Hong Kong Med J 2016 Oct;22(5):478–85 | Epub 19 Aug 2016
DOI: 10.12809/hkmj164876
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Extracorporeal blood purification for sepsis
HP Shum, FRCP, FHKAM (Medicine)1;
WW Yan, FRCP, FHKAM (Medicine)1;
TM Chan, MD, FHKAM (Medicine)2
1 Department of Intensive Care, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr HP Shum (shumhp@ha.org.hk)
Abstract
It has been speculated that extracorporeal blood
purification therapies might improve the clinical
outcome for patients with severe sepsis, with or
without acute kidney injury, since the removal of
inflammatory mediators and/or bacterial toxins
from circulation could modulate the inflammatory
responses that result in organ damage. Despite initial
enthusiasm based on promising preliminary results,
subsequent investigations did not show sustainable
survival benefit. We review the principles and
development of blood purification techniques for
sepsis and septic acute kidney injury.
Introduction
The concepts underlying the pathogenesis of
septic acute kidney injury (AKI) are complex.
It is characterised by renal macro- and micro-circulatory
disturbance, surge of inflammatory
markers, and de-regulation of oxidative stress,
followed by a bioenergetic adaptive response and
controlled cell cycle arrest aimed at preventing cell
death.1 Continuous renal replacement therapy is
commonly performed in the critical care setting for
patients with septic AKI. The use of low- or normal-volume
continuous venovenous haemodialysis or
haemofiltration, however, has failed to demonstrate
any improvement of patient outcome in severe
sepsis.2 3 Extracorporeal blood purification therapies have been proposed to improve the outcome
for patients with severe sepsis with and without
AKI. The underlying principle is the removal of
excessive inflammatory mediators and/or bacterial
toxins from the blood compartment in order to
modulate the inflammatory response. This involves
various techniques including haemoperfusion/haemoadsorption, high-adsorption haemofiltration,
high-volume haemofiltration (HVHF), high cut-off
(HCO) membrane haemofiltration/haemodialysis,
plasma exchange, and coupled plasma filtration
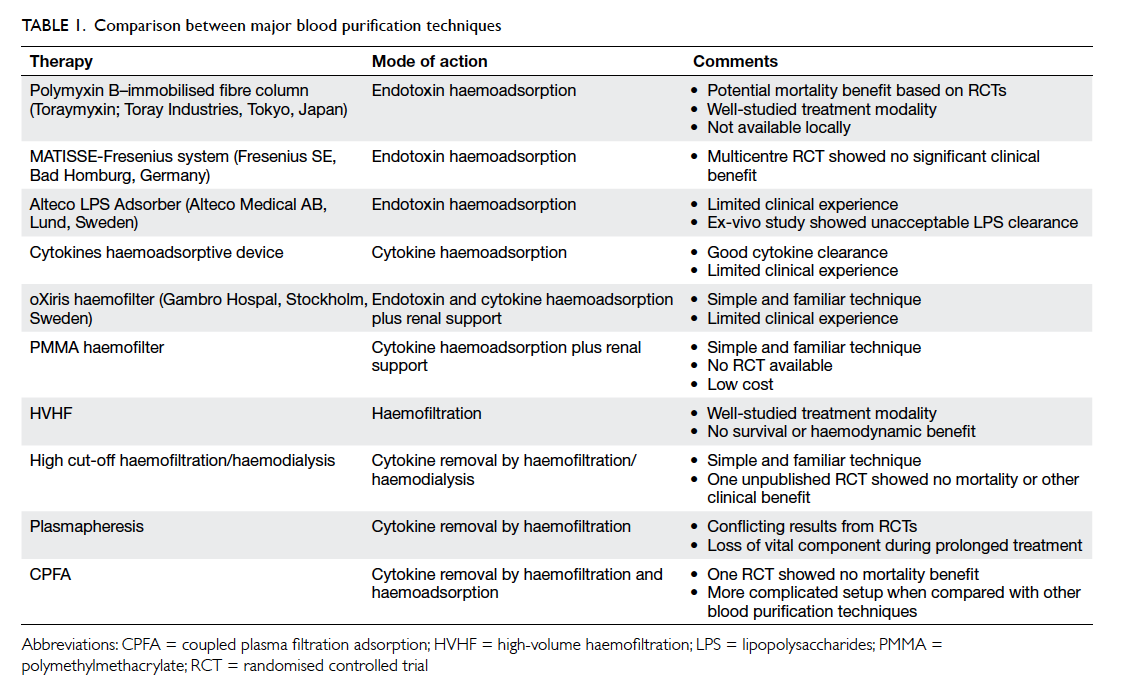
adsorption (CPFA) [Table 1]. These techniques are gaining popularity in Europe and Japan. This
overview discusses the concept and latest advances
in blood purification for sepsis and septic AKI.
Therapeutic concept of extracorporeal blood purification
During sepsis, triacylated peptides, diacylated
peptides, or lipopolysaccharides (LPS) are released
by pathogens, and are recognised by the Toll-like
receptors located on the surface of antigen-presenting
cells.4 5 Toll-like receptors also recognise
locally produced damage-associated molecular
patterns (DAMPs) from ischaemic renal tissue
and circulating DAMPs released from extensive
extrarenal tissue damage in sepsis.6 This triggers
the activation of leukocytes, endothelial cells, and
epithelial cells that release more inflammatory
mediators such as tumour necrosis factor–alpha
(TNF-α), interleukin-1 (IL-1), IL-6, IL-8 and IL-10,
causing cellular and tissue damage.7 8 This is called a ‘cytokine storm’, and can also occur in non-infectious
conditions such as severe trauma, extensive burns,
acute necrotising pancreatitis, and post–cardiac
arrest. A cytokine storm per se, in the absence
of life-threatening triggering factors, can induce
haemodynamic instability and multi-organ failure
as illustrated by Suntharalingam et al.9 Moreover,
immunoparalysis might occur after a cytokine storm
and contribute to severe secondary nosocomial
infections.10 As demonstrated in a postmortem by
Boomer et al,11 patients who die of severe sepsis
have biochemical and immunohistochemical
findings consistent with immunosuppression. This
gives rise to the concept of immunomodulation in
sepsis. Low-dose steroid administration has been
shown to improve septic shock reversal but is not
associated with any survival benefits and is currently
out of favour.12 13 The clinical benefit of intravenous immunoglobulins and anti–TNF-α in the treatment
of severe sepsis is controversial and inconclusive.14 15 Blood purification may offer non-specific clearance
of inflammatory mediators and/or microbial toxins
and thus help to restore immune homeostasis. Five
theories have been proposed to explain the potential
benefit of blood purification in sepsis. First, Ronco
et al16 proposed the “cytokine peak concentration
hypothesis” and suggested that eliminating the peaks
in cytokine blood concentration during the early
phase of sepsis could stop the inflammatory cascade,
limit organ damage, and consequently decrease the
incidence of multi-organ failure syndrome. Second,
Honoré and Matson17 proposed the “threshold
immunomodulation hypothesis” that indicated
cytokines will equilibrate between the blood and
tissue compartments. This provided an explanation
for the clinical benefit of blood purification
techniques even without any significant changes in
cytokine level within the blood compartment. Third,
Di Carlo and Alexander18 proposed the “mediator
delivery hypothesis” and suggested that high-volume
fluid replacement during haemofiltration might
promote lymphatic flow and displace inflammatory
mediators to the blood compartment, making them
available for removal. Fourth, Peng et al19 suggested
that blood purification therapies could act directly at
the cellular level to restore immune function. Finally,
Rimmelé and Kellum20 proposed the “cytokinetic
model” which indicated that blood purification
techniques remove cytokines from the blood
compartment and widen the cytokine/chemokine
concentration gradient between blood and infected
tissue. This improves leukocyte trafficking towards
the infective foci, and thus promotes bacterial killing.
Haemoperfusion/haemoadsorption
This technique binds toxins and other mediators in
the extracorporeal circuit and removes them from
the blood compartment.20 The sorbents, which
consist of microfibres or resin-covered beads, are
normally contained in cartridges that are placed
in series within the extracorporeal circuit. They
have a selective or non-selective binding capacity
for cytokines, chemokines, super-antigens, or
endotoxins by means of hydrophobic interaction,
van der Waals forces, or ionic interactions.20 Initial
clinical applications were complicated by severe
thrombocytopenia and leukopenia but these were
subsequently managed using a biocompatible
coating.
Polymyxin B–immobilised fibre column
Polymyxin B (PMX)–immobilised fibre column
haemoperfusion (Toraymyxin, Toray Industries,
Tokyo, Japan) is the most commonly used approach,
and has been used for the treatment of septic shock
since 1994 in Japan and since 2002 in Europe. It
has gained popularity worldwide in recent years,
especially after the landmark EUPHAS (Early Use
of Polymyxin B Hemoperfusion in Abdominal
Sepsis) study.21 The PMX is a group of cyclic cationic
polypeptide antibiotics derived from Bacillus
polymyxa. Endotoxins are heat and pH stable, and
thus can be difficult to remove from protein-rich
solutions such as blood. The PMX is capable of
binding and neutralising endotoxins. Nephrotoxicity
and neurotoxicity, however, are very common and
thus limit their clinical use.22 To overcome this
problem, PMX is immobilised onto polystyrene
fibres that effectively remove endotoxin without
leaching. The blood is perfused at a rate of 80 to 100
mL/min through a PMX-immobilised fibre column.
Anticoagulation is achieved using unfractionated
heparin, low-molecular-weight heparin, or the
protease inhibitor nafamostat mesylate. Treatment
usually lasts for 2 to 27 hours once or in some
patients up to 4 times, depending on the clinical
response. Three meta-analyses (approximately 1000
patients) were published before 2015: Studies by
Mitaka and Tomita23 (17 studies, 975 patients) and
Cruz et al24 (28 studies, 1425 patients) included
both randomised controlled trials (RCTs) and
observational studies. When reported, Gram-negative
infections were identified in approximately
70% of patients (range, 37.9%-100% in individual
studies). In general, PMX treatment led to significant
haemodynamic improvement with a reduction
in the use of inotropic agents/vasopressors in
patients with sepsis. Moreover, it was associated
with a decreased endotoxin level, modulation
of inflammatory markers, and improvement of
the PaO2/FiO2 ratio (ratio of the partial pressure of oxygen in arterial blood to the inspired oxygen fraction) in most included studies.23 24
Treatment by PMX significantly reduced 28-day
mortality compared with conventional therapy. The
meta-analysis by Zhou et al25 (8 studies, 370 patients)
included RCTs only and focused on mortality,
and showed significant survival benefit compared
with conventional treatment. Only a few clinically
important adverse effects were reported during
PMX haemoperfusion, including cartridge clotting,
hypotension, and hypersensitivity. Nonetheless,
the largest multicentre RCT (232 patients) testing
the performance of PMX haemoperfusion in
peritonitis-induced septic shock was published in
April 2015, and reported contrasting findings.26 No
significant differences in 28-day mortality (27.7%
in PMX-treated group vs 19.5% in controls; P=0.14),
haemodynamic patterns, or organ failure evolution
were observed. This negative result was similar to a
large retrospective study (642 patients) by Iwagami
et al27 who examined the effect of postoperative
PMX haemoperfusion on peritonitis-induced
septic shock. Patients treated with one or two PMX
haemoperfusion sessions showed similar mortality at
day 28 (17%) to propensity-matched patients without
PMX treatment (16.3%). EUPHRATES (safety and
efficacy of PMX haemoperfusion for septic shock
study), a very large multicentre US-based phase III
trial in patients with confirmed endotoxaemia, is
currently underway and results should be available
after July 2017.28 Based on current evidence, the
clinical benefit of PMX haemoperfusion in Gram-negative
sepsis is unclear. Moreover, the cost of
individual haemoperfusion cartridges is very high
(approximately HK$40 000 per cartridge) and limits
its clinical use in local settings. Currently, PMX-immobilised
fibre column haemoperfusion is not
available in Hong Kong.
MATISSE-Fresenius system
The MATISSE-Fresenius system (Fresenius SE, Bad
Homburg, Germany) binds endotoxins to human
albumin. The extracorporeal circuit is maintained
by the Fresenius haemoadsorption machine using
the MATISSE haemoadsorber that contains human
serum albumin immobilised on polymethacrylate
beads. Trends in the improvement of morbidity
and organ dysfunction were reported in initial
non-randomised studies,29 30 although a subsequent
multicentre RCT could not identify any significant
clinical benefit, which then limited its clinical use.31
Currently, the MATISSE-Fresenius system is not
available in Hong Kong.
Alteco Lipopolysaccharide Adsorber
The Alteco LPS Adsorber (Alteco Medical AB,
Lund, Sweden) captures endotoxins using specially
designed synthetic peptides. This device was
launched in 2006. Tailor-made synthetic peptides
with a high affinity for endotoxins are attached to the
surface of the polyethylene plates using a covalent
bonding technique. Clinical experience with this
device is scarce, and is limited mainly to case reports
and case series.32 33 34 In general, these case series
report a shorter vasopressor infusion duration in
adsorber-treated patients compared with controls.
Only one underpowered RCT has been published
by local investigators.35 The study was terminated
early and showed no significant clinical benefit
(disease severity score, vasopressor use, length of
study, and 28-day mortality) following the addition
of the Alteco LPS Adsorber to conventional therapy
in patients who had intra-abdominal sepsis with
shock.35 The side-effect profile of this novel device
was acceptable but a recent ex-vivo experimental
study showed that the Alteco LPS Adsorber could
not achieve acceptable LPS clearance in serum,
heparinised plasma, or whole blood.36 Therefore,
the potential benefit of the Alteco LPS Adsorber in
sepsis is not clear.
Cytokines haemoadsorptive device
Several cytokine-absorbing columns have been tested
in animal studies, showing excellent adsorption rates
for inflammatory cytokines such as TNF-α, IL-1β,
IL-6, and IL-8.37 Human data are limited to case
reports and case series.38 39 40 CytoSorb (CytoSorbents
Corporation; Monmouth Junction [NJ], US) is
a novel synthetic haemabsorption column that
targets inflammatory mediators.41 It is currently
the only European-approved extracorporeal device
for cytokine haemoadsorption. Case reports show
good cytokine clearance and haemodynamic
improvement with this device.41 42 43 44 Further studies
focused on clinically relevant endpoints are highly
recommended. CytoSorb is not available in Hong
Kong.
High-adsorption haemofiltration
The AN69 and polymethylmethacrylate (PMMA)
membrane haemofilters are the currently
available options for performing high-adsorption
haemofiltration in septic patients. Both have a
high cytokine adsorption capacity but surface
treatment can further modify their haemoadsorptive
properties.45 46 47
oXiris haemofilter
oXiris (Gambro Hospal, Stockholm, Sweden) is an
AN69-based membrane haemofilter that is surface-treated
with a polyethyleneimine and grafted with
heparin (Table 2). The AN69 core membrane has superior cytokine-binding capacity compared with
the traditional polysulphone membrane. Surface
treatment with polyethyleneimine enhances
endotoxin capture,48 while heparin coating reduces
membrane thrombogenicity, and prolongs the filter
life and improves efficiency. A case-control study by
Shum et al49 involving Gram-negative septicaemic
patients (n=6) showed that oXiris continuous
venovenous haemofiltration (CVVH) was associated
with a greater reduction in Sequential Organ Failure
Assessment score compared with conventional
polysulphone-based CVVH (n=24). Subsequent
large case series (n=40) suggested that oXiris
treatment had a positive effect on haemodynamics
with a reduction in cytokine levels (IL-6).50
Treatment usually lasts for 72 hours (manufacturer’s
recommendation) and costs approximately HK$8000
per haemofilter. As of mid 2015, at least five public
hospitals in Hong Kong have clinical experience with
oXiris haemofilters in the treatment of septic shock.
A large-scale RCT will be necessary to determine the
potential benefit of this device, however.
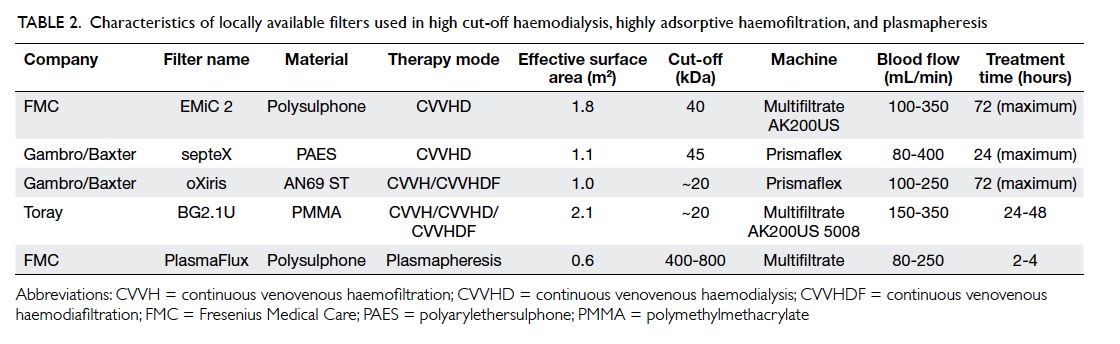
Table 2. Characteristics of locally available filters used in high cut-off haemodialysis, highly adsorptive haemofiltration, and plasmapheresis
Polymethylmethacrylate haemofilter
The PMMA membrane has a higher cytokine
adsorption capacity than the traditional
polyacrylonitrile and polysulphone membrane.51
Membrane binding site saturation is one of the
main concerns during treatment involving highly
adsorptive haemofiltration. The PMMA haemofilter
can maintain its cytokine adsorption capacity
for at least 24 hours after being changed.52 The
initial clinical experience of PMMA continuous
haemodiafiltration in the treatment of sepsis is
encouraging, with significant haemodynamic
improvement and potential survival benefit.53 54 A
local RCT (Australian New Zealand Clinical Trial
Registry ACTRN12611000652976) that is aimed at
investigating the clinical benefit of PMMA-based
CVVH in patients with septic shock and AKI is
currently underway. Treatment usually lasts for 24
to 48 hours and costs approximately HK$300 per
haemofilter.
High-volume haemofiltration
In 2002, HVHF was defined as >35 mL/kg/h, based
on recommendations from the Acute Dialysis
Quality Initiative Workgroup.55 Nonetheless in
clinical practice, 35 mL/kg/h is not that high and
can be achieved with ease, especially in those with
low body weight. To clarify this issue, Honore et al56 defined continuous HVHF as 50 to 70 mL/kg/h, and 100 to 120 mL/kg/h for 4 to 8 hours followed
by conventional CVVH as pulse HVHF. In addition,
HVHF is regarded as effective blood purification
therapy because circulating inflammatory mediators
are mostly water-soluble and range between 5 kDa
and 60 kDa. They are more effectively removed by
convective means than by diffusion techniques.
Moreover, haemofilter membranes have some
adsorptive properties that allow the removal of
mediators with a molecular weight higher than the
membrane cut-off point. It is clear that conventional
haemofiltration with low ultrafiltration rates is
ineffective for cytokine removal.2 3 Increasing the
ultrafiltration flow rate can increase the adsorption
capacity of the haemofilter because of its effect on
transmembrane pressure (greater membrane site
recruitment) and the exposure of more available
adsorptive surface area.57 Only two RCTs that
investigated the potential benefit of HVHF over
conventional CVVH in septic patients were available
before the publication of the landmark trial (high-volume
versus standard-volume haemofiltration
for septic shock patients with acute kidney injury
study) in 2013.57 58 Cole et al57 performed the first
randomised crossover clinical trial that involved 11
patients with septic shock and multi-organ failure.
Patients were assigned to either 8 hours of HVHF
(6 L/h) or 8 hours of standard CVVH (1 L/h) in a
random order. The results showed that HVHF was
associated with a greater reduction in vasopressor
use. A study by Boussekey et al58 (HVHF 65 mL/kg/h
vs control 35 mL/kg/h; n=20) yielded similar
findings and showed no survival benefit of HVHF
over conventional CVVH. Multiple non-randomised
studies showed decreased mortality with HVHF for
septic shock patients but most of the studies were
relatively small.59 60 61 Despite the initially encouraging
results, HVHF has not gained in popularity because
the use of a large volume of ultrapure replacement
solution equates to significant increases in treatment
cost, risk of severe electrolyte disturbance, and
nursing workload. The landmark IVORIE study was
published in 2013.62 This multicentre RCT involved
140 critically ill septic shock patients who were
randomised to receive either HVHF at 70 mL/kg/h
or standard CVVH treatment at 35 mL/kg/h. It
showed neither significant survival benefit nor
haemodynamic improvement for HVHF compared
with standard treatment. Subsequently two meta-analyses
(4 studies with approximately 500
patients) published in 2014 concluded that neither
HVHF nor pulse HVHF offered any added clinical
benefit when compared with standard-volume
haemofiltration.63 64 Therefore, the routine use of
HVHF for treatment of sepsis is not recommended.
High cut-off haemodialysis/haemofiltration
Inflammatory mediators are relatively large (TNF-α:
17 kDa, IL-6: 26 kDa, and IL-8: 8 kDa), and are
classified as middle molecules. The conventional
high-flux haemofilter has a cut-off point at
approximately 20 kDa and is unlikely to achieve
good cytokine clearance.2 3 The nominal cut-off point
for HCO membranes ranges from 60 to 150 kDa
and the clinical cut-off point in blood ranges from
40 to 100 kDa.65 This can greatly increase
the sieving coefficients of various inflammatory
mediators at the expense of loss of albumin (66 kDa), antithrombin-III (60 kDa), protein C (62 kDa), and many other vital proteins. Reducing the pore size slightly can limit vital protein loss but also
decrease cytokine removal. Ex-vivo studies showed
that HCO haemofiltration displayed the greatest
consistency in cytokine removal when compared
with standard haemofiltration.66 The CPFA and
haemoadsorption appeared to offer a similar level of
cytokine clearance to the HCO technique. Albumin
loss was comparable between HCO haemofiltration,
HCO haemodialysis, and HCO haemodiafiltration.66
Morgera et al67 published the first study on the use of
HCO haemofiltration among septic shock patients
and showed good IL-6 (but not TNF-α) clearance.
Subsequently, Morgera et al68 conducted an RCT
that involved 30 septic AKI patients who were
randomised to HCO or conventional haemofiltration.
The HCO group showed a significant decline in
vasopressor use and cytokine level.68 The largest
RCT was the High Cut-Off Continuous Veno-venous
Hemodialysis (CVVHD) in Patients Treated
for Acute Renal Failure After Systemic Inflammatory
Response Syndrome (SIRS)/Septic Shock (HICOSS)
study.69 The estimated sample size was 120 patients
but the study was terminated early because of a lack
of difference between the groups after 81 patients
had been recruited. There was no difference in
28-day mortality, vasopressor use, duration of
mechanical ventilation, length of stay in intensive
care unit, or albumin level between the groups.69 This
underpowered RCT (due to premature termination)
cannot provide a clear answer about the potential
benefit of HCO haemofiltration/haemodialysis in
septic patients and a further large-scale prospective
RCT is recommended. Only the septeX (Gambro
Hospal, Stockholm, Sweden) and EMiC 2 (Fresenius
SE, Bad Homburg, Germany) HCO haemofilters are
available in Hong Kong (Table 2). Treatment usually lasts for
24 to 72 hours and costs approximately HK$8000 per
HCO haemofilter.
Plasmapheresis and coupled plasma filtration adsorption
The nominal cut-off point for the plasma filter ranges
from 400 to 800 kDa and therefore can achieve good
cytokine removal with significant albumin loss
(Table 2). Only three RCTs have been published
to date. Busund et al70 published the largest RCT
involving 106 adult septic patients randomised to
receive either two sessions of plasmapheresis or
standard therapy. Plasmapheresis offered better
28-day survival compared with the control group
(67% vs 47%). Studies by Reeves et al71 and Long et al72 showed no survival benefit, however. Therefore,
the debate regarding the benefit of plasmapheresis
in sepsis continues. One important drawback of
plasmapheresis is the significant loss of albumin,
fibrinogen, antithrombin, and immunoglobulin
that takes a long time to regenerate in the absence
of post-treatment replacement.73 This problem can
be resolved with the use of CPFA (Lynda, Bellco,
Mirandola, Italy). This CPFA therapy comprises
a plasma filter, a non-selective hydrophobic resin
cartridge with high affinity for inflammatory
mediators, and a high-flux haemofilter for
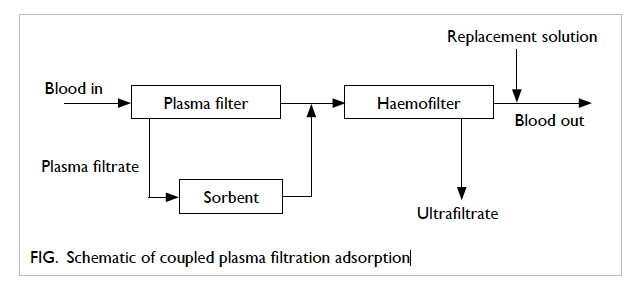
convective solute removal (Fig).74 Only filtrated
plasma has direct contact with the sorbents that
have no biocompatibility problems when compared
with direct haemoperfusion. Treatment lasts for
approximately 10 hours and requires cartridge
changes due to saturation problems. Livigni et al75
published the only multicentre RCT focused on
patients with septic shock. Patients were randomised
to standard treatment with or without CPFA. The
CPFA therapy was performed daily for 5 days and
lasted at least 10 hours/day. The estimated sample
size was 330 patients but the study was terminated
early on the grounds of futility after 192 patients had
been recruited. No significant benefits for mortality,
organ dysfunction, or intensive care unit stay were
observed. Therefore, based on the available evidence,
the routine use of CPFA for treatment of septic
shock is not recommended. The CPFA is currently
not available in Hong Kong.
Conclusion
Building on the concept of excessive inflammatory
mediator release, blood purification techniques
have emerged as an adjunctive therapy for patients
with severe sepsis and septic AKI. They are effective
in clearing endotoxin or inflammatory mediators
and are well tolerated. Despite initially promising
results, most blood purification techniques have not
provided any sustainable mortality benefits. In severe
sepsis, source control, early appropriate antibiotics,
and haemodynamic support are the three most
important treatment components.76 As a supportive
treatment, blood purification techniques may not
significantly affect patient mortality. Since the
outcome for septic patients has improved over time,
much larger sample sizes will be needed to detect
the relatively small effects of these new therapies
on sepsis.77 Large-scale, well-designed, prospective
RCTs are the way forward.
The application of these novel techniques should be
individualised but more specific recommendations
must await further evidence.
Declaration of interests
All authors have disclosed no conflicts of interest.
References
1. Shum HP, Yan WW, Chan TM. Recent knowledge on the
pathophysiology of septic acute kidney injury: A narrative
review. J Crit Care 2016;31:82-9. Crossref
2. Cole L, Bellomo R, Hart G, et al. A phase II randomized,
controlled trial of continuous hemofiltration in sepsis. Crit
Care Med 2002;30:100-6. Crossref
3. Payen D, Mateo J, Cavaillon JM, et al. Impact of continuous
venovenous hemofiltration on organ failure during the
early phase of severe sepsis: a randomized controlled trial.
Crit Care Med 2009;37:803-10. Crossref
4. Prince LR, Whyte MK, Sabroe I, Parker LC. The role of
TLRs in neutrophil activation. Curr Opin Pharmacol
2011;11:397-403. Crossref
5. Nakayama H, Kurokawa K, Lee BL. Lipoproteins in
bacteria: structures and biosynthetic pathways. FEBS J
2012;279:4247-68. Crossref
6. Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute
kidney injury revisited: pathophysiology, prevention and
future therapies. Curr Opin Crit Care 2014;20:588-95. Crossref
7. Angus DC, van der Poll T. Severe sepsis and septic shock.
N Engl J Med 2013;369:2063. Crossref
8. Gomez H, Ince C, De Backer D, et al. A unified theory
of sepsis-induced acute kidney injury: inflammation,
microcirculatory dysfunction, bioenergetics, and the
tubular cell adaptation to injury. Shock 2014;41:3-11. Crossref
9. Suntharalingam G, Perry MR, Ward S, et al. Cytokine
storm in a phase 1 trial of the anti-CD28 monoclonal
antibody TGN1412. N Engl J Med 2006;355:1018-28. Crossref
10. Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus
reactivation in critically ill immunocompetent patients.
JAMA 2008;300:413-22. Crossref
11. Boomer JS, To K, Chang KC, et al. Immunosuppression
in patients who die of sepsis and multiple organ failure.
JAMA 2011;306:2594-605. Crossref
12. Wang C, Sun J, Zheng J, et al. Low-dose hydrocortisone
therapy attenuates septic shock in adult patients but does
not reduce 28-day mortality: a meta-analysis of randomized
controlled trials. Anesth Analg 2014;118:346-57. Crossref
13. Kalil AC, Sun J. Low-dose steroids for septic shock and
severe sepsis: the use of Bayesian statistics to resolve clinical
trial controversies. Intensive Care Med 2011;37:420-9. Crossref
14. Alejandria MM, Lansang MA, Dans LF, Mantaring JB 3rd.
Intravenous immunoglobulin for treating sepsis, severe
sepsis and septic shock. Cochrane Database Syst Rev
2013;(9):CD001090.
15. Bernard GR, Francois B, Mira JP, et al. Evaluating the
efficacy and safety of two doses of the polyclonal anti-tumor
necrosis factor-alpha fragment antibody AZD9773
in adult patients with severe sepsis and/or septic shock:
randomized, double-blind, placebo-controlled phase IIb
study. Crit Care Med 2014;42:504-11. Crossref
16. Ronco C, Tetta C, Mariano F, et al. Interpreting the
mechanisms of continuous renal replacement therapy in
sepsis: the peak concentration hypothesis. Artif Organs
2003;27:792-801. Crossref
17. Honoré PM, Matson JR. Extracorporeal removal for sepsis:
Acting at the tissue level—the beginning of a new era for
this treatment modality in septic shock. Crit Care Med
2004;32:896-7. Crossref
18. Di Carlo JV, Alexander SR. Hemofiltration for cytokine-driven
illnesses: the mediator delivery hypothesis. Int J
Artif Organs 2005;28:777-86.
19. Peng Z, Singbartl K, Simon P, et al. Blood purification in
sepsis: a new paradigm. Contrib Nephrol 2010;165:322-8. Crossref
20. Rimmelé T, Kellum JA. Clinical review: blood purification
for sepsis. Crit Care 2011;15:205. Crossref
21. Cruz DN, Antonelli M, Fumagalli R, et al. Early use
of polymyxin B hemoperfusion in abdominal septic
shock: the EUPHAS randomized controlled trial. JAMA
2009;301:2445-52. Crossref
22. Shoji H. Extracorporeal endotoxin removal for the
treatment of sepsis: endotoxin adsorption cartridge
(Toraymyxin). Ther Apher Dial 2003;7:108-14. Crossref
23. Mitaka C, Tomita M. Polymyxin B–immobilized fiber
column hemoperfusion therapy for septic shock. Shock
2011;36:332-8. Crossref
24. Cruz DN, Perazella MA, Bellomo R, et al. Effectiveness
of polymyxin B–immobilized fiber column in sepsis: a
systematic review. Crit Care 2007;11:R47. Crossref
25. Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification
and mortality in sepsis: a meta-analysis of randomized
trials. Crit Care Med 2013;41:2209-20. Crossref
26. Payen DM, Guilhot J, Launey Y, et al. Early use of
polymyxin B hemoperfusion in patients with septic shock
due to peritonitis: a multicenter randomized control trial.
Intensive Care Med 2015;41:975-84. Crossref
27. Iwagami M, Yasunaga H, Doi K, et al. Postoperative
polymyxin B hemoperfusion and mortality in patients with
abdominal septic shock: a propensity-matched analysis.
Crit Care Med 2014;42:1187-93. Crossref
28. Klein DJ, Foster D, Schorr CA, Kazempour K, Walker PM,
Dellinger RP. The EUPHRATES trial (Evaluating the Use of
Polymyxin B Hemoperfusion in a Randomized controlled
trial of Adults Treated for Endotoxemia and Septic shock):
study protocol for a randomized controlled trial. Trials
2014;15:218. Crossref
29. Ullrich H, Jakob W, Frohlich D, et al. A new endotoxin
adsorber: first clinical application. Ther Apher 2001;5:326-34. Crossref
30. Staubach KH, Boehme M, Zimmermann M, Otto V. A
new endotoxin adsorption device in Gram-negative sepsis:
use of immobilized albumin with the MATISSE adsorber.
Transfus Apher Sci 2003;29:93-8. Crossref
31. Reinhart K, Meier-Hellmann A, Beale R, et al. Open
randomized phase II trial of an extracorporeal endotoxin
adsorber in suspected Gram-negative sepsis. Crit Care
Med 2004;32:1662-8. Crossref
32. Kulabukhov VV. Use of an endotoxin adsorber in the
treatment of severe abdominal sepsis. Acta Anaesthesiol
Scand 2008;52:1024-5. Crossref
33. Yaroustovsky M, Abramyan M, Popok Z, et al. Preliminary
report regarding the use of selective sorbents in complex
cardiac surgery patients with extensive sepsis and
prolonged intensive care stay. Blood Purif 2009;28:227-33. Crossref
34. Ala-Kokko TI, Laurila J, Koskenkari J. A new endotoxin
adsorber in septic shock: observational case series. Blood
Purif 2011;32:303-9. Crossref
35. Shum HP, Leung YW, Lam SM, Chan KC, Yan WW. Alteco
endotoxin hemoadsorption in Gram-negative septic shock
patients. Indian J Crit Care Med 2014;18:783-8. Crossref
36. Harm S, Falkenhagen D, Hartmann J. Endotoxin
adsorbents in extracorporeal blood purification: do they
fulfill expectations? Int J Artif Organs 2014;37:222-32. Crossref
37. Taniguchi T. Cytokine adsorbing columns. Contrib
Nephrol 2010;166:134-41. Crossref
38. Kellum JA, Venkataraman R, Powner D, Elder M,
Hergenroeder G, Carter M. Feasibility study of cytokine
removal by hemoadsorption in brain-dead humans. Crit
Care Med 2008;36:268-72. Crossref
39. Kobe Y, Oda S, Matsuda K, Nakamura M, Hirasawa H.
Direct hemoperfusion with a cytokine-adsorbing device
for the treatment of persistent or severe hypercytokinemia:
a pilot study. Blood Purif 2007;25:446-53. Crossref
40. Tsuchida K, Takemoto Y, Sugimura K, Yoshimura R,
Nakatani T. Direct hemoperfusion by using Lixelle column
for the treatment of systemic inflammatory response
syndrome. Int J Mol Med 2002;10:485-8. Crossref
41. Morris C, Gray L, Giovannelli M. Early report: The use
of CytoSorb haemabsorption column as an adjunct in
managing severe sepsis: initial experiences, review and
recommendations. J Intensive Care Soc 2015;16:257-64. Crossref
42. Hinz B, Jauch O, Noky T, Friesecke S, Abel P, Kaiser R.
CytoSorb, a novel therapeutic approach for patients with
septic shock: a case report. Int J Artif Organs 2015;38:461-4. Crossref
43. Wiegele M, Krenn CG. CytoSorb in a patient with
Legionella pneumonia–associated rhabdomyolysis: a case
report. ASAIO J 2015;61:e14-6. Crossref
44. Hetz H, Berger R, Recknagel P, Steltzer H. Septic shock
secondary to beta-hemolytic streptococcus-induced
necrotizing fasciitis treated with a novel cytokine
adsorption therapy. Int J Artif Organs 2014;37:422-6. Crossref
45. Bouman CS, van Olden RW, Stoutenbeek CP. Cytokine
filtration and adsorption during pre- and postdilution
hemofiltration in four different membranes. Blood Purif
1998;16:261-8. Crossref
46. De Vriese AS, Colardyn FA, Philippé JJ, Vanholder RC,
De Sutter JH, Lameire NH. Cytokine removal during
continuous hemofiltration in septic patients. J Am Soc
Nephrol 1999;10:846-53.
47. Hirasawa H, Oda S, Matsuda K. Continuous
hemodiafiltration with cytokine-adsorbing hemofilter
in the treatment of severe sepsis and septic shock.
Contrib Nephrol 2007;156:365-70. Crossref
48. Mitzner S, Schneidewind J, Falkenhagen D, Loth F,
Klinkmann H. Extracorporeal endotoxin removal by
immobilized polyethylenimine. Artif Organs 1993;17:775-81. Crossref
49. Shum HP, Chan KC, Kwan MC, Yan WW. Application
of endotoxin and cytokine adsorption haemofilter in
septic acute kidney injury due to Gram-negative bacterial
infection. Hong Kong Med J 2013;19:491-7. Crossref
50. Turani F, Candidi F, Barchetta R, et al. Continuous renal
replacement therapy with the adsorbent membrane oXiris
in septic patients: a clinical experience. Crit Care 2013;17 Suppl 2:63. Crossref
51. Matsuda K, Hirasawa H, Oda S, Shiga H, Nakanishi K.
Current topics on cytokine removal technologies. Ther
Apher 2001;5:306-14. Crossref
52. Nakada TA, Hirasawa H, Oda S, Shiga H, Matsuda K.
Blood purification for hypercytokinemia. Transfus Apher
Sci 2006;35:253-64. Crossref
53. Nakada TA, Oda S, Matsuda K, et al. Continuous
hemodiafiltration with PMMA hemofilter in the treatment
of patients with septic shock. Mol Med 2008;14:257-63. Crossref
54. Matsuda K, Moriguchi T, Harii N, Yanagisawa M, Harada D,
Sugawara H. Comparison of efficacy between continuous
hemodiafiltration with a PMMA high-performance
membrane dialyzer and a PAN membrane hemofilter in
the treatment of septic shock patients with acute renal
failure. Contrib Nephrol 2011;173:182-90. Crossref
55. Kellum JA, Mehta RL, Angus DC, Palevsky P, Ronco C,
ADQI Workgroup. The first international consensus
conference on continuous renal replacement therapy.
Kidney Int 2002;62:1855-63. Crossref
56. Honore PM, Joannes-Boyau O, Boer W, Collin V. High-volume
hemofiltration in sepsis and SIRS: current concepts
and future prospects. Blood Purif 2009;28:1-11. Crossref
57. Cole L, Bellomo R, Journois D, Davenport P, Baldwin I,
Tipping P. High-volume haemofiltration in human septic
shock. Intensive Care Med 2001;27:978-86. Crossref
58. Boussekey N, Chiche A, Faure K, et al. A pilot randomized
study comparing high and low volume hemofiltration
on vasopressor use in septic shock. Intensive Care Med
2008;34:1646-53. Crossref
59. Cornejo R, Downey P, Castro R, et al. High-volume
hemofiltration as salvage therapy in severe hyperdynamic
septic shock. Intensive Care Med 2006;32:713-22. Crossref
60. Joannes-Boyau O, Rapaport S, Bazin R, Fleureau C, Janvier
G. Impact of high volume hemofiltration on hemodynamic
disturbance and outcome during septic shock. ASAIO J
2004;50:102-9. Crossref
61. Piccinni P, Dan M, Barbacini S, et al. Early isovolaemic
haemofiltration in oliguric patients with septic shock.
Intensive Care Med 2006;32:80-6. Crossref
62. Joannes-Boyau O, Honoré PM, Perez P, et al. High-volume
versus standard-volume haemofiltration for septic shock
patients with acute kidney injury (IVOIRE study): a
multicentre randomized controlled trial. Intensive Care
Med 2013;39:1535-46. Crossref
63. Lehner GF, Wiedermann CJ, Joannidis M. High-volume
hemofiltration in critically ill patients: a systematic review
and meta-analysis. Minerva Anestesiol 2014;80:595-609.
64. Clark E, Molnar AO, Joannes-Boyau O, Honoré PM, Sikora
L, Bagshaw SM. High-volume hemofiltration for septic
acute kidney injury: a systematic review and meta-analysis.
Crit Care 2014;18:R7. Crossref
65. Haase M, Bellomo R, Morgera S, Baldwin I, Boyce N. High
cut-off point membranes in septic acute renal failure: a
systematic review. Int J Artif Organs 2007;30:1031-41.
66. Atan R, Crosbie D, Bellomo R. Techniques of extracorporeal
cytokine removal: a systematic review of the literature.
Blood Purif 2012;33:88-100. Crossref
67. Morgera S, Rocktäschel J, Haase M, et al. Intermittent high
permeability hemofiltration in septic patients with acute
renal failure. Intensive Care Med 2003;29:1989-95. Crossref
68. Morgera S, Haase M, Kuss T, et al. Pilot study on the
effects of high cutoff hemofiltration on the need for
norepinephrine in septic patients with acute renal failure.
Crit Care Med 2006;34:2099-104. Crossref
69. Honore P, Beck W, editors. High cut-off continuous venovenous
hemodialysis (CVVHD) in patients treated for
acute renal failure after systemic inflammatory response
syndrome (SIRS)/septic shock (HICOSS). Proceedings of
the 10th Congress of the World Federation of Societies of
Intensive and Critical Care Medicine (WFSICCM); 2009
Aug 28-Sep 1; Florence, Italy.
70. Busund R, Koukline V, Utrobin U, Nedashkovsky E.
Plasmapheresis in severe sepsis and septic shock: a
prospective, randomised, controlled trial. Intensive Care
Med 2002;28:1434-9. Crossref
71. Reeves JH, Butt WW, Shann F, et al. Continuous
plasmafiltration in sepsis syndrome. Plasmafiltration in
Sepsis Study Group. Crit Care Med 1999;27:2096-104. Crossref
72. Long EJ, Shann F, Pearson G, Buckley D, Butt W. A
randomised controlled trial of plasma filtration in severe
paediatric sepsis. Crit Care Resusc 2013;15:198-204.
73. Stegmayr B, Abdel-Rahman EM, Balogun RA. Septic shock
with multiorgan failure: from conventional apheresis to
adsorption therapies. Semin Dial 2012;25:171-5. Crossref
74. Formica M, Inguaggiato P, Bainotti S, Wratten ML.
Coupled plasma filtration adsorption. Contrib Nephrol
2007;156:405-10. Crossref
75. Livigni S, Bertolini G, Rossi C, et al. Efficacy of coupled
plasma filtration adsorption (CPFA) in patients with septic
shock: a multicenter randomised controlled clinical trial.
BMJ Open 2014;4:e003536. Crossref
76. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis
campaign: international guidelines for management
of severe sepsis and septic shock: 2012. Crit Care Med
2013;41:580-637. Crossref
77. Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R.
Mortality related to severe sepsis and septic shock among
critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308-16. Crossref