Hong Kong Med J 2016 Oct;22(5):486–95 | Epub 26 Aug 2016
DOI: 10.12809/hkmj164844
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Diabetic retinopathy screening: global and local perspective
Rita A Gangwani, FRCS (Edin), FHKAM (Ophthalmology)1;
JX Lian, MPH, PhD1;
Sarah M McGhee, PhD, FFPH(UK)2;
David Wong, FRCOphth, FRCS1,3;
Kenneth KW Li, FRCS (Edin), FHKAM (Ophthalmology)1,4
1 Department of Ophthalmology, The University of Hong Kong, Pokfulam, Hong Kong
2 School of Public Health, The University of Hong Kong, Pokfulam, Hong Kong
3 Royal Liverpool University, Liverpool, United Kingdom
4 Department of Ophthalmology, United Christian Hospital, Kwun Tong, Hong Kong
Corresponding author: Dr Rita A Gangwani (dr.rita_gangwani@hotmail.com)
Abstract
Diabetes mellitus has become a global epidemic. It
causes significant macrovascular complications such
as coronary artery disease, peripheral artery disease,
and stroke; as well as microvascular complications
such as retinopathy, nephropathy, and neuropathy.
Diabetic retinopathy is known to be the leading
cause of blindness in the working-age population
and may be asymptomatic until vision loss occurs.
Screening for diabetic retinopathy has been shown
to reduce blindness by timely detection and effective
laser treatment. Diabetic retinopathy screening
is being done worldwide either as a national
screening programme or hospital-based project or
as a community-based screening programme. In this
article, we review different methods of screening
including grading used to detect the severity of sight-threatening
retinopathy and the newer screening
methods. This review also includes the method of
systematic screening being carried out in Hong
Kong, a system that has helped to identify diabetic
retinopathy among all attendees in public primary
care clinics using a Hong Kong–wide public patients’
database.
Introduction
Diabetes mellitus (DM) is becoming a global
epidemic. In 2010, the World Health Organization
(WHO) estimated that the global prevalence of
DM is approximately 6.4% or 280 million people
worldwide.1 The figures from 2014 are even more
alarming: approximately 347 million people globally
are diagnosed to have DM.2 Sedentary lifestyles, lack
of physical activity, obesity, and lack of awareness
have contributed to an increased prevalence of DM,
particularly in developing countries.2
Diabetes mellitus is a chronic disease
characterised by hyperglycaemia. Of the two types
of DM, type 1 (insulin-dependent or juvenile type)
is characterised by a total lack of insulin due to
destruction of islets of Langerhans in the pancreas,
due to an autoimmune process the cause of which
may be unknown, and is not preventable with
current knowledge.3 4 Type 2 DM, the more common
type (non–insulin-dependent or adult-onset)
characterised by resistance to the action of insulin
and failure of insulin production, usually occurs due
to excess body weight and lack of physical activity
and is preventable.3 4
Diabetes mellitus causes both macrovascular
complications such as coronary artery disease,
peripheral arterial disease, and stroke; and
microvascular complications such as diabetic
nephropathy, neuropathy, and retinopathy.5 Diabetic
retinopathy (DR) is one of the most common
microvascular complications and one of the most
common causes of blindness in populations of
working age (20-70 years). While certain risk factors
for DR, like the type and duration of DM, cannot be
modified, control of other modifiable risk factors
such as glycaemic control (haemoglobin A1c [HbA1c]), hypertension,
and hyperlipidaemia is effective and essential to
reduce DR-related blindness.6 7 8 9
Diabetic retinopathy consists of the early non-proliferative
diabetic retinopathy (NPDR) stage, which can be mild, moderate, or severe; the advanced
stage as proliferative DR (PDR); and maculopathy or
diabetic macular oedema. Vision loss in DR occurs
mainly due to macular oedema and PDR. Some
studies consider PDR and diabetic macular oedema
or diabetic maculopathy to be sight-threatening DR
(STDR) while some other studies include moderate-to-severe NPDR additionally within the category
of STDR. Blindness caused by DR is preventable.
Since DR is usually asymptomatic, early detection
and timely treatment are essential to prevent
blindness.10 11 12 The Diabetic Retinopathy Study and
The Early Treatment Diabetic Retinopathy Study
(ETDRS) showed the effectiveness of scatter laser
photocoagulation in patients with severe NPDR
and PDR, and focal laser treatment in patients with
diabetic macular oedema.10 11 12 Diabetic macular
oedema is associated with the breakdown of the
blood-retinal barrier. Inflammation plays a significant
role and is mediated by multiple cytokines including
inflammatory cytokines and vascular endothelial
growth factor (VEGF).13 Several clinical trials have
demonstrated the effectiveness of anti-VEGF drugs
(such as ranibizumab, bevacizumab, pegaptanib and
aflibercept) in restoring the integrity of the blood-retinal
barrier and effectively reducing diabetic
macular oedema and improving vision.14
Blindness due to DR has important
implications for the individual and is a huge socio-economic
burden on the health care system and
society.15 16 Although new treatments with anti-VEGF therapy for diabetic macular oedema and PDR
can be very effective, they are very costly considering
most people need maintenance treatment over some
months and years.16 Additionally, chronic cases do
not respond well to anti-VEGF therapy.
The WHO recommends that screening should
be done for any condition that is an important
health problem, has an effective treatment that can
be delivered early, usually before symptoms of the
condition are apparent, when facilities for diagnosis
and treatment are available, when screening is
feasible and cost-effective, and when subjects can
be followed up longitudinally.17 Diabetic retinopathy
fulfils most of these criteria and some studies have
shown that screening can reduce the rate of blindness
due to DR.18 19
Since photocoagulation is effective to treat
retinopathy and prevent blindness, it has been
considered unethical to conduct a randomised
controlled trial of screening versus no screening.20
Therefore, few studies have examined the cost-effectiveness
of screening for DR directly and
most have used computer-based cost-effectiveness
models to simulate the experience of cohorts of
diabetic patients.20 21 Such studies have calculated
the cost-effectiveness of screening and treatment in
terms of cost per quality-adjusted life year gained,
sight year saved, or case of blindness avoided.21 22
One study carried out in Hong Kong showed that
systematic screening at no charge to the subject is
more cost-effective from the societal perspective
than screening with a small co-payment.23
The important measures to prevent vision
loss due to DR therefore include: (1) early detection
of retinopathy by some form of screening, (2)
subsequent monitoring of the condition with regular
fundus examination, and (3) timely and effective
laser treatment when deemed necessary.
Screening of DR is carried out at most
places throughout the world, but there is no single
recommended method that is suitable for every
country. The DR classification and grading method,
particularly for STDR, has minor differences across
different countries too. The aim of this paper was to
review the methods of screening for DR from both a
global and local perspective.
History of diabetic retinopathy screening
In 1989, the St Vincent Declaration in Europe
aimed to reduce DM-related blindness by one third
in 5 years.24 The Diabetes 2000 programme of the
American Academy of Ophthalmology (AAO) was
implemented to promote screening and treatment
for DR.25 The English national screening programme
(ENSP) started systematic DR screening in 2003 and
aimed to reduce blindness by 40% within 5 years.26 27
Systematic screening means that every eligible
person is contacted and offered screening regularly
and every effort is made to screen the whole group
at risk. This usually requires a register of all those
with DM and the maintenance of active contact
with them. In 2004, 15 years after the St Vincent
Declaration, the Liverpool declaration aimed to
reduce DR-related blindness further by ensuring
that systematic screening reached at least 80% of
the diabetic population in all European countries.
As a result, there is now universal access to laser
therapy in these countries.28 In South-East Asia,
Thailand has launched the new Thailand Healthy
Lifestyle Strategy Plan (2011-2020) to decrease the
prevalence, complications, and disability of five
major non-communicable diseases including DM
and is now introducing a mobile eye care project to
enable people from rural communities to have access
to DM screening.29 30
In order to ensure standardisation and quality
of DR screening, guidelines have been developed
by national organisations such as the American
Diabetes Association (ADA), the AAO, and the
ENSP, and many screening programmes are now
being carried out worldwide.25 26 30 31
Recommendations for diabetic retinopathy screening
According to ADA and AAO, adults and children of
≥10 years of age who had type 1 DM should have
an initial and comprehensive eye examination by an
ophthalmologist or optometrist within 5 years of
the diagnosis. Similarly, type 2 DM patients should
undergo DR screening within 5 years of the diagnosis.
An initial comprehensive eye examination should
include dilated fundal examination and follow-up
examinations at least yearly thereafter.25 28 31 In
the presence of any retinopathy (NPDR, PDR, or
macular oedema), referral to an ophthalmologist
is required and more frequent examinations are
recommended. Pregnant women with pre-existing
DM should undergo dilated fundus examination in
the first trimester with close follow-up throughout
pregnancy and for 1 year postpartum.25 Women
who develop gestational DM do not require an
eye examination during pregnancy and are not at
increased risk of developing DR during pregnancy.25
Patients with mental and physical disability are
not excluded from DR screening; ENSP has special
provision for such groups.32
Many of the current guidelines, such as AAO
and ENSP, recommend annual screening for DR.25 31 32 Iceland is one of the pioneers in DR screening and
introduced a risk-adjusted screening interval for DR.
The Icelandic model adjusts the screening interval
from 6 months up to 60 months according to the
individual risk of STDR taking into account the level
of HbA1c, systolic blood pressure, type of DM, stage
of DR, gender, and duration of DM.33 Screening
intervals in the Icelandic model could therefore be
more variable than the fixed intervals in the AAO or
ENSP in the UK.33 34
Types of diabetic retinopathy screening
Opportunistic versus systematic screening
Opportunistic screening is sporadic and occurs
when a test is offered by a doctor or health care
professional or when the patient asks the doctor
for the test. Opportunistic screening may not be
checked for quality assurance and may not include all
those at risk. In contrast and as previously described,
systematic screening consists of quality-assured predetermined
screening processes that include the
active identification of those at risk, maintenance of
a register of eligible subjects, and invitation to attend
the screening programme. Everyone who participates
in the systematic screening undergoes the same
method of screening. The selection, invitation, and
follow-up processes are determined in advance and
constitute a system that provides feedback and/or
referral with call and recall for screening at specified
intervals.
Historically, opportunistic screening has been
done. Systematic screening, which includes the
whole population at risk in its target group, ensures
much better coverage of DM patients.
Methods used for screening
Screening for DR has been performed using different
methods. This includes direct ophthalmoscopy,
dilated stereoscopic fundoscopy, fundus analogue
photography and now, more commonly, the use
of digital photography with wide-angle imaging.
The digital fundus photography can be performed
with pupil dilatation (mydriatic) or without
pupil dilatation (non-mydriatic) and also with a
stereoscopic or non-stereoscopic technique. Non-mydriatic
fundus cameras have been commonly
employed in DR screening as they have the advantage
of not requiring pupil dilatation and can capture a
wide angle of the retina. In the presence of media
opacity such as cataract, however, the image quality
of non-mydriatic cameras is less satisfactory and
may lead to ungradable images. For this reason,
mydriatic fundus photography is preferred in the
diabetic population given that cataract is more
prevalent as it has the advantage of having a lower
percentage of ungradable images due to media
opacity. Nevertheless, pupil dilatation is more time-consuming and carries a small risk of precipitating
an acute angle-closure glaucoma attack.
The previously accepted gold standard for
DR screening is dilated seven-field 30° stereoscopic
fundus photographs with grading by experienced
readers using the recommended ETDRS process
(Fig 1).35 This procedure remains the gold standard for academic research but is seldom adopted for
population screening because it is time-consuming.
Furthermore, seven-field stereoscopic fundus
photographs give rise to too many screening
failures and are therefore not suitable for mass
screening, especially in a population with a high
prevalence of cataract. Slit-lamp biomicroscopic
fundus examination by an ophthalmologist is also
considered the clinical gold standard and is equally
effective but not practical for large-scale screening.
Additionally, clinical verification and validation
are difficult because of the problem of accurate
clinical documentation. The detection ability of
colour fundus photography using a fundus camera
to detect DR was compared with that of doctors in
diabetic clinics using ophthalmoscopy. The camera
detection rate was 4 times higher through undilated
pupils and more than twice as high through dilated
pupils.36 Although improved detection rates by
ophthalmoscopy may improve clinical detection or
diagnosis of DR, ophthalmoscopy can easily overlook
signs of early DR in a busy diabetic clinics.36
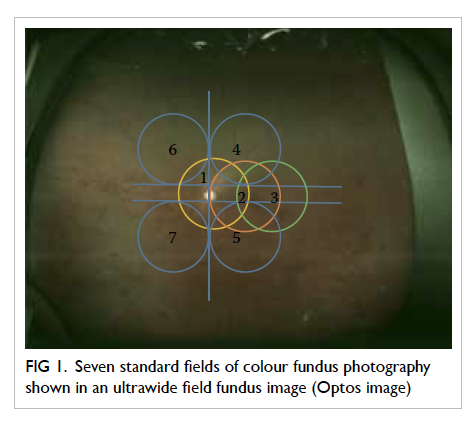
Figure 1. Seven standard fields of colour fundus photography shown in an ultrawide field fundus image (Optos image)
Various studies have compared single-field
and two-field screening retinal photographs to
seven-field stereo photographs.37 38 39 40 Single-field 45°
photographs centred at the fovea, when compared
with seven-field photographs, had a sensitivity of 74%
to 86% and specificity of 92% to 95%.37 38 Some other
studies have shown high sensitivity and specificity to
detect DR using two-field fundus photographs.39 40
Two-field 45° to 50° photographs consist of images
covering the temporal area including the macula
and optic disc and the second-field covering the
nasal area including the optic disc (Fig 2). Two-field
photography has the advantage of detecting DR
in the nasal retina that could otherwise have been
missed by single-field photography.

Figure 2. Two-field fundus photographs of the right eye of a patient with diabetic retinopathy
Centred at (a) fovea and (b) optic disc
The ENSP for diabetic eye disease in the UK
developed a screening protocol for DR using non-stereoscopic
45°, two-field fundus photography
(centred at the macula and optic disc).26 41 Other
studies have also utilised single- or three-field digital
fundus photography as a screening tool for DR
screening.42 43
Recently, ultrawide field fundus imaging
(UWFI) has shown that a 100° to 200° field view of
the retina can be acquired without pupil dilatation
(Optos P200MA and Optos P200C imagers; Optos, Fife,
UK) [Fig 3]. It has the benefits of reducing ungradable
images, increasing disease detection, and shortening
image evaluation times.44 45 Since it can detect more
retinopathy and can detect other peripheral retinal
pathology, such as retinal detachment and ocular
tumours, UWFI provides a more ‘complete’ retinal
examination. Although the image quality of the photo
is not as good as traditional fundus photography, it
is gradually improving. It is also very expensive and
there is colour distortion of the images. With further
advancements, it may play a role in DR screening in
the future.
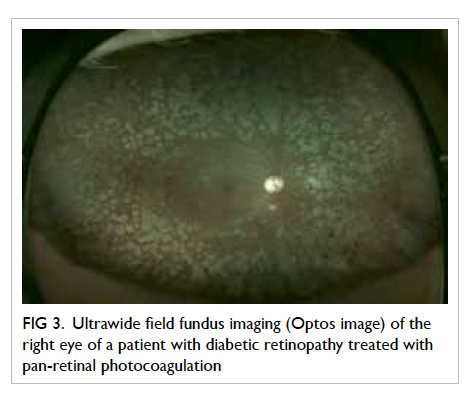
Figure 3. Ultrawide field fundus imaging (Optos image) of the right eye of a patient with diabetic retinopathy treated with pan-retinal photocoagulation
Another recent technique, cell phone–based
technique, has been used in which a handheld
condensing lens paired with a smartphone camera
can capture images at low cost.46
Methods used in screening programmes
Several countries have implemented national
screening programmes including Iceland, ENSP
in the UK, and the OPHDIAT (a telemedical
network screening system for DR) in France.47 48 49 In
the OPHDIAT programme, fundus photographs
are first taken with non-mydriatic cameras at
satellite screening centres by technicians before
they are transferred via a telemedicine network to
ophthalmologists for grading.49 In India, similar
telescreening is being carried out for DR in South
India, in which 45° single-field digital fundus
photographs are taken and images transmitted
digitally for grading by retina specialists.50 In the
UK, DR graders are not medically trained but they
undergo vigorous training by ophthalmologists and
have to carry out a minimum number of grading
episodes. There are also very stringent quality
control processes, top-up training, and revalidation
processes in place to guarantee quality. Once
patients enter the screening programme, most are
not required to undergo clinical examination by an
ophthalmologist unless in cases of STDR or if there are
ungradable fundus photographs or there is any
other eye disease that warrants management by an
ophthalmologist.
There is an additional role for general
practitioners, diabetic nurses, dieticians, and
others in a DR-related programme, such as the risk
assessment and management programme (RAMP)
in the Hospital Authority, Hong Kong. The RAMP is
a primary health care programme that aims to screen
patients for chronic systemic diseases including, in
particular, hypertension and DM including DR.51
Type 2 DM is a disease of multiple aetiologies in
which both genetic and environmental factors,
particularly lifestyle, play a significant role. Lifestyle
modification is therefore important. The RAMP in
Hong Kong tries to implement a comprehensive
package by being holistic—screening for renal
diseases, examining feet and eyes, monitoring blood
pressure and other cardiovascular risk factors, and
educating patients about lifestyle modification.
The RAMP programme in Hong Kong has been
successful in controlling HbA1c and blood pressure
in many subjects and should help to reduce the
incidence, prevalence, and severity of DR.52
Thus, DR screening can be effectively
performed by ophthalmologists, optometrists, or
specially trained graders, and other professionals
play an important part in its wider aspects.
Classification of diabetic retinopathy in screening programmes
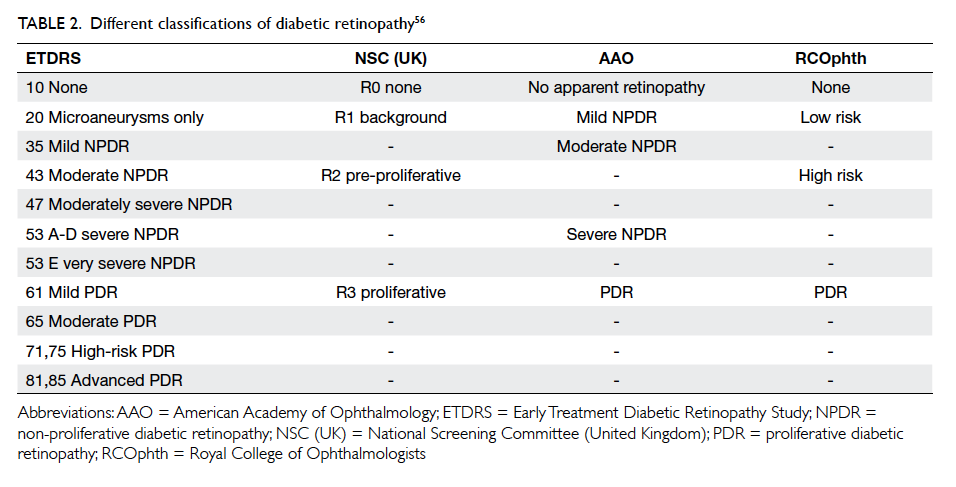
The most commonly adopted clinical classification of
DR is NPDR and PDR. From a screening perspective,
however, DR is best classified as (1) STDR or vision-threatening
diabetic retinopathy (VTDR) or (2) non-STDR (or non-VTDR), as STDR warrants referral
to an ophthalmologist for further management
while patients with non-STDR can remain in the
screening programme for further monitoring. Yau et al6 highlighted the methods of screening and DR grading used in various clinical studies and found
that most studies use ETDRS and its modification or
the AAO International Clinical Diabetic Retinopathy
Disease Severity Scale. Using this classification, DR
severity is categorised as NPDR (levels 20-53) or
PDR (≥level 60). For diabetic macular oedema, there
is more diversity. Some studies consider diabetic
macular oedema to be present if there is retinal
thickening within one disc diameter of the centre of
the macula or if there is a history of macular oedema
with a history of photocoagulation.6 Other studies
consider the presence of macular oedema if there
are hard exudates within one disc diameter of the
macula or in addition to hard exudates, presence of
microaneurysm and blot haemorrhage within one
disc diameter from the foveal centre or the presence
of focal photocoagulation scars in the macular
area.53 54
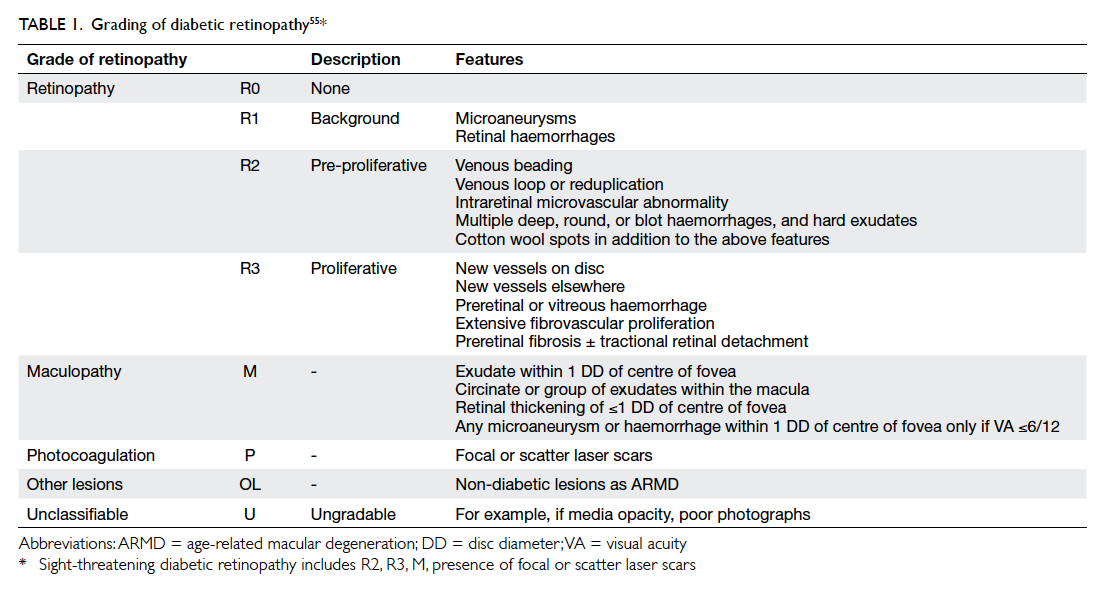
The ENSP grading system has grades of no DR
(R0), mild NPDR (R1), pre-PDR (R2) that includes
moderate and severe NPDR grades, and PDR (R3)
[Table 1].55 Maculopathy is said to be present when
there is hard exudate within one disc diameter
of the centre of the fovea or microaneurysm or
dot haemorrhage within one disc diameter of the
centre of fovea in the presence of visual acuity of
≤6/12 in the absence of any other obvious cause.55
The screening outcomes in ENSP include: annual
screening, referral to an ophthalmologist (for pre-PDR, which could be moderate or severe NPDR,
and maculopathy), and fast-track referral (for PDR).
Although there are different DR classifications, most
can be converted using a conversion table (Table 2).56
Systematic diabetic retinopathy screening in Hong Kong
Screening procedure
Diabetes is prevalent in Hong Kong with an estimated
10% of the population afflicted.57 As previously
described, the Hospital Authority, the major
public health care provider in Hong Kong, started
a multidisciplinary RAMP for patients receiving
DM care in primary care out-patient clinics known
as general out-patient clinics. All enrolled patients
in RAMP undergo comprehensive screening for
diabetic complications including systematic DR
screening, following the ENSP guideline, which was
started in Hong Kong in 2010. As well as RAMP
attempting to educate and modify the patient risk
factors such as blood sugar level (HbA1c), blood
pressure, blood lipids, body weight and smoking
habit, patients receive treatment from doctors and
further counselling from nurses about the results of
tests including the retinopathy result.
The DR screening procedure consists of
checking habitual and pinhole visual acuity in
each eye using an ETDRS chart. Pupils are dilated
and non-stereoscopic digital colour retinal fundus
photographs are taken for each patient: two
photographs are taken for each eye—one centred at
the macula and the other centred at the optic disc.
Grading procedure in diabetic retinopathy screening in risk assessment and management programme in Hong Kong
Based on the ENSP, all fundus photographs are graded
on the digital monitors with a spatial resolution of
1024 x 768 pixels, by trained optometrists and an
ophthalmologist, for presence/absence and severity
of DR.26 55 The fundus photographs undergo grading
by a primary grader, secondary grader, and arbitration
grader as per ENSP. Fundus photographs that are not
assessable are considered as ungradable. Patients
with STDR (grades R2, R3, maculopathy, and ungradable)
are referred to specialist ophthalmology
clinics of the Hospital Authority for further
management. By applying the Icelandic model,
patients with grades R0 or R1 are usually scheduled
for their next screening appointment in 12 months
or later unless they are considered at high risk for
STDR, based on risk factors including HbA1c and
blood pressure, in which case, they will be screened
at a shorter screening interval. Some RAMP clinics
use the Icelandic model as a reference to stratify the
individual risk of STDR based on the level of risk
factors.
Grader requirements and quality assurance
The graders undergo a structured training
programme to identify different features of DR,
periodic assessments, and continuous monitoring
of grading performance. The graders are required
to achieve and maintain sensitivity of ≥95% and
specificity of ≥85% at all levels of grading. For quality
assurance, a set of images were sent to an international
DR grading centre, the Ophthalmic Reading Centre,
Royal Liverpool University Hospital, Liverpool, UK,
and the grades given were compared with those
given by the local Hong Kong graders. There was a
high level of agreement between the local graders
and those from the international grading centre
(unpublished data).
This screening system in Hong Kong has the
advantage of taking digital fundus photographs,
which is feasible, affordable in terms of time and
cost, reproducible, and allows a relatively easy
grading process. The major challenge in this method
is the number of referrals generated—particularly for
patients with maculopathy. In ENSP, patients who
have a single-dot haemorrhage or exudate close to
the fovea are referred to the specialist ophthalmology
clinic. Most of these patients, particularly mild
cases who do not have clinically significant macular
oedema, do not require treatment or intervention.
In addition, patients with grade R2, which includes
grades of moderate and severe NPDR, may or may
not need immediate treatment. Nevertheless, the
system used in Hong Kong is cautious in that the
patients with STDR who require treatment will be
identified earlier rather than later.
Prevalence of diabetic retinopathy/sight-threatening diabetic retinopathy from systematic screening
From August 2010 to March 2014, a total of 262 661
screening episodes were performed with a total
number of 174 532 patients receiving DR screening.
The prevalence of any DR at first screening
was 39% (68 058/174 532) and of STDR 9.8%
(17 116/174 532).58
The future of diabetic retinopathy screening
Screening of DR is currently performed by
trained professionals, such as ophthalmologists,
optometrists, or specially trained graders. Since
it requires screening of large populations and is
time-consuming, the role of automated grading is
currently being explored. In this, a computer system
uses image processing and pattern recognition
techniques to detect the lesions of DR. The pattern
recognition consists of two distinct methods: the
digital image processing method and the neural
network method. The image processing method is
suitable for detecting and counting early lesions of
DR such as haemorrhages, microaneurysms, hard
exudates, and cotton wool spots. The neural network
method is suitable for solving pattern recognition
problems such as lesion patterns of various stages
of severity of DR; thus the neural network method
is helpful in grading DR.59 Results from computer-aided
analysis of the retina or automated analysis of
diabetic subjects, based on the appearance of blood
vessels in their ocular fundus, are encouraging.60 61
An internet-based tele-ophthalmology system could
correctly identify clinically significant macular
oedema and PDR based on Joint Photographic
Experts Group–compressed stereoscopic
photographic files when compared with standard
ETDRS-graded stereoscopic slide film photography.62
Researchers are now focusing on automated
diagnosis of retinopathy by content-based image
retrieval that is the process of retrieving related
images from a large database collection based on
their pictorial content.62
Use of optical coherence tomography in screening
Optical coherence tomography (OCT) provides
high-resolution in-vivo imaging of the different
cellular layers of the macula (Fig 4).25 It is an important
non-invasive procedure that has revolutionised the
management of diabetic macular oedema in a way
that helps to assess and monitor macular thickness,
monitor macular oedema, identify vitreomacular
traction and other forms of macular abnormalities
in patients with diabetic macular oedema.25 63
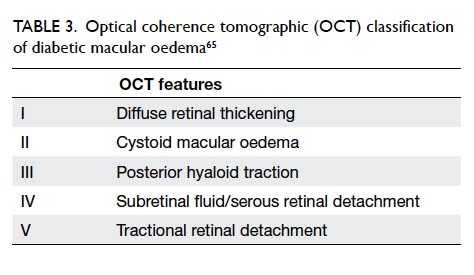
Classification by OCT of diabetic macular oedema
helps to objectively quantify and monitor the
severity of macular oedema (Table 3).63 64 65 In the DR screening programmes using non-stereoscopic
digital retinal photos, the presence of maculopathy
is judged using 2-dimensional fundus photographs
and many cases identified in this way do not warrant
treatment. Recently ENSP has started introducing
OCT as a screening tool for maculopathy at their
screening sites (unpublished data).
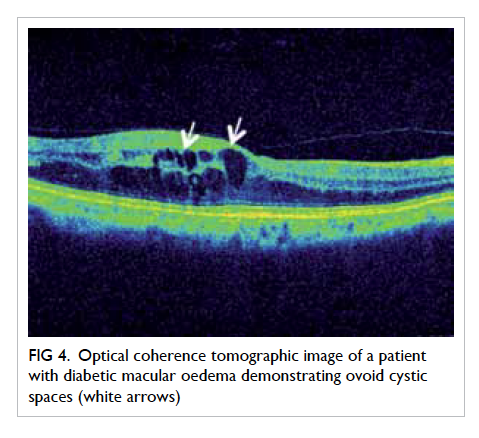
Figure 4. Optical coherence tomographic image of a patient with diabetic macular oedema demonstrating ovoid cystic spaces (white arrows)
Conclusion
This review has summarised the recommendations
and methods of DR screening adopted in various
countries globally and in Hong Kong. Development
of low-cost cameras with integration of DR screening
in public health care programmes could facilitate the
availability of DR screening to populations of different
income groups in various countries, particularly in
developing countries. Sustainability of a quality-assured
screening programme, ensuring that patients
are compliant with appropriate screening intervals
and treatment, is one of the greatest challenges that
can be overcome by educating the population and
empowering primary eye care workers and health
care workers. Continued efforts are required by all
eye care professionals.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Gakidou E, Mallinger L, Abbott-Klafter J, et al. Bulletin of
the World Heath Organization. Management of diabetes
and associated cardiovascular risk factors in seven
countries: a comparison of data from national health
examination surveys. Available from: http://www.who.int/bulletin/volumes/89/3/10-080820/en/#. Accessed 22 Oct
2015.
2. World Health Organization. Diabetes programme:
diabetes. Available from: http://www.who.int/diabetes/en/. Accessed 13 Jan 2016.
3. World Health Organization. Media centre: diabetes.
Available from: http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed 13 Jan 2016.
4. Dodson PM. Diabetic retinopathy: screening to treatment.
Oxford: Oxford University Press; 2008. Crossref
5. Fowler MJ. Microvascular and macrovascular
complications of diabetes. Clin Diabetes 2008;26:77-82. Crossref
6. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence
and major risk factors of diabetic retinopathy. Diabetes
Care 2012;35:556-64. Crossref
7. Zhu CH, Zhang SS, Kong Y, Bi YF, Wang L, Zhang Q.
Effects of intensive control of blood glucose and blood
pressure on microvascular complications in patients with
type II diabetes mellitus. Int J Ophthalmol 2013;6:141-5.
8. Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50:
risk factors for incidence and progression of retinopathy in
Type II diabetes over 6 years from diagnosis. Diabetologia
2001;44:156-63. Crossref
9. Matthews DR, Stratton IM, Aldington SJ, Holman RR,
Kohner EM, Group UKPDS. Risks of progression of
retinopathy and vision loss related to tight blood pressure
control in type 2 diabetes mellitus: UKPDS 69. Arch
Ophthalmol 2004;122:1631-40. Crossref
10. Preliminary report on effects of photocoagulation therapy.
The Diabetic Retinopathy Study Research Group. Am J
Ophthalmol 1976;81:383-96. Crossref
11. Treatment techniques and clinical guidelines for
photocoagulation of diabetic macular edema. Early
Treatment Diabetic Retinopathy Study report number
2. Early Treatment Diabetic Retinopathy Study Research
Group. Ophthalmology 1987;94:761-74.
12. Early photocoagulation for diabetic retinopathy. ETDRS
report number 9. Early Treatment Diabetic Retinopathy
Study Research Group. Ophthalmology 1991;98(5
Suppl):766S-785S.
13. Wenick AS, Bressler NM. Diabetic macular edema: current
and emerging therapies. Middle East Afr J Ophthalmol
2012;19:4-12. Crossref
14. Stewart MW. Anti-VEGF therapy for diabetic macular
edema. Curr Diab Rep 2014;14:510. Crossref
15. Garg S, Davis RM. Diabetic retinopathy screening update.
Clin Diabetes 2009;27:140-5. Crossref
16. Happich M, Reitberger U, Breitscheidel L, Ulbig M,
Watkins J. The economic burden of diabetic retinopathy
in Germany in 2002. Graefes Arch Clin Exp Ophthalmol
2008;246:151-9. Crossref
17. Wilson JM, Jungner G. Principles and practice of screening
for disease. Geneva: World Health Organization; 1968.
18. Rohan TE, Frost CD, Wald NJ. Prevention of blindness
by screening for diabetic retinopathy: a quantitative
assessment. BMJ 1989;299:1198-201. Crossref
19. Stefánsson E, Bek T, Porta M, Larsen N, Kristinsson JK,
Agardh E. Screening and prevention of diabetic blindness.
Acta Ophthalmol Scand 2000;78:374-85. Crossref
20. Crijns H, Casparie AF, Hendrikse F. Continuous computer
simulation analysis of the cost-effectiveness of screening
and treating diabetic retinopathy. Int J Technol Assess
Health Care 1999;15:198-206. Crossref
21. Jones S, Edwards RT. Diabetic retinopathy screening: a
systematic review of the economic evidence. Diabet Med
2010;27:249-56. Crossref
22. Javitt JC, Aiello LP. Cost-effectiveness of detecting
and treating diabetic retinopathy. Ann Intern Med
1996;124:164-9. Crossref
23. Lian J, McGhee SM, Gangwani RA, et al. The impact of
a co-payment on the cost-effectiveness of screening for
diabetic retinopathy. J Public Health (Oxf) 2015 Nov 14.
Epub ahead of print. Crossref
24. Diabetes care and research in Europe: the Saint Vincent
declaration. Diabet Med 1990;7:360. Crossref
25. American Academy of Ophthalmology. Diabetic
retinopathy preferred practice pattern guidelines 2014.
Available from: http://one.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp-2014. Accessed 10 Jan
2015.
26. Harding S, Greenwood R, Aldington S, et al. Grading
and disease management in national screening for
diabetic retinopathy in England and Wales. Diabet Med
2003;20:965-71. Crossref
27. Harding S, Garvican L, Talbot J. The impact of national
diabetic retinopathy screening on ophthalmology: the
need for urgent planning. Eye (Lond) 2005;19:1009-11. Crossref
28. Screening for diabetic retinopathy in Europe 15 years
after the St. Vincent Declaration. Available from: http://reseau-ophdiat.aphp.fr/Document/Doc/confliverpool.pdf.
Accessed 6 Oct 2015.
29. Ministry of Public Health. Thailand healthy lifestyle
strategic plan 2011-2020. Bangkok, Thailand: The War
Veterans Organizations of Thailand; 2011: 58.
30. World Diabetes Foundation mobile eye care WDF08-395.
Available from: http://www.worlddiabetesfoundation.org/projects/thailand-wdf08-395. Accessed 8 Apr 2013.
31. American Diabetes Association. Standards of medical care
in diabetes—2014. Diabetes Care 2014;37 Suppl 1:S14-80. Crossref
32. Diabetes—diabetic eye screening. Available from: http://www.nhs.uk/conditions/Diabetes/Pages/diabetic-eye-screening.aspx. Accessed 13 Jan 2016.
33. Aspelund T, Thornórisdóttir O, Olafsdottir E, et al.
Individual risk assessment and information technology
to optimise screening frequency for diabetic retinopathy.
Diabetologia 2011;54:2525-32. Crossref
34. McGhee S, Harding SP, Wong D. Individual risk assessment
and information technology to optimise screening
frequency for diabetic retinopathy by Aspelund et al.
(2011) Diabetologia 54:2525-2532. Graefes Arch Clin Exp
Ophthalmol 2012;250:477-8. Crossref
35. Grading diabetic retinopathy from stereoscopic color
fundus photographs—an extension of the modified Airlie
House classification. ETDRS report number 10. Early
Treatment Diabetic Retinopathy Study Research Group.
Ophthalmology 1991;98(5 Suppl):786S-806S. Crossref
36. Ryder RE, Vora JP, Atiea JA, Owens DR, Hayes TM, Young
S. Possible new method to improve detection of diabetic
retinopathy: Polaroid non-mydriatic retinal photography.
Br Med J (Clin Res Ed) 1985;291:1256-7. Crossref
37. Ku JJ, Landers J, Henderson T, Craig JE. The reliability of
single-field fundus photography in screening for diabetic
retinopathy: the Central Australian Ocular Health Study.
Med J Aust 2013;198:93-6. Crossref
38. Williams GA, Scott IU, Haller JA, Maguire AM, Marcus
D, McDonald HR. Single-field fundus photography
for diabetic retinopathy screening: a report by the
American Academy of Ophthalmology. Ophthalmology
2004;111:1055-62. Crossref
39. Scanlon PH, Malhotra R, Greenwood RH, et al. Comparison
of two reference standards in validating two field mydriatic
digital photography as a method of screening for diabetic
retinopathy. Br J Ophthalmol 2003;87:1258-63. Crossref
40. Boucher MC, Gresset JA, Angioi K, Olivier S. Effectiveness
and safety of screening for diabetic retinopathy with
two nonmydriatic digital images compared with the
seven standard stereoscopic photographic fields. Can J
Ophthalmol 2003;38:557-68. Crossref
41. Scanlon PH. The English national screening programme
for sight-threatening diabetic retinopathy. J Med Screen
2008;15:1-4. Crossref
42. Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM.
The sensitivity and specificity of single-field nonmydriatic
monochromatic digital fundus photography with remote
image interpretation for diabetic retinopathy screening:
a comparison with ophthalmoscopy and standardized
mydriatic color photography. Am J Ophthalmol
2002;134:204-13. Crossref
43. Vujosevic S, Benetti E, Massignan F, et al. Screening for
diabetic retinopathy: 1 and 3 nonmydriatic 45-degree
digital fundus photographs vs 7 standard early treatment
diabetic retinopathy study fields. Am J Ophthalmol
2009;148:111-8. Crossref
44. Silva PS, Cavallerano JD, Tolls D, et al. Potential efficiency
benefits of nonmydriatic ultrawide field retinal imaging
in an ocular telehealth diabetic retinopathy program.
Diabetes Care 2014;37:50-5. Crossref
45. Kim JD, Warren C, Shachar T, Goldberg MR, Kim JE.
Comparison of non-mydriatic ultra-widefield fundus
photography to standard early treatment of diabetic
retinopathy study 7-fields in diabetic retinopathy screening.
Invest Ophthalmol Vis Sci 2015;56:600.
46. Haddock LJ, Kim DY, Mukai S. Simple, inexpensive
technique for high-quality smartphone fundus
photography in human and animal eyes. J Ophthalmol
2013;2013:518479. Crossref
47. Olafsdottir E, Stefánsson E. Biennial eye screening in
patients with diabetes without retinopathy: 10-year
experience. Br J Ophthalmol 2007;91:1599-601. Crossref
48. Kristinsson JK. Diabetic retinopathy. Screening and
prevention of blindness. A doctoral thesis. Acta
Ophthalmol Scand Suppl 1997;(223):1-76.
49. Massin P, Chabouis A, Erginay A, et al. OPHDIAT:
a telemedical network screening system for diabetic
retinopathy in the Ile-de-France. Diabetes Metab
2008;34:227-34. Crossref
50. Raman R, Bhojwani DN, Sharma T. How accurate is the
diagnosis of diabetic retinopathy on telescreening? The
Indian scenario. Rural Remote Health 2014;14:2809.
51. Fung CS, Chin WY, Dai DS, et al. Evaluation of the quality
of care of a multi-disciplinary risk factor assessment and
management programme (RAMP) for diabetic patients.
BMC Fam Pract 2012;13:116. Crossref
52. Jiao FF, Fung CS, Wong CK, et al. Effects of the
Multidisciplinary Risk Assessment and Management
Program for Patients with Diabetes Mellitus (RAMP-DM)
on biomedical outcomes, observed cardiovascular events
and cardiovascular risks in primary care: a longitudinal
comparative study. Cardiovasc Diabetol 2014;13:127. Crossref
53. Klein BE. Overview of epidemiologic studies of diabetic
retinopathy. Ophthalmic Epidemiol 2007;14:179-83. Crossref
54. Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R,
Mohan V. Prevalence of diabetic retinopathy in urban India:
the Chennai Urban Rural Epidemiology Study (CURES)
eye study, I. Invest Ophthalmol Vis Sci 2005;46:2328-33. Crossref
55. Kinshuck D. Examining and grading retinopathy,
for professionals. Available from: http://www.diabeticretinopathy.org.uk/gradingretinopathy.htm.
Accessed 20 Aug 2015.
56. The Royal College of Ophthalmologists. Diabetic
retinopathy guidelines. Available from: https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-301-FINAL-DR-GUIDELINES-DEC-2012-updated-July-2013.pdf. Accessed 6 Nov 2015.
57. International Diabetes Federation. IDF diabetes atlas
6th edition, 2013. Available from: http://www.idf.org/diabetesatlas. Accessed 20 Aug 2015.
58. Lian JX, Gangwani RA, McGhee SM, et al. Systematic
screening for diabetic retinopathy (DR) in Hong Kong:
prevalence of DR and visual impairment among diabetic
population. Br J Ophthalmol 2016;100:151-5. Crossref
59. Lee SC, Lee ET, Kingsley RM, et al. Comparison of
diagnosis of early retinal lesions of diabetic retinopathy
between a computer system and human experts. Arch
Ophthalmol 2001;119:509-15. Crossref
60. Niemeijer M, van Ginneken B, Cree MJ, et al. Retinopathy
online challenge: automatic detection of microaneurysms
in digital color fundus photographs. IEEE Trans Med
Imaging 2010;29:185-95. Crossref
61. Li Q, You J, Zhang D. Vessel segmentation and width
estimation in retinal images using multiscale production of
matched filter responses. Expert Syst Appl 2012;39:7600-10. Crossref
62. Chaum E, Karnowski TP, Govindasamy VP, Abdelrahman
M, Tobin KW. Automated diagnosis of retinopathy by
content-based image retrieval. Retina 2008;28:1463-77. Crossref
63. Virgili G, Menchini F, Murro V, Peluso E, Rosa F, Casazza
G. Optical coherence tomography (OCT) for detection
of macular oedema in patients with diabetic retinopathy.
Cochrane Database Syst Rev 2011;(7):CD008081. Crossref
64. Al Kharousi N, Wali UK, Azeem S. Current applications of
optical coherence tomography in ophthalmology. Available
from: http://www.intechopen.com/books/optical-coherence-tomography/current-applications-of-optical-coherence-tomography-in-ophthalmology. Accessed 13 Jan 2016.
65. Kim BY, Smith SD, Kaiser PK. Optical coherence
tomographic patterns of diabetic macular edema. Am J
Ophthalmol 2006;142:405-12. Crossref