Hong Kong Med J 2016 Dec;22(6):546–55 | Epub 31 Oct 2016
DOI: 10.12809/hkmj154788
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Clinical outcome of neoadjuvant chemoradiation in locally advanced rectal cancer at a
tertiary hospital
William WK Yeung, FRCR, FHKAM (Radiology)1;
Brigette BY Ma, FHKCP, MD (CUHK)1;
Janet FY Lee, FHKAM (Surgery), MD (CUHK)2;
Simon SM Ng, FHKAM (Surgery), MD (CUHK)2;
Michael HY Cheung, FRCS, FHKAM (Surgery)3;
WM Ho, MRCP, FHKAM (Medicine)1;
Maverick WK Tsang, FRCR, FHKAM (Radiology)1;
Simon Chu, FRCS, FHKAM (Surgery)2;
Daisy CM Lam, MB, BS, FRCR1;
Frankie KF Mo, PhD1
1 Department of Clinical Oncology, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong
2 Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong
3 Department of Surgery, North District Hospital, Sheung Shui, Hong Kong
Corresponding author: Dr William WK Yeung (wilyeung@netvigator.com)
Abstract
Objectives: To review the clinical outcome of locally
advanced rectal cancer treated with neoadjuvant
chemoradiation followed by definitive surgery with
or without adjuvant chemotherapy and to elucidate
the prognostic factors for treatment outcome.
Methods: This historical cohort study was
conducted at a tertiary public hospital in Hong
Kong. All patients who had undergone neoadjuvant
chemoradiation for locally advanced rectal cancer
in our department from November 2005 to
October 2014 were recruited. Local recurrence–free
survival, distant metastasis–free survival, disease-free
survival, and overall survival of patients were
documented.
Results: A total of 135 patients who had received
neoadjuvant chemoradiation during the study
period were reviewed. There were 130 patients
who had completed neoadjuvant chemoradiation
and surgery. The median follow-up time was 35.1
months. The 3- and 5-year local recurrence–free
survival, distant metastasis–free survival, disease-free
survival, as well as overall survival rates were
91.8% and 86.7%, 73.9% and 72.1%, 70.1% and 64.6%,
as well as 86.5% and 68.4%, respectively. The rate of
pathological complete response was 13.8%. The T and N downstaging rate was
49.2% and 63.1%, respectively. The rate of conversion
from threatened circumferential resection margin to
clearance of margin was 90.6%. Of the 42 cases that
were initially deemed to require abdominal perineal
resection, 15 (35.7%) were converted to sphincter-sparing
surgery.
Conclusions: The treatment outcome of
neoadjuvant chemoradiation for locally advanced
rectal cancer was comparable with overseas data
in terms of local control rate and overall survival.
This strategy may increase the chance of achieving
a clear surgical margin by downstaging the tumour,
especially in patients who presented with threatened
circumferential margin.
New knowledge added by this study
- This is a local study from a tertiary oncology centre on the clinical outcome of neoadjuvant chemoradiation in the treatment of locally advanced rectal cancer.
- Neoadjuvant chemoradiation is effective in downstaging advanced rectal cancers, especially those with threatened circumferential resection margin, facilitating definitive surgery to achieve a clearance of the final pathological margin.
Introduction
According to the Hong Kong Cancer Registry,1 there
were 1797 new cases of rectal/anal cancer in 2013.
The incidence rate per 100 000 persons was 25.0
(crude rate) and 13.3 (age-standardised rate). The
total number of deaths from rectal/anal cancer was
597, and the mortality rate was 8.3 (crude rate) or 3.7
(age-standardised rate) per 100 000 persons. In Hong
Kong, colorectal cancer is the first most common
cancer in incidence and the second in mortality rate
for both sexes.
Conventional treatment of rectal cancer is
mainly surgery. In locally advanced cancer, adjuvant
therapy with concurrent chemoradiation has been
shown to improve local control and disease-free
survival (DFS) in phase III clinical trials.2 3 4 5 The
major indication for adjuvant chemoradiation is
pathological T3 or T4 and/or regional nodal disease
without distant metastasis.
Preoperative radiotherapy with or without
concurrent chemotherapy has been shown to reduce
the local recurrence rate of locally advanced rectal
cancer.6 7 8 9 10 11 12 Preoperative radiotherapy comprises a
short or long course.
Short-course preoperative radiotherapy was
given in 5 Gy per fraction for five fractions over
1 week, followed by surgery about 1 week after
completion of radiotherapy. Since the introduction of
total mesorectal excision (TME), the local recurrence
rate has been significantly reduced. In the new era of
TME surgery, a Dutch rectal trial confirmed that a
short course of preoperative radiotherapy, followed
by TME surgery, was also beneficial in reducing
local recurrence rate from 8.2% to 2.4% over 2
years compared with TME surgery alone in locally
advanced rectal cancer.13 14
Long-course preoperative radiotherapy
involves a conventional fractionation of 1.8 Gy per
fraction, five fractions per week, up to a total dose of
45 to 50 Gy. It is given with concurrent chemotherapy
consisting of mostly a fluoropyrimidine-containing
regimen. Surgery is usually performed approximately
4 to 10 weeks after completion of chemoradiation.
A randomised German trial (CAO/ARO/AIO 94)10 compared preoperative long-course
chemoradiation with postoperative chemoradiation.
At a median follow-up of 4 years, no significant
difference was reported in the 5-year overall survival
(OS). Nonetheless, treatment compliance, grade 3/4 acute and late toxicity profile, tumour and nodal
downstaging, and rates of pelvic recurrence all
favoured the preoperative chemoradiation arm.
In addition, the sphincter preservation rate in the
194 patients with low-lying tumours declared by
the surgeon prior to randomisation requiring an
abdominoperineal resection (APR) was enhanced
with preoperative treatment (39% vs 19%; P=0.004).
Since 2005, our hospital has adopted a treatment
policy of long-course neoadjuvant (or preoperative)
chemoradiation (nCRT) for selected cases of locally
advanced rectal cancer. The objective of this
study was to review the clinical outcome of these
patients with locally advanced rectal cancer treated
with nCRT in our department from November 2005
to October 2014 and to elucidate the prognostic
factors for treatment outcome retrospectively.
Methods
Eligible patients included those without distant
metastasis and who were staged preoperatively on
radiological grounds with T3 or T4 disease and/or
having nodal involvement. There might have been
other extra specific reasons for recommending nCRT,
including threatened circumferential resection
margin (CRM), sphincter-sparing surgery, avoidance of
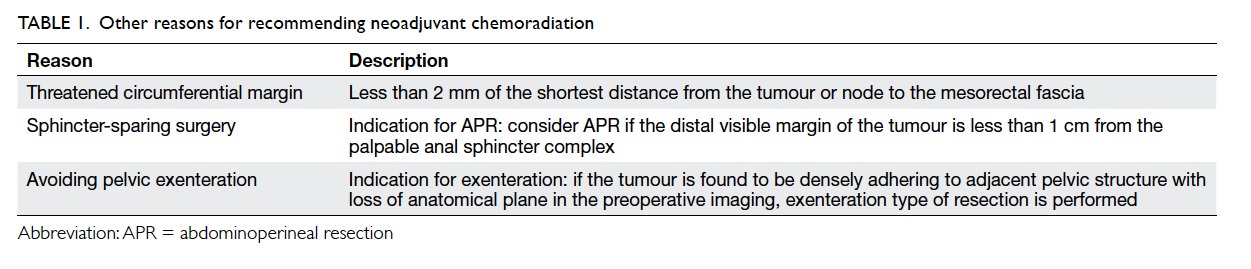
pelvic exenteration, and unresectability (Table 1). Patients were required to be medically fit and agree
to the nCRT.
The nCRT scheme adopted in our department
consisted of the following.
Radiotherapy
Simulation procedure was done in an immobilised
prone position with full bladder, using simulation
computed tomography (CT) scan. The three-dimensional conformal
radiotherapy planning was performed
on the simulation CT scan imaging, using three
coplanar fields with shielding conformal to the target
volume. The radiotherapy was given in two phases.
Phase 1 included the whole pelvis. A total dose of 45
Gy was delivered at 1.8 Gy per day, five fractions per
week over 5 weeks. Phase II included only the gross
tumour and the enlarged pelvic nodes with margins.
A booster dose of 5.4 Gy was administered in the
same fractionation as phase 1.
Chemotherapy
Concurrent chemotherapy was given in the first and
fifth weeks of radiation. It comprised an intravenous
(IV) bolus of 5-fluorouracil (5-FU; 400 mg/m2) and
leucovorin (20 mg/m2) on days 1 to 4.
The surgery was scheduled about 4 to 10 weeks
after completion of nCRT. Adjuvant chemotherapy
with four cycles of 5-FU and leucovorin was
administered to most patients. In some selected
cases with pathological node-positive disease
following surgery, four cycles of capecitabine and
oxaliplatin (‘XELOX’ regimen) were given.
In this study, clinical data were collected
retrospectively from the medical records of all
patients who had undergone nCRT for locally
advanced rectal cancer in the Department of Clinical
Oncology at the Prince of Wales Hospital, Hong Kong
from November 2005 to October 2014. The surgery
was performed either at Prince of Wales Hospital or
the referring hospital. There was variation in practice
for pretreatment staging method, re-staging on
completion of nCRT (follow-up CT scan was
arranged to exclude distant metastasis at least 2 weeks
after nCRT; optional magnetic resonance imaging
[MRI] was considered at least 4 weeks after nCRT), and
follow-up among different hospitals. The patients’
demographic information, tumour characteristics,
and treatment details were retrieved. The initial type
of surgery recommended by the referring surgical
team at presentation and any extra specific reasons
(intentions) for referral for nCRT were reviewed.
The final pathology at the definitive surgery (the
pathological T and N staging, the tumour size, any
pathological complete response [pCR], the resection
margins), the treatment-related toxicity (radiation- or
chemotherapy-related, surgical complications),
recurrence (local, regional, distant relapse), and
disease status at follow-up were reviewed.
The key study endpoints included loco-regional
recurrence–free survival, distant metastasis–free
survival, DFS, and OS. Other secondary endpoints
included the rate of pCR, tumour downstaging (T
and N staging), conversion of threatened CRM to
clearance of margins (R0), conversion to sphincter-sparing
surgery for lower rectal cancers, conversion
from a potential pelvic exenteration to non-exenterating
surgery, and the rate of conversion
from unresectable to resectable tumour. For toxicity
endpoints, the rate of grade 3 or above acute
toxicity according to the National Cancer Institute Common Terminology
Criteria for Adverse Events version 4.0, and
the rate of grade 3 or above late radiation toxicity
according to the Toxicity criteria of the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer, and perioperative complications as
represented by rate of 30-day postoperative mortality
and morbidity (delayed wound healing, anastomotic
complication, reoperation) were also assessed.
Statistical analysis
Descriptive statistics were used to report the
incidence rates of secondary endpoints that were
calculated directly. The survival rates and time-to-event rates were estimated with the Kaplan-Meier method. Univariate analysis based on the proportional hazard model was performed to
investigate the relationship between different
outcome (survival) and prognostic factors. The
hazard ratio and the corresponding 95% confidence
interval were shown. The prognostic factors included
pretreatment T stage, pretreatment N stage,
histological grade, threatened CRM, completion
of nCRT, time from nCRT to surgery, pathological
T stage, pathological N stage, pathological group
stage, pCR, pathological margin, number of involved
nodes, and completion of adjuvant chemotherapy. For
those significant prognostic factors, multivariate
analysis using Cox regression with stepwise selection
was performed.
This study was approved by the Joint Chinese
University of Hong Kong–New Territories East
Cluster Clinical Research Ethics Committee with
informed consent waived. The principles outlined in
the Declaration of Helsinki have also been followed.
Results
A total of 135 patients who had received nCRT in our
department from November 2005 to October 2014
were reviewed, of whom 130 had completed nCRT
and surgery with or without adjuvant chemotherapy.
Of the five patients who did not have surgery, two
refused surgery after nCRT and three progressed after
nCRT without undergoing surgery.
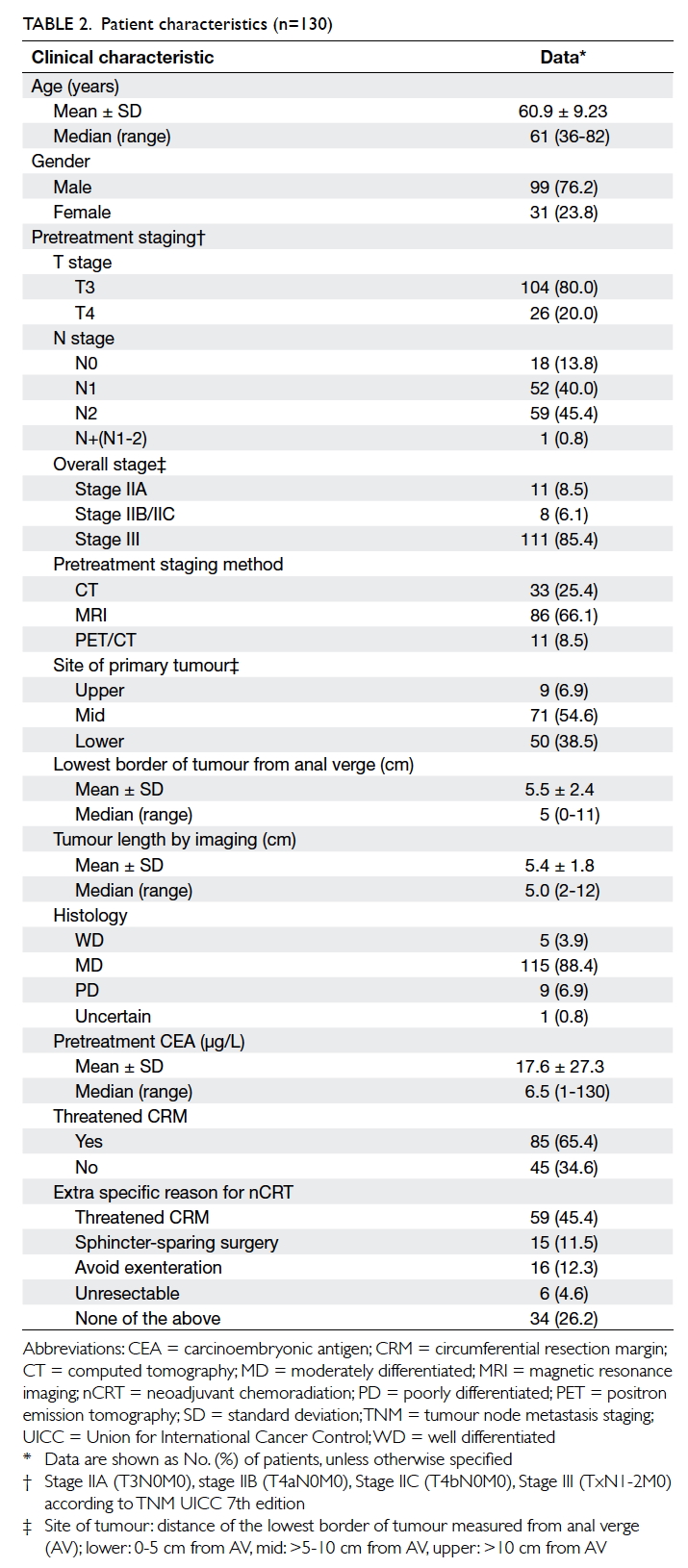
Patient characteristics are shown in Table 2.
The mean age was 60.9 (standard deviation
[SD], 9.23) years. The male-to-female ratio was 3.2:1. For
the pretreatment stage, 80% and 20% were T3 and
T4 respectively, while 13.8%, 40.0% and 45.4% were
N0, N1 and N2 stage, respectively. For the overall
group stage, the incidences of stage IIA, IIB/C, and
III were 8.5%, 6.1%, and 85.4%, respectively. A total
of 65.4% cases had threatened CRM at pretreatment
imaging.
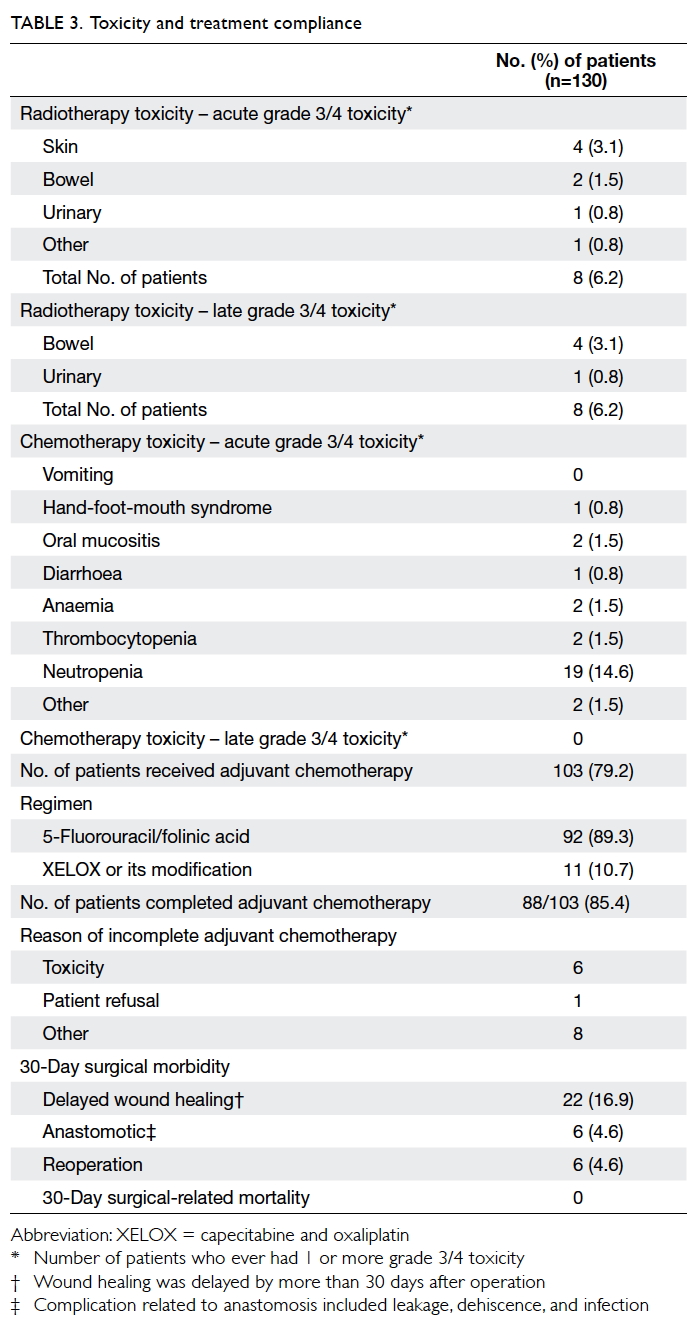
Of the 130 patients who had surgery, 128
(98.5%) completed nCRT. For the radiotherapy-related
toxicities, the combined incidence of grade 3
or above acute toxicity to the skin, bowel, and urinary
toxicity was 6.2%. Similarly, the radiotherapy-related
grade 3 or above late toxicity to the bowel and
urinary tract was 6.2%. For chemotherapy-related
grade 3 or above acute toxicity, the incidences of
neutropenia, anaemia, and thrombocytopenia
were 14.6%, 1.5%, and 1.5%, respectively. The most
common non-haematological grade 3 or above
acute toxicities were hand-foot-mouth syndrome
(0.8%), mucositis (1.5%), and diarrhoea (0.8%).
Adjuvant chemotherapy was given to 103 (79.2%)
patients, of whom 92 (89.3%) received the regimen
of IV bolus 5-FU and leucovorin. With regard to
surgical complications, 22 (16.9%) patients had
delayed wound healing (>30 days after operation),
six (4.6%) had anastomotic complication, and six
(4.6%) required reoperation. There was no 30-day
postoperative mortality reported (Table 3).
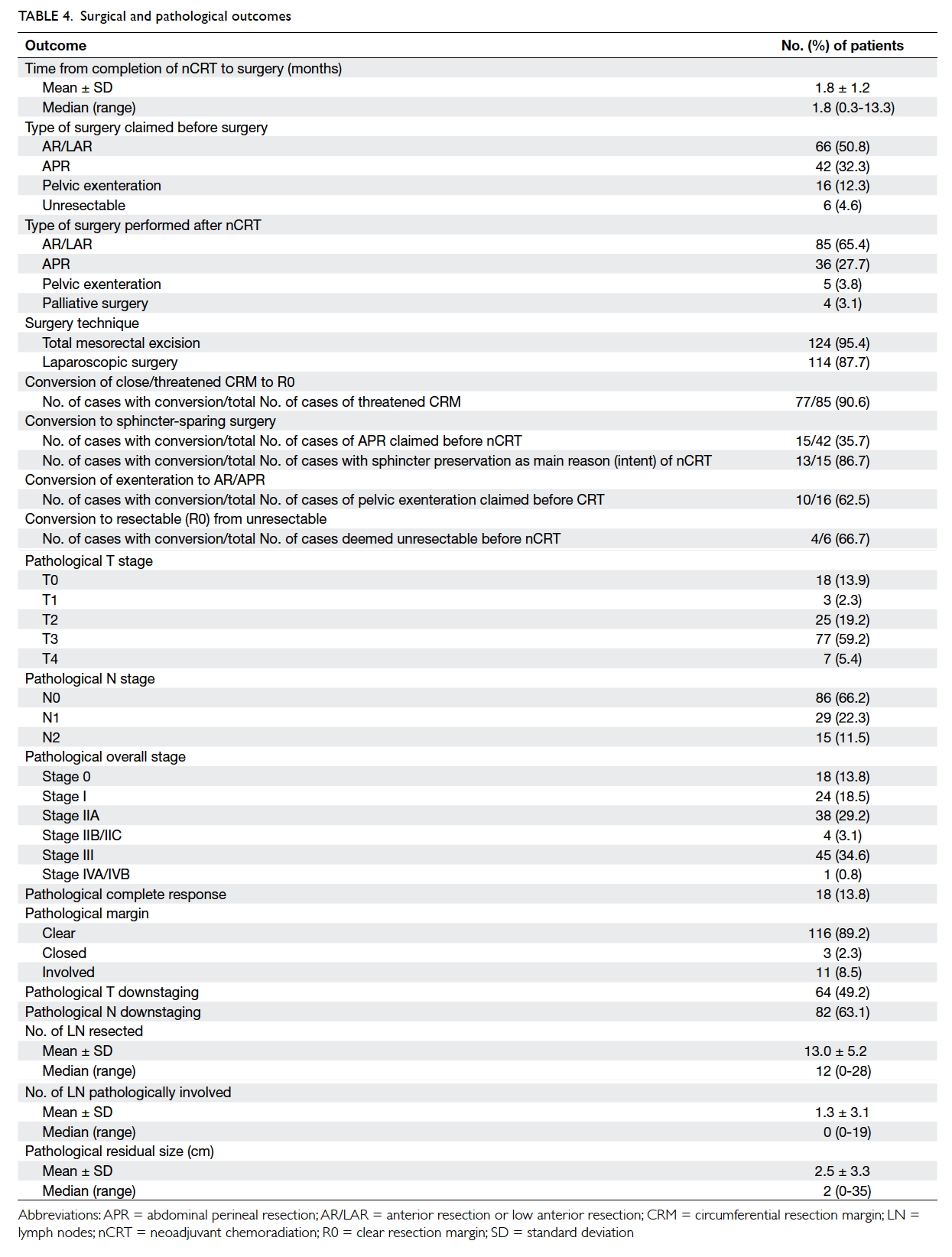
Of the 130 cases, 124 (95.4%) underwent TME surgery and 114 (87.7%) had laparoscopic surgery. The mean time from the
date of completion of nCRT to surgery was 7.2
(SD, 4.8) weeks. Comparing the type of surgery
recommended before starting nCRT and those
finally carried out after nCRT, the rate of anterior
resection/low anterior resection increased to
65.4% from 50.8%, and the rate of APR/pelvic
exenteration decreased to 27.7%/3.8% from
32.3%/12.3% respectively. The overall rate of
surgical conversion was reported in several clinical
contexts: (1) percentage achieving a R0 resection, (2)
percentage undergoing sphincter-sparing surgery, and
(3) percentage avoiding pelvic exenteration. First, of
the total number of patients who were found to have
threatened CRM before treatment, 90.6% finally
achieved a R0 resection. Of the 42 patients who
were initially deemed on presentation to require an
APR, 35.7% underwent sphincter-sparing surgery.
In a subgroup of the 15 patients who had received
nCRT with the intention of sphincter preservation,
86.7% (n=13) underwent sphincter-sparing
surgery rather than APR. Among these 13 cases with
successful sphincter-sparing surgery, one had pCR
and all had clear resection margins. They remained
alive and free of loco-regional and distant recurrence
at the end of this study. Of the 16 patients who were
initially assessed to require pelvic exenteration,
62.5% (n=10) underwent non-exenterating
surgery. There were six patients in whom tumour
was deemed unresectable and who were referred for
nCRT to improve resectability. Complete resection
with negative margins was subsequently achieved in
four (66.7%) of the six patients while the other two
had a positive margin in the palliative surgery.
The final pathological staging in the surgical
specimen is reported (Table 4). The rates of pCR
and clear resection margin were 13.8% and 89.2%,
respectively. The rate of T downstaging was
49.2% and that for N stage was 63.1% (Table 3).
The median follow-up time was 35.1 months. Of
the 130 patients, local recurrence, loco-regional
recurrence, distant metastasis, disease recurrence,
and death occurred in 10 (crude rate, 7.7%), 15
(11.5%), 30 (23.1%), 34 (26.2%), and 23 (17.7%)
patients, respectively. The Kaplan-Meier estimates
of the 3-year local recurrence–free survival, regional
recurrence–free survival, loco-regional recurrence–free survival, distant metastasis–free survival, DFS,
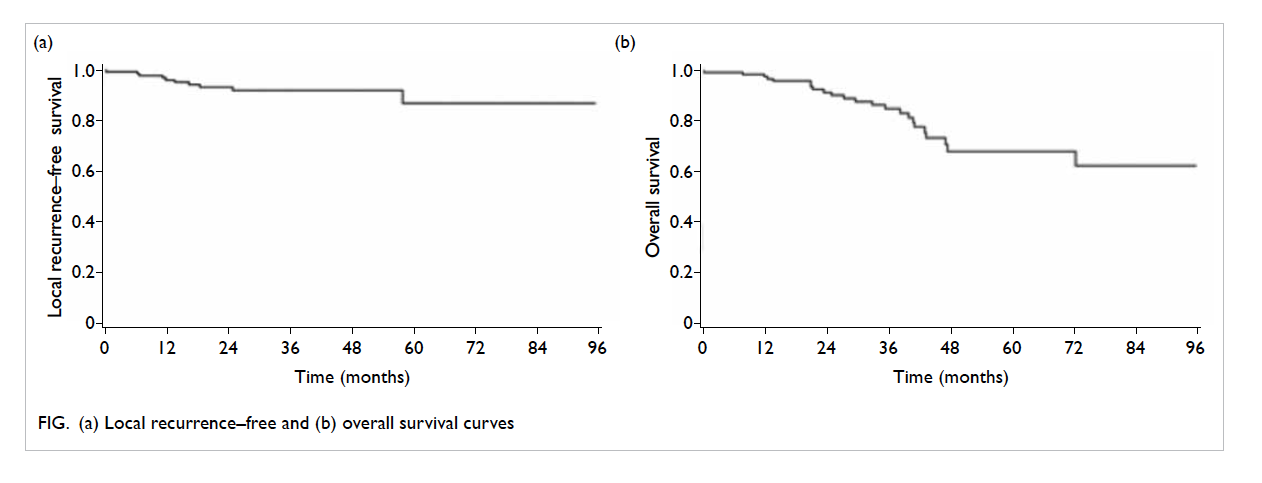
and OS were 91.8%, 92.6%, 87.9%, 73.9%, 70.1%, and
86.5%, respectively. The respective 5-year survival
rates were 86.7%, 85.3%, 81.0%, 72.1%, 64.6%, and
68.4%. The corresponding Kaplan-Meier curves for
local recurrence-free survival and OS is also shown in
the Figure (the curves for loco-regional recurrence–free survival, distant metastasis–free survival, and
DFS are shown in the Appendix).
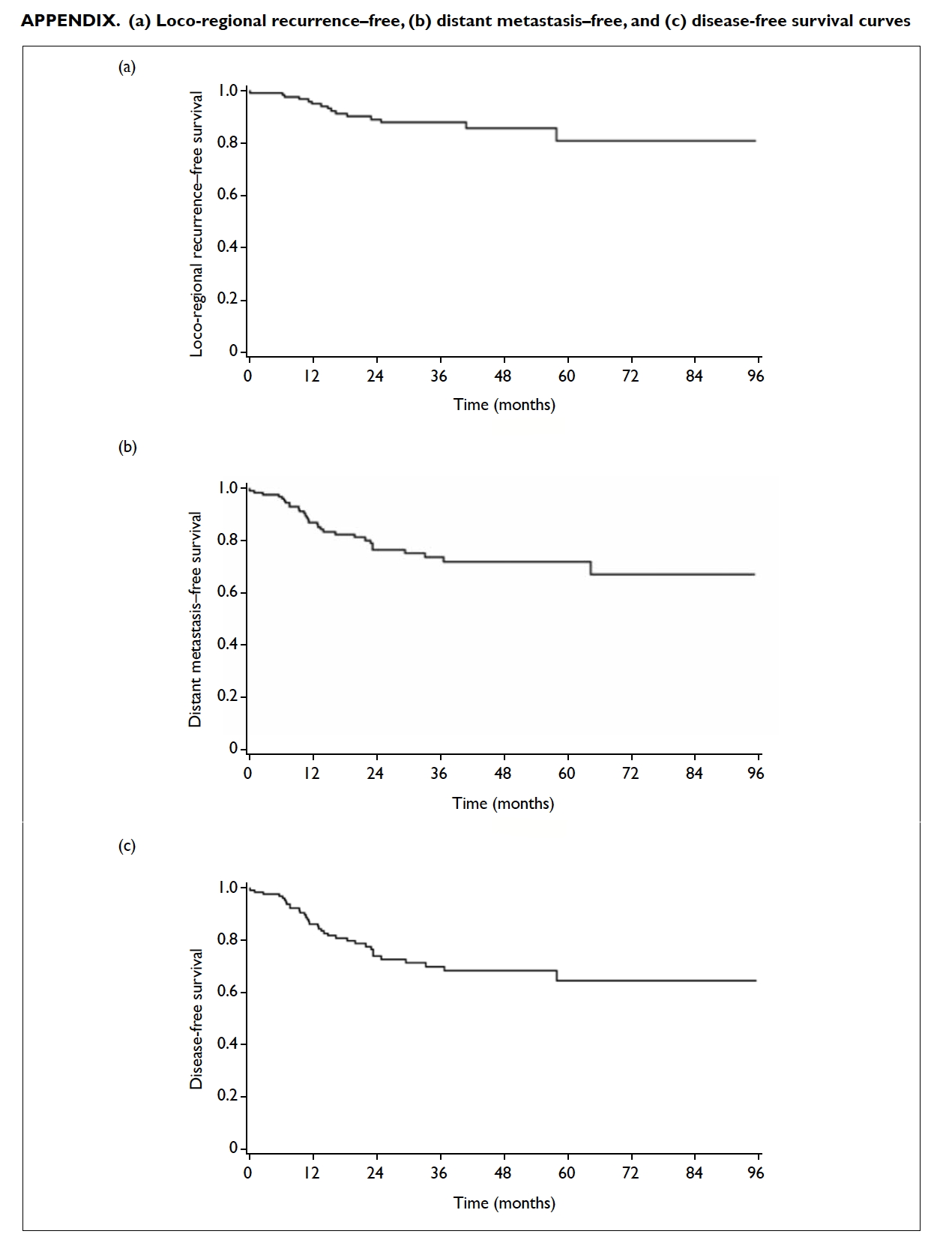
Appendix. (a) Loco-regional recurrence–free, (b) distant metastasis–free, and (c) disease-free survival curves
Analysis of prognostic factors
The variables (factors including age and gender were
tested but not significant in univariate model) in
the univariate analysis included the pretreatment
T stage, pretreatment N stage, histological
grade, presence of threatened CRM, completion
of nCRT, time from nCRT to surgery (continuous
variable), pathological T stage, pathological N stage,
pathological group stage, pCR, pathological
margin, number of involved nodes (continuous
variable), and completion of adjuvant chemotherapy.
Those significant prognostic factors were studied by
multivariate analysis.
In the multivariate analysis, the pathological
clear margin, completion of nCRT, and the number
of involved nodes were significantly associated
with local recurrence–free survival. The number of
involved nodes, pathological clear margin, and time
from nCRT to surgery were significantly associated
with loco-regional recurrence–free survival. The
number of involved nodes, the pretreatment
T4, pathological stage III/IV, and completion
of adjuvant chemotherapy were significantly
associated with distant metastasis–free survival.
The number of involved nodes, pathological stage
III/IV, and completion of adjuvant chemotherapy
were significantly associated with DFS. Finally, the
number of involved nodes, the pretreatment T4,
and pathological stage III/IV were significantly
associated with OS (Table 5).
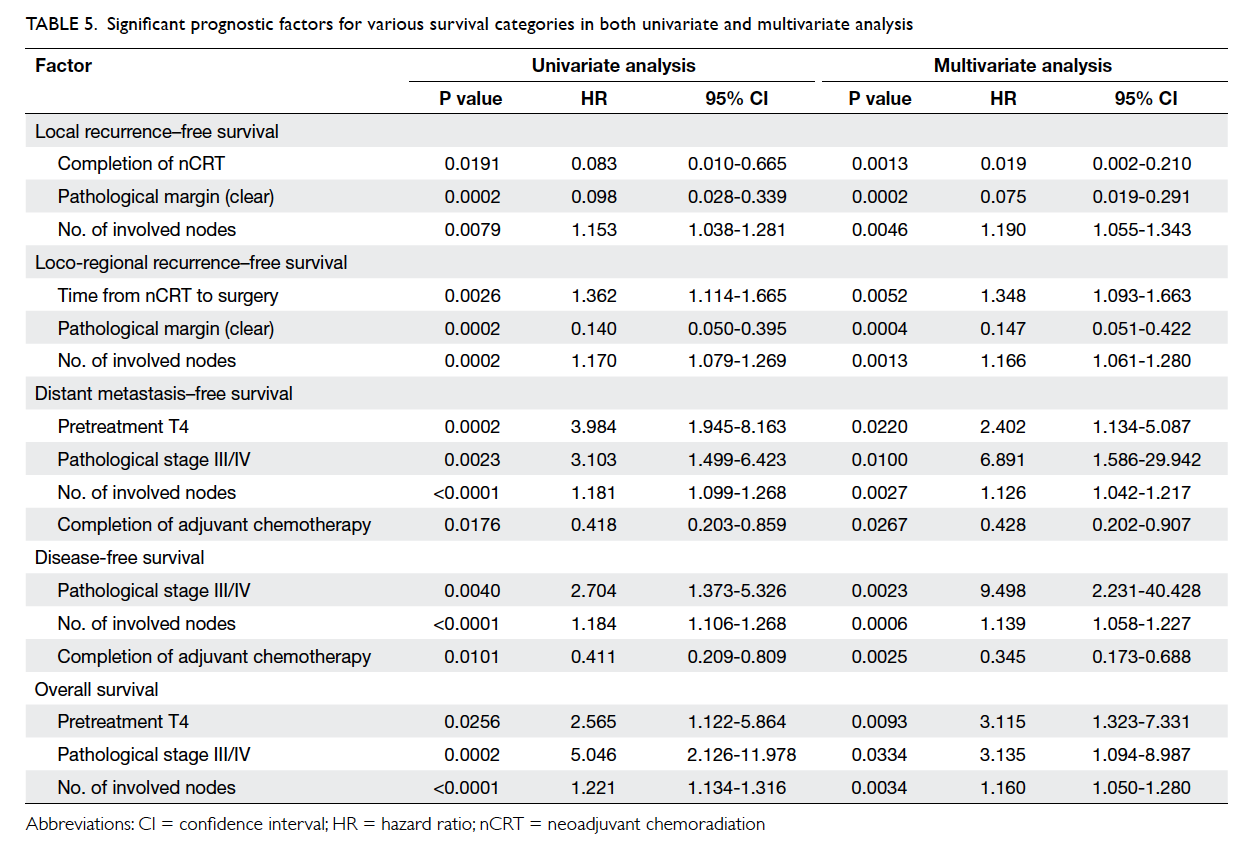
Table 5. Significant prognostic factors for various survival categories in both univariate and multivariate analysis
Discussion
Although the current study was retrospective,
survival data were comparable with figures reported
in international studies. In the major randomised
trials, 5-year local recurrence rate in the arm with
preoperative short-course radiotherapy was in the
range of 11% to 14%, and OS was in the range of
42% to 76%.6 7 8 9 In the randomised trials that had an
arm with nCRT, the 4- or 5-year local recurrence
rates were 5.7% to 15.6% and the OS were 66.2% to
76%.10 15 16 17 18 In this study, the 5-year local recurrence rate and loco-regional recurrence rate was 13.3% and
19%, respectively. These were close to the reported
figures from randomised studies.10 15 16 17 18 The 5-year OS in this study was 68.4% and is comparable with
international studies.10 15 16 17 18
The pCR rate was 13.8% in this study, again
comparable with randomised trials10 15 16 17 18 and
reviews.19 Together with the favourable downstaging
effects, the completion resection rate was
high (89.2%). This is the primary aim of nCRT in
advanced rectal cancer. The role of nCRT in sphincter preservation
for low-lying tumours has been a
controversial issue in some randomised trials,10 11 15 16
and critical reviews.20 21 In a German study,10 among the 194 patients with tumours that were determined
by the surgeon before randomisation to require an
APR, a statistically significant increase in sphincter
preservation was achieved among patients who
received nCRT compared with those who received
postoperative chemoradiation (39% vs 19%;
P=0.004). Although long-course nCRT is expected
to result in tumour downsizing, a Polish trial11
did not find that long-course chemoradiation was
superior to short-course preoperative radiotherapy
in reducing the APR rate. The possible explanations
for this finding include the possibility that the degree
of downsizing was not sufficient to alter the surgical
approach, due to surgeon’s concern about residual
microscopic disease despite an apparently good
response after nCRT, or the surgeons had made
their clinical decision based on the pretreatment
staging information. In our study the overall rate of
conversion from APR to sphincter-sparing surgery
was 35.7% and was comparable with that (39%) in the
German trial10; and for the subgroup of patients with
an intention to spare the sphincter, the conversion
rate was even higher, up to 86.7%, with a good
clinical outcome.
The extent of extramural tumour spread and
lymph node and CRM status are powerful predictive
factors for local recurrence, distant metastases,
and OS in patients with rectal cancer.22 23 24 25 26 27 28 From our
study, it was evident that the number of involved
nodes in the final pathology was an independent
factor in OS, DFS, local or loco-regional recurrence–free survival, and
distant metastasis–free survival. For local or loco-regional
recurrence, the pathological clear margin,
the completion of nCRT, and the time from nCRT
to surgery were independent prognostic factors.
Although in this study there was an attempt to
find the optimal cut-off time for surgery after the
completion of nCRT, this was not possible because
of the small sample size. Increasing the time interval
from completion of nCRT to surgery was associated
with a detrimental effect on loco-regional recurrence
(hazard ratio=1.348).
In this study, completion of adjuvant
chemotherapy was a prognostic factor for distant
metastasis. This implies that adjuvant chemotherapy
might be important in reducing distant metastasis.
It remains controversial whether adjuvant
chemotherapy should be given after nCRT and
surgery. A 2x2 factorial randomised trial (EORTC
trial 22921)29 30 31 32 that assessed the value of preoperative
chemo-radiotherapy versus preoperative
radiotherapy and postoperative chemotherapy versus
no postoperative chemotherapy in patients with
cT3-4 disease could not demonstrate any prolonged
progression-free or OS from adjuvant chemotherapy
in patients with resectable T3-T4 rectal cancer.
Its follow-up report of 785 eligible patients who
underwent R0 resection showed that patients with
a good prognosis (ypT0-2) seemed to benefit from
adjuvant chemotherapy, especially if the tumour was
located in the mid-rectum.33 Nonetheless, an updated
analysis of the EORTC 22921 trial18 recently failed
to confirm the benefit of adjuvant chemotherapy
for ypT0-2 patients after a median follow-up of 10.4
years. In the I-CNR-RT phase III randomised trial,34
there was no benefit of adjuvant chemotherapy
(6 cycles of 5-FU and folinic acid) compared with
observation only after nCRT. The result may be
partly attributed to the low compliance to complete
the planned number of chemotherapy cycles. The
British Chronicle trial35 is unique in comparing
XELOX postoperatively against observation alone
in locally advanced rectal cancer treated with nCRT.
After a median follow-up of 44.8 months, there was
no statistically significant benefit of adjuvant XELOX
in the 3-year DFS rate. A Korean study reported
the results of ADORE phase II study in which 321
patients of ypT3-4/ypN0 or ypTx/ypN1-2 after nCRT
with 5-FU alone were randomised to receive adjuvant
chemotherapy with 5-FU or FOLFOX.36 37 After a median follow-up of 38.2 months, the 3-year DFS
rate was better in the FOLFOX arm (P=0.047).
Although adjuvant treatment of patients with
rectal cancer remains controversial, the National Comprehensive Cancer Network guidelines
recommend 5-FU–based chemotherapy with
oxaliplatin as the preferred adjuvant treatment for all
patients with rectal cancer, who receive neoadjuvant
5-FU–based chemoradiation, regardless of surgical
pathology results. The recently reported German
CAO/ARO/AIO-04 trial also revealed the benefit of
adding oxaliplatin to both neoadjuvant and adjuvant
treatment with significant improvement in DFS of
patients with clinically staged cT3-4 or cN1-2 rectal
cancer compared with conventional 5-FU–based
combined modality regimen.38
There were limitations to this study. The
data were collected retrospectively and there was
no blinding during data collection. It is possible
that potential confounding factors like smoking
and co-morbidity were inadequately controlled for.
Toxicity data were not collected systematically and
thus could be underreported. If the data can be
collected prospectively, a tailor-made toxicity form
will be designed and more toxicity can be captured.
The median follow-up time was relatively short.
Magnetic resonance imaging is now a standard
staging tool in rectal cancer. The use of MRI as initial
staging was only 66.1% in this cohort. Therefore, pretreatment
staging might not accurately reflect the
true staging at presentation. In this study, there was
limited reporting of late toxicity of radiation such as
sexual and sphincter dysfunction. The full extent of
the late toxicity of radiation requires longer follow-up.
Due to the small sample size, the adjustment of
the potential confounding factors for survival was a
limitation of the study.
Conclusions
The treatment outcome following nCRT for
locally advanced non-metastatic rectal cancer
in our experience was comparable with overseas
data in terms of local control rate and OS. The
high conversion rate from having a threatened
circumferential margin to clear resection margin,
and the high T and N downstaging rates,
suggest that this approach is effective in facilitating
surgery to obtain complete surgical clearance.
In the subgroup with an intention of sphincter preservation,
the conversion rate from APR to
sphincter-sparing surgery was high. The rate of acute
toxicities was within expectations and manageable
and there were no treatment-related deaths.
Acknowledgements
Although not named in the author list, we thank the
other colleagues who contributed to the treatment
of this group of patients and those who helped with
data collection.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Hong Kong Cancer Registry. Statistics. Available from:
http://www3.ha.org.hk/cancereg/statistics.html. Accessed Aug 2016.
2. Prolongation of the disease-free interval in surgically
treated rectal carcinoma. Gastrointestinal Tumor Study
Group. N Engl J Med 1985;312:1465-72. Crossref
3. Douglass HO Jr, Moertel CG, Mayer R, et al. Survival after
postoperative combination treatment of rectal cancer. N
Engl J Med 1986;315:1294-5. Crossref
4. Krook JE, Moertel CG, Gunderson LL, et al. Effective
surgical adjuvant therapy for high-risk rectal carcinoma. N
Engl J Med 1991;324:709-15. Crossref
5. Fisher B, Wolmark N, Rockette H, et al. Postoperative
adjuvant chemotherapy or radiation therapy for rectal
cancer: results from NSABP protocol R-01. J Natl Cancer
Inst 1988;80:21-9. Crossref
6. Påhlman L, Glimelius B. Pre- or postoperative radiotherapy
in rectal and rectosigmoid carcinoma. Report from a
randomized multicenter trial. Ann Surg 1990;211:187-95. Crossref
7. Cedermark B, Johansson H, Rutqvist LE, Wilking N. The
Stockholm I trial of preoperative short term radiotherapy
in operable rectal carcinoma. A prospective randomized
trial. Stockholm Colorectal Cancer Study Group. Cancer
1995;75:2269-75. Crossref
8. Improved survival with preoperative radiotherapy in
resectable rectal cancer. Swedish Rectal Cancer Trial. N
Engl J Med 1997;336:980-7. Crossref
9. Martling A, Holm T, Johansson H, Rutqvist LE, Cedermark
B, Stockholm Colorectal Cancer Study Group. The
Stockholm II trial on preoperative radiotherapy in rectal
carcinoma: long-term follow-up of a population-based
study. Cancer 2001;92:896-902. Crossref
10. Sauer R, Becker H, Hohenberger W, et al. Preoperative
versus postoperative chemoradiotherapy for rectal cancer.
N Engl J Med 2004;351:1731-40. Crossref
11. Sauer R, Liersch T, Merkel S, et al. Preoperative versus
postoperative chemoradiotherapy for locally advanced
rectal cancer: results of the German CAO/ARO/AIO-94
randomized phase III trial after a median follow-up of 11
years. J Clin Oncol 2012;30:1926-33. Crossref
12. Sebag-Montefiore D, Stephens RJ, Steele R, et al.
Preoperative radiotherapy versus selective postoperative
chemoradiotherapy in patients with rectal cancer (MRC
CR07 and NCIC-CTG C016): a multicentre, randomised
trial. Lancet 2009;373:811-20. Crossref
13. Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative
radiotherapy combined with total mesorectal excision for
resectable rectal cancer. N Engl J Med 2001;345:638-46. Crossref
14. Van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative
radiotherapy combined with total mesorectal excision
for resectable rectal cancer: 12-year follow-up of the
multicentre, randomised controlled TME trial. Lancet
Oncol 2011;12:575-82. Crossref
15. Bujko K, Nowacki MP, Nasierowska-Guttmejer A,
Michalski W, Bebenek M, Kryj M. Long-term results of
a randomized trial comparing preoperative short-course
radiotherapy with preoperative conventionally fractionated
chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. Crossref
16. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized
trial of short-course radiotherapy versus long-course
chemoradiation comparing rates of local recurrence in
patients with T3 rectal cancer: Trans-Tasman Radiation
Oncology Group trial 01.04. J Clin Oncol 2012;30:3827-33. Crossref
17. Gérard JP, Conroy T, Bonnetain F, et al. Preoperative
radiotherapy with or without concurrent fluorouracil and
leucovorin in T3-4 rectal cancers: results of FFCD 9203. J
Clin Oncol 2006;24:4620-5. Crossref
18. Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based
adjuvant chemotherapy after preoperative
chemoradiotherapy in rectal cancer: long-term results
of the EORTC 22921 randomised study. Lancet Oncol
2014;15:184-90. Crossref
19. Damin DC, Lazzaron AR. Evolving treatment strategies for
colorectal cancer: a critical review of current therapeutic
options. World J Gastroenterol 2014;20:877-87. Crossref
20. Gerard JP, Rostom Y, Gal J, et al. Can we increase the
chance of sphincter saving surgery in rectal cancer with
neoadjuvant treatments: lessons from a systematic review
of recent randomized trials. Crit Rev Oncol Hematol
2012;81:21-8. Crossref
21. Bujko K, Kepka L, Michalski W, Nowacki MP. Does rectal
cancer shrinkage induced by preoperative radio(chemo)therapy increase the likelihood of anterior resection? A
systematic review of randomised trials. Radiother Oncol
2006;80:4-12. Crossref
22. Dukes CE, Bussey JH. The spread of rectal cancer and its effect on
prognosis. Br J Cancer 1958;12:309-20. Crossref
23. Gunderson LL, Sosin H. Areas of failure found at
reoperation (second or symptomatic look) following
“curative surgery” for adenocarcinoma of the rectum: clinicopathologic correlation and implications for
adjuvant therapy. Cancer 1974;34:1278-92. Crossref
24. Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM,
Donaldson G. Patterns of recurrence of rectal cancer after
potentially curative surgery. Cancer 1983;52:1317-29. Crossref
25. Quirke P, Durdey P, Dixon MF, Williams NS. Local
recurrence of rectal adenocarcinoma due to inadequate
surgical resection. Histopathological study of lateral
tumour spread and surgical excision. Lancet 1986;2:996-9. Crossref
26. Merkel S, Mansmann U, Siassi M, Papadopoulos
T, Hohenberger W, Hermanek P. The prognostic
inhomogeneity in pT3 rectal carcinomas. Int J Colorectal
Dis 2001;16:298-304. Crossref
27. Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of
circumferential resection margin involvement vary
between surgeons and predict outcomes in rectal cancer
surgery. Ann Surg 2002;235:449-57. Crossref
28. Wibe A, Rendedal PR, Svensson E, et al. Prognostic
significance of the circumferential resection margin
following total mesorectal excision for rectal cancer. Br J
Surg 2002;89:327-34. Crossref
29. Bosset JF, Collette L, Calais G, et al. Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med
2006;355:1114-23. Crossref
30. Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal
effect of chemotherapy with preoperative radiotherapy for
rectal cancer: preliminary results—EORTC 22921. J Clin
Oncol 2005;23:5620-7. Crossref
31. Bosset JF, Calais G, Mineur L, et al. Preoperative radiation
(Preop RT) in rectal cancer: effect and timing of additional
chemotherapy (CT) 5-year results of the EORTC 22921
trial. Proc Am Soc Clin Oncol 2005;23:247S (abstract no. 3505).
32. Bosset JF, Calais G, Daban A, et al. Preoperative
chemoradiotherapy versus preoperative radiotherapy in
rectal cancer patients: assessment of acute toxicity and
treatment compliance. Report of the 22921 randomised
trial conducted by the EORTC Radiotherapy Group. Eur J
Cancer 2004;40:219-24. Crossref
33. Collette L, Bosset JF, den Dulk M, et al. Patients with
curative resection of cT3-4 rectal cancer after preoperative
radiotherapy or radiochemotherapy: does anybody benefit
from adjuvant fluorouracil-based chemotherapy? A trial
of the European Organisation for Research and Treatment
of Cancer Radiation Oncology Group. J Clin Oncol
2007;25:4379-86. Crossref
34. Sainato A, Cernusco Luna Nunzia V, Valentini V, et
al. No benefit of adjuvant fluorouracil leucovorin
chemotherapy after neoadjuvant chemoradiotherapy in
locally advanced cancer of the rectum (LARC): long term
results of a randomized trial (I-CNR-RT). Radiother Oncol
2014;113:223-9. Crossref
35. Glynne-Jones R, Counsell N, Quirke P, et al. Chronicle:
results of a randomised phase III trial in locally advanced
rectal cancer after neoadjuvant chemoradiation
randomising postoperative adjuvant capecitabine
plus oxaliplatin (XELOX) versus control. Ann Oncol
2014;25:1356-62. Crossref
36. Hong YS, Nam B, Kim K, et al. Adjuvant chemotherapy
with oxaliplatin/5-fluorouracil/leucovorin (FOLFOX)
versus 5-fluorouracil/leucovorin for rectal cancer patients
whose postoperative yp stage 2 or 3 after preoperative
chemotherapy: updated results of 3-year disease-free
survival from a randomized phase II study (The ADORE). J
Clin Oncol 2014;32(5 Suppl):abstract no. 3502.
37. Hong YS, Nam BH, Kim KP, et al. Oxaliplatin, fluorouracil,
and leucovorin versus fluorouracil and leucovorin as
adjuvant chemotherapy for locally advanced rectal cancer
after preoperative chemoradiotherapy (ADORE): an open-label,
multicentre, phase 2, randomised controlled trial.
Lancet Oncol 2014;15:1245-53. Crossref
38. Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to
fluorouracil-based preoperative chemoradiotherapy and
postoperative chemotherapy of locally advanced rectal
cancer (the German CAO/ARO/AIO-04 study): final
results of the multicentre, open-label, randomised, phase
3 trial. Lancet Oncol 2015;16:979-89. Crossref