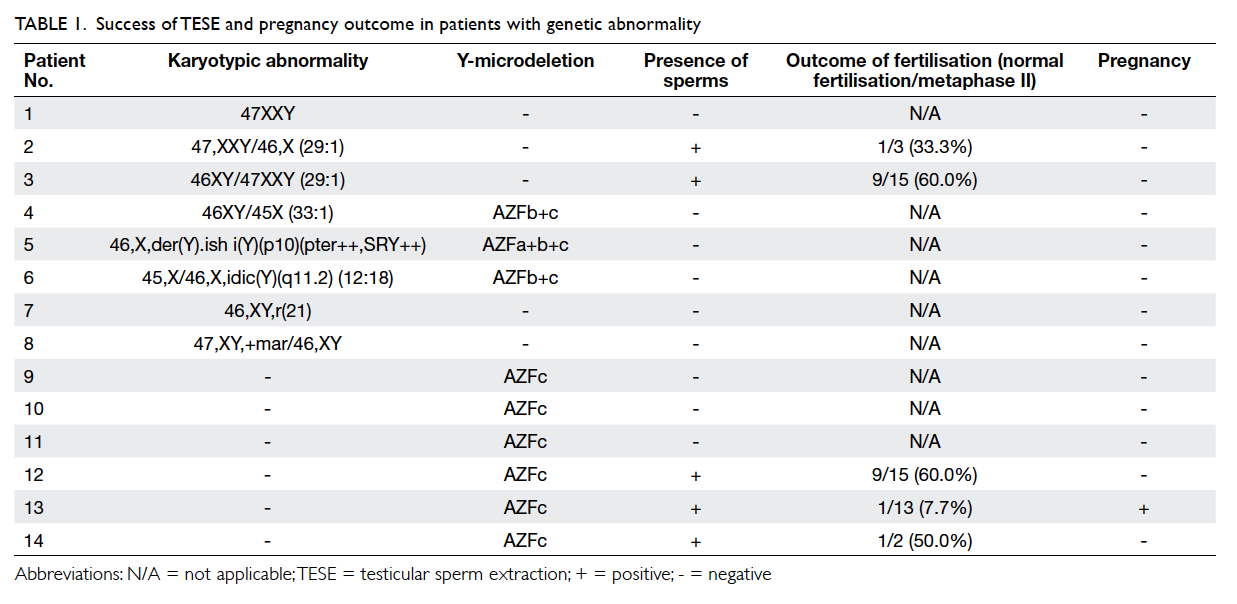
information was missing in nine men. Of 76 men
with genetic information available, eight (10.5%) had
karyotypic abnormality—six were sex chromosomal
and two were autosomal, as shown
in Table 1. The most common sex chromosomal abnormality was Klinefelter syndrome (3/6, 50%;
1 non-mosaic and 2 mosaic). The two men with
autosomal chromosome abnormality were a ring
chromosome 21 and a mosaic supernumerary marker
chromosome. Of these 76 men, nine (11.8%) had
microdeletion of the Y chromosome. The most
common Y-microdeletion was AZFc microdeletion
(6/9, 66.7%). Three men had both chromosomal
abnormality and Y-microdeletion.
Of the 89 patients, sperms were successfully
retrieved in 40 (44.9%). There was no statistically
significant difference in the sperm retrieval rate
in those with karyotype abnormalities and AFZc
microdeletion compared with those without (Table 2).
The same was true when those with sex chromosomal
abnormality were compared with those having
normal karyotype. Sperms were retrieved in the two
patients with mosaic Klinefelter syndrome but not
the one with non-mosaic Klinefelter syndrome. For
those with Y-microdeletion, sperms were retrieved
in 50% (3/6) with AZFc microdeletions, but no
sperms were found in the men with AZFa+b+c and
AZFb+c microdeletions. The men with AZFa+b+c
and AZFb+c microdeletions all had co-existing sex
chromosomal abnormalities (Table 1).
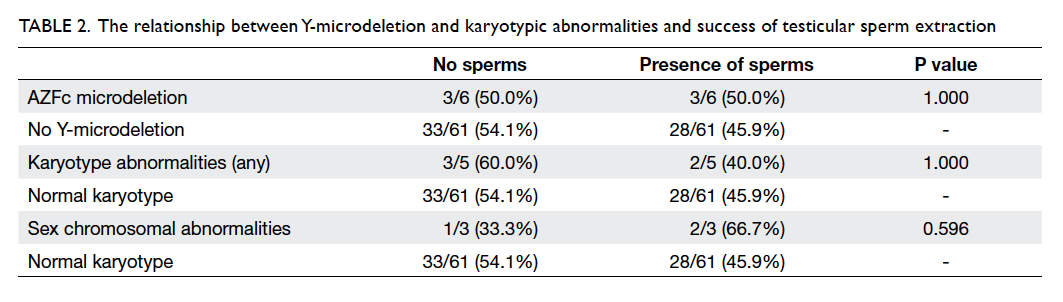
Table 2. The relationship between Y-microdeletion and karyotypic abnormalities and success of testicular sperm extraction
The mean age of the female partners was 37.2
(SD, 6.2) years. The median dosage of gonadotropins
used was 1950 IU (interquartile range, 1650-2550
IU), duration of stimulation 12 days (11-14 days),
peak oestradiol level 11 341 pmol/L (7859.5-19 260.5
pmol/L), and the number of metaphase II oocytes
obtained was 8 (4-13). Pregnancy test was positive
in 15/89 (16.9%) and the clinical pregnancy rate was
12/89 (13.5%). Among those with sperms found,
pregnancy test was positive in 15/40 (37.5%) and the
clinical pregnancy rate was 12/40 (30.0%). Clinical
pregnancy rate per transfer was 12/35 (34.3%). Two
patients had biochemical pregnancy and one had
ectopic pregnancy. There were 10 live births—seven
singletons and three pairs of twins. One patient
underwent second-trimester medical termination
of pregnancy for fetal alobar holoprosencephaly.
One had an ongoing pregnancy at 12 weeks but
was subsequently lost to follow-up. The ongoing
pregnancy rate per cycle was 10/89 (11.2%). The
clinical pregnancy rate was not statistically different
between those who had karyotypic abnormalities
(0/5, 0% vs 8/61, 13.1%; P=1.000) and Y-microdeletion
(1/6, 16.7% vs 8/61, 13.1%; P=1.000) compared with
those who did not. Among the five patients who did
not have embryo transfer, two had failed fertilisation,
two had no transferrable embryos, and one had no
oocyte retrieved.
Discussion
Ho et al17 highlighted the importance of male
factor in infertility assessment and treatment in a
recent case series in a local male infertility clinic.
The incidence of azoospermia was reported to be
up to 36.2%, of which 52.1% had a non-obstructive
cause and would require TESE.17 With increasing
awareness of male infertility, it is hoped that more
men will seek professional help and share the burden
in fertility treatment. Nevertheless, existing data
available in the literature on outcomes of IVF-TESE
are fragmentary5 and local data are still lacking.
Karyotypic abnormalities and Y-microdeletion
have been associated with severe male factor
infertility. Fu et al21 demonstrated high rates of
chromosomal abnormalities and Y chromosome
microdeletions in Chinese infertile men with
azoospermia or severe oligozoospermia. In another
local study from our centre, the prevalence of
chromosomal abnormality and Y-microdeletion
were up to 21.1% and 8.5%, respectively in the
azoospermic group.6 The prevalence of sex
chromosomal abnormality and Y-microdeletion in
the present study was 6/76 (7.9%) and 9/76 (11.8%),
respectively; the former was lower than that reported
by our centre previously. This may be because
we only included men who had TESE performed
in the current study, and it is possible that some
men, particularly those who were found to have
genetic abnormality, did not further pursue assisted
reproductive treatment considering the anticipated
poor prognosis.
Testing for chromosomal abnormalities
and Y-microdeletion are recommended as an
essential part of the workup of men with NOA or
severe oligospermia by the American Society for
Reproductive Medicine,13 American Urological
Association,12 Canadian Urological Association,11 and International Federation of Fertility Societies
guidelines.14 In particular, microdeletion of the
AZFa or AZFb regions were associated with poor
prognosis of sperm retrieval and no sperms have
been retrieved in these patients.22 Although our
study did not show any statistically significant
difference in the sperm retrieval rates in those
with karyotype abnormalities and Y-microdeletion
compared with those without, it should be noted
that all patients who had sperms retrieved were
having AZFc microdeletion consistent with existing
reports, and those who had AZFabc and AZFbc did
not have sperms retrieved. Nonetheless the three
men with AZFabc and AZFbc microdeletions all had
co-existing sex chromosomal abnormalities that
may also have affected spermatogenesis. Men with
AZFa or AZFb microdeletion are therefore often
advised against TESE because of a very low chance
of successful retrieval of mature spermatozoa. Given
proper counselling, these patients may opt not to
pursue further assisted reproductive techniques, and
go directly for options including donor insemination
or adoption. On the other hand, the majority of
men with AZFc microdeletion have sperm available
for use. In some studies, AZFc deletion was even
associated with an increased likelihood of sperm
retrieval.22 The sperm retrieval rate in men with
Klinefelter syndrome via microdissection TESE
has also been reported to be similar or even higher
than in those with NOA and normal karyotype.23 24 25
In our experience, sperms were only found in the
two patients with mosaic Klinefelter syndrome but
not the one with non-mosaic Klinefelter syndrome.
Previous studies showed that sperms are more likely
to be retrieved in younger men with Klinefelter
syndrome.26 27 In our study, the man with non-mosaic Klinefelter syndrome was 42 years old and
might have passed the window of opportunity for
successful sperm retrieval. The two patients with
mosaic Klinefelter syndrome were 34 and 47 years
old, respectively.
This study provides important information
on the prognosis for men with NOA. The sperm
retrieval rate of 44.9% is very similar to a previous
report at our centre where sperms were found in
12/26 (46.2%) of men.28 In that study, the pregnancy
rate was 14.3% per cycle when spermatozoa were
injected. Our study showed that the chance for a man
with NOA undergoing TESE to father his own child
is 13.5%, very similar to that of 13.4% reported by
Vloeberghs et al.5 Indeed, Vloeberghs et al5 have only included men with normal karyotype and absence of
Y-microdeletion, who were included in our study,
and we only included men who underwent
first cycle of IVF-TESE. The clinical pregnancy rate
would have been higher if we also included men who
underwent further attempts. Nonetheless, men were
generally advised against further TESE after failure
to retrieve sperms in the first attempt owing to the
poor prognosis.
Another important issue is the potential for the
genetic abnormality to be transmitted vertically via
assisted reproductive technology. Couples wherein
the male partner has Y-microdeletion should be
counselled about the potential for inheritance of
compromised fertility by male offspring and proper
genetic counselling should be in place before
embarking on IVF-TESE.
The practice of our centre was synchronous
TESE on the day of oocyte retrieval. Several studies
have shown comparable fertilisation and pregnancy
rates when TESE is performed beforehand, either
on the day before oocyte retrieval29 or even prior to
initiation of controlled ovarian hyperstimulation.30
The merit of the latter approach is that women do not
have to go through controlled ovarian stimulation if
no sperms can be retrieved, and therefore can avoid
the risks of ovarian hyperstimulation syndrome and
the costs involved. The available cryopreservation-thawing
procedure for sperms leads to sperm loss
however, and there is still a possibility that the cycle
will have to be cancelled if there are no viable sperms
after thawing.
As 50% of couples undergo IVF-TESE in
vain, with resulting psychological and financial
implications, research into various factors that could
predict successful sperm retrieval is important.
Previous findings from our retrospective study did
not suggest any significant differences in the age,
history of mumps or orchitis/oligozoospermia,
volume of both testes, serum FSH and testosterone
levels in men with or without spermatozoa in IVF-TESE
cycles.28 Indeed, no individual biochemical
or hormonal marker has been found to reliably
predict success in TESE.31 Although histopathology
of testicular biopsy has been shown to predict TESE
outcome, it is invasive and usually done at the time
of TESE itself rather than beforehand in many
patients, limiting its role as a predictive marker.31
The detection of Y chromosome microdeletion,
especially AZFa and AZFb, was important to guide
prognosis as discussed above. Ramasamy et al32
showed that high serum FSH level in men did not
affect the success of microdissection TESE and should not be
used to deny men the possibility to father a child with
their own genetic material.14 Similarly, testicular size
may represent poor spermatogenesis in general but
does not consistently predict sperm retrieval rate.31
In a meta-analysis, serum inhibin B had a sensitivity
of 0.65 and a specificity of 0.83 in prediction of
successful TESE but it was still suboptimal as a
single predictive criterion.33 Seminal anti-Müllerian
hormone and inhibin B are secreted by the Sertoli
cells into the seminiferous tubules and therefore in
theory are more direct markers of spermatogenesis,
but their predictive value for successful TESE was
not confirmed in a prospective study of 139 men with
NOA by Mitchell et al.34 A combination of factors
have been shown to fare better, and authors have
described the use of a predictive score involving the
total testicular volume, FSH, and inhibin B to predict
the sperm retrieval rate in NOA.35
There are several limitations to our study.
The number of men who declined genetic testing
or TESE was not known. These men may be those
with anticipated poor prognosis such that our study
has included those with ‘better’ prognosis and
therefore a higher sperm retrieval rate. In addition,
while some authors suggest that hormonal profile
or testicular volume may have provided prognostic
information,35 36 our information on hormonal profile of men was incomplete. Prior to TESE, FSH might
have been checked up to 2 years because of the long
waiting list for IVF, and therefore was not analysed
in this study. Other important limitations are the
small number of cases and retrospective nature of
our study that precludes proper statistical analysis.
When pregnancy outcome is analysed, it is essential
to remember the other confounding variables of the
female partner such as age and diagnostic category
that limit the conclusions that can be drawn.
Moreover, as the study spanned over 13 years, there
have been changes such as surgical techniques. Men
included in the earlier years underwent TESE while
those more recently underwent microdissection TESE. Further
studies looking into different prognostic factors to
predict successful sperm retrieval are needed.
Conclusions
The sperm retrieval rate and clinical pregnancy
rate per cycle in men undergoing IVF-ICSI-TESE
in our unit were 44.9% and 13.5%, respectively.
Karyotype and AZFc microdeletion abnormalities
did not predict the success of sperm retrieval or
clinical pregnancy rate in couples undergoing
IVF-ICSI-TESE in the present case series, but are
important in patient counselling. Consistent with
existing literature, no sperms could be retrieved in
individuals with AZFa and AZFb microdeletions.
Acknowledgements
We would like to thank Mr Tak-ming Cheung for
data management, and the laboratory colleagues
of Prenatal Diagnostic Laboratory at Tsan Yuk
Hospital who have helped trace the karyotype and
Y-microdeletion results.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Infertility diagnosis by age of patients receiving RT
procedures (other than DI and AIH) in 2013. Council on
Human Reproductive Technology. Available from: http://www.chrt.org.hk/english/publications/files/table17_2013.pdf. Accessed 6 Jul 2015.
2. Devroey P, Van Steirteghem A. A review of ten years
experience of ICSI. Hum Reprod Update 2004;10:19-28. Crossref
3. Devroey P, Liu J, Nagy Z, et al. Pregnancies after testicular
sperm extraction and intracytoplasmic sperm injection in
non-obstructive azoospermia. Hum Reprod 1995;10:1457-60. Crossref
4. Donoso P, Tournaye H, Devroey P. Which is the best sperm
retrieval technique for non-obstructive azoospermia? A
systematic review. Hum Reprod Update 2007;13:539-49. Crossref
5. Vloeberghs V, Verheyen G, Haentjens P, Goossens A,
Polyzos NP, Tournaye H. How successful is TESE-ICSI in
couples with non-obstructive azoospermia? Hum Reprod
2015;30:1790-6. Crossref
6. Ng PP, Tang MH, Lau ET, et al. Chromosomal anomalies
and Y-microdeletions among Chinese subfertile men in
Hong Kong. Hong Kong Med J 2009;15:31-8.
7. Chiang HS, Wei HJ, Chen YT. Genetic screening for patients
with azoospermia and severe oligo-asthenospermia. Int J
Androl 2000;23 Suppl 2:20-5. Crossref
8. Chandley AC. Chromosome anomalies and Y chromosome
microdeletions as causal factors in male infertility. Hum
Reprod 1998;13 Suppl 1:45-50. Crossref
9. Tse JY, Yeung WS, Lau EY, Ng EH, So WW, Ho PC.
Deletions within the azoospermia factor subregions of the
Y chromosome in Hong Kong Chinese men with severe
male-factor infertility: controlled clinical study. Hong
Kong Med J 2000;6:143-6.
10. Lee SH, Ahn SY, Lee KW, Kwack K, Jun HS, Cha KY.
Intracytoplasmic sperm injection may lead to vertical
transmission, expansion, and de novo occurrence of Y-chromosome
microdeletions in male fetuses. Fertil Steril
2006;85:1512-5. Crossref
11. Jarvi K, Lo K, Grober E, et al. The workup and management
of azoospermic males. Can Urol Assoc J 2015;9:229-35. Crossref
12. Jarow J, Sigman M, Kolettis PN, et al. The evaluation of the
azoospermic male: AUA Best Practice Statement (revised
7/22/11). US, Maryland: American Urological Association
Education and Research, Inc; 2011.
13. Practice Committee of the American Society for Reproductive
Medicine in collaboration with the Society for Male
Reproduction and Urology. Evaluation of the azoospermic
male. Fertil Steril 2008;90(5 Suppl):S74-7. Crossref
14. Standards and Practice Committee. International
Federation of Fertility Societies. Global standards
of infertility care. Standard 17. Investigation and
management of non-obstructive azoospermia.
Recommendations for practice. June 2014.
15. World Health Organization. WHO laboratory manual
for the examination of human semen and sperm-cervical
mucus interaction. 4th ed. Cambridge: Cambridge
University Press; 1999.
16. Cooper TG, Noonan E, von Eckardstein S, et al. World
Health Organization reference values for human semen
characteristics. Human Reprod Update 2010;16:231-45. Crossref
17. Ho KL, Tsu JH, Tam PC, Yiu MK. Disease spectrum and
treatment patterns in a local male infertility clinic. Hong
Kong Med J 2015;21:5-9.
18. Tse JY, Yeung WS, Ng EH, et al. A comparative study of
Y chromosome microdeletions in infertile males from two
Chinese populations. J Assist Reprod Genet 2002;19:376-83. Crossref
19. Li HW, Lee VC, Lau EY, Yeung WS, Ho PC, Ng EH. Role of
baseline antral follicle count and anti-Mullerian hormone
in prediction of cumulative live birth in the first in vitro
fertilisation cycle: a retrospective cohort analysis. PLoS
One 2013;8:e61095. Crossref
20. Crabbé E, Verheyen G, Silber S, et al. Enzymatic digestion
of testicular tissue may rescue the intracytoplasmic sperm
injection cycle in some patients with non-obstructive
azoospermia. Hum Reprod 1998;13:2791-6. Crossref
21. Fu L, Xiong DK, Ding XP, et al. Genetic screening
for chromosomal abnormalities and Y chromosome
microdeletions in Chinese infertile men. J Assist Reprod
Genet 2012;29:521-7. Crossref
22. Stahl PJ, Masson P, Mielnik A, Marean MB, Schlegel PN,
Paduch DA. A decade of experience emphasizes that
testing for Y microdeletions is essential in American men
with azoospermia and severe oligozoospermia. Fertil Steril
2010;94:1753-6. Crossref
23. Bakircioglu ME, Ulug U, Erden HF, et al. Klinefelter
syndrome: does it confer a bad prognosis in treatment of
nonobstructive azoospermia? Fertil Steril 2011;95:1696-9. Crossref
24. Sabbaghian M, Modarresi T, Hosseinifar H, et al.
Comparison of sperm retrieval and intracytoplasmic
sperm injection outcome in patients with and without
Klinefelter syndrome. Urology 2014;83:107-10. Crossref
25. Ozveri H, Kayabasoglu F, Demirel C, Donmez E. Outcomes
of micro-dissection TESE in patients with non-mosaic
Klinefelter’s syndrome without hormonal treatment. Int J
Fertil Steril 2015;8:421-8.
26. Rohayem J, Fricke R, Czeloth K, et al. Age and markers of
Leydig cell function, but not of Sertoli cell function predict
the success of sperm retrieval in adolescents and adults
with Klinefelter’s syndrome. Andrology 2015;3:868-75. Crossref
27. Mehta A, Paduch DA. Klinefelter syndrome: an argument
for early aggressive hormonal and fertility management.
Fertil Steril 2012;98:274-83. Crossref
28. Ng HY, Lau YL, Yeung SB, So WK, Tam PC, Ho PC.
Testicular sperm extraction and intracytoplasmic sperm
injection in non-obstructive azoospermia. Chinese Med J
(Engl) 2000;113:246-50.
29. Levran D, Ginath S, Farhi J, Nahum H, Glezerman
M, Weissman A. Timing of testicular sperm retrieval
procedures and in vitro fertilization–intracytoplasmic
sperm injection outcome. Fertil Steril 2001;76:380-3. Crossref
30. Karacan M, Alwaeely F, Erkan S, et al. Outcome of
intracytoplasmic sperm injection cycles with fresh
testicular spermatozoa obtained on the day of or the
day before oocyte collection and with cryopreserved
testicular sperm in patients with azoospermia. Fertil Steril
2013;100:975-80. Crossref
31. Bernie AM, Ramasamy R, Schlegel PN. Predictive factors
of successful microdissection testicular sperm extraction.
Basic Clin Androl 2013;23:5. Crossref
32. Ramasamy R, Lin K, Gosden LV, Rosenwaks Z, Palermo
GD, Schlegel PN. High serum FSH levels in men with
nonobstructive azoospermia does not affect success of
microdissection testicular sperm extraction. Fertil Steril
2009;92:590-3. Crossref
33. Toulis KA, Iliadou PK, Venetis CA, et al. Inhibin B
and anti-Müllerian hormone as markers of persistent
spermatogenesis in men with non-obstructive
azoospermia: a meta-analysis of diagnostic accuracy
studies. Hum Reprod Update 2010;16:713-24. Crossref
34. Mitchell V, Boitrelle F, Pigny P, et al. Seminal plasma levels of
anti-Müllerian hormone and inhibin B are not predictive of
testicular sperm retrieval in nonobstructive azoospermia:
a study of 139 men. Fertil Steril 2010;94:2147-50. Crossref
35. Boitrelle F, Robin G, Marcelli F, et al. A predictive score
for testicular sperm extraction quality and surgical ICSI
outcome in non-obstructive azoospermia: a retrospective
study. Hum Reprod 2011;26:3215-21. Crossref
36. Yang Q, Huang YP, Wang HX, et al. Follicle-stimulating
hormone as a predictor for sperm retrieval rate in patients
with nonobstructive azoospermia: a systematic review and
meta-analysis. Asian J Androl 2015;17:281-4. Crossref