Hong Kong Med J 2016 Oct;22(5):435–44 | Epub 12 Aug 2016
DOI: 10.12809/hkmj154739
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Can surgical need in patients with Naja atra (Taiwan or Chinese cobra) envenomation be
predicted in the emergency department?
HY Su, MD1;
MJ Wang, PhD2;
YH Li, PhD3;
CN Tang, MD4;
MJ Tsai, MD, PhD5
1 Department of Emergency Medicine, E-Da Hospital and I-Shou University, Kaohsiung, Taiwan; Department of Emergency Medicine, Buddhist Tzu Chi General Hospital, Hualien, Taiwan
2 Department of Medical Research, Buddhist Tzu Chi General Hospital, Hualien, Taiwan
3 Department of Public Health, Tzu Chi University, Hualien, Taiwan
4 Department of Family Medicine, Buddhist Tzu Chi General Hospital, Hualien, Taiwan
5 Department of Emergency Medicine, Ditmanson Medical Foundation Chiayi Christian Hospital, Chiayi, Taiwan; Department of Sports Management, Chia Nan University of Pharmacy and Science, Tainan, Taiwan
Corresponding author: Dr MJ Tsai (tshi33@gmail.com)
An earlier version of this paper was presented at the 7th Asian Conference on Emergency Medicine held in Tokyo, Japan on 23-25 October 2013.
Abstract
Objectives: To investigate the clinical predictors
and the aetiologies for surgery in patients with Naja atra (Taiwan or Chinese cobra) envenomation.
Methods: This case series was conducted in the only
tertiary care centre in eastern Taiwan. Patients who
presented to the emergency department with Naja atra bite between January 2008 and September 2014
were included. Clinical information was collected
and compared between surgical and non-surgical
patients.
Results: A total of 28 patients with Naja atra
envenomation presented to the emergency
department during the study period. Of these, 60.7%
(n=17) required surgery. Necrotising fasciitis (76.5%)
was the main finding in surgery. Comparisons
between surgical and non-surgical patients showed
skin ecchymosis (odds ratio=34.36; 95% confidence
interval, 2.20-536.08; P=0.012) and a high total dose
of antivenin (≥6 vials; odds ratio=14.59; 95%
confidence interval, 1.10-192.72; P=0.042) to be
the most significant predictors of surgery. The rate
of bacterial isolation from the surgical wound was
88.2%. Morganella morganii (76.5%), Enterococcus
faecalis (58.8%), and Bacteroides fragilis (29.4%) were
the most common pathogens involved. Bacterial
susceptibility testing indicated that combined broad-spectrum
antibiotics were needed to cover mixed
aerobic and anaerobic bacterial infection.
Conclusions: Patients with Naja atra envenomation
who present with skin ecchymosis or the need for
a high dose of antivenin may require early surgical
assessment. Combined broad-spectrum antibiotics
are mandatory.
New knowledge added by this study
- Among the six major venomous snakebites in Taiwan, Naja atra envenomation most commonly leads to surgical intervention.
- Ecchymosis on the bite wound may be a good indicator for surgical need in N atra envenomation.
- Adequate antibiotic treatment may play an important role in the early management of N atra envenomation.
- Surgical debridement and broad-spectrum antibiotic treatment are suggested in patients with N atra envenomation who develop ecchymosis. Surgery is more likely when high-dose antivenin has been used.
Introduction
Snakebites are an important public health and
wilderness medical issue in Taiwan. Because of
the warm and humid climate in Taiwan, there are
more than 40 terrestrial snake species, of which 15
are venomous. Six of the venomous species are of
high clinical importance, including Protobothrops
mucrosquamatus (Taiwan Habu), Trimeresurus
stejnegeri (Taiwan bamboo viper), Naja atra (Taiwan
or Chinese cobra), Bungarus multicinctus (banded
krait), Deinagkistrodon acutus (hundred pacer), and
Daboia russelii siamensis (Russell’s viper).1 2
Naja atra belongs to the Elapidae family, and
in addition to Taiwan, it inhabits southern China,
Hong Kong, northern Laos, and northern Vietnam.3
Cobra venom contains a mixture of components,
including cardiotoxin, cobrotoxin, haemotoxin,
and phospholipase A2.4 Patients envenomed by a
cobra experience varying degrees of neurotoxicity
and cytotoxicity depending upon the proportions
of the venom components. Due to evolution and
geographical variations, different cobra species
cause distinct clinical effects. For example, Naja philippinensis (northern Philippine cobra) causes a
purely neurotoxic effect without local cytotoxicity.5
In contrast, N atra envenomation is associated
with more cytotoxic effects.3 6 7 Although an
equine-derived bivalent F(ab)2 antivenin has been
produced by the Centers for Disease Control, ROC (Taiwan) to neutralise the venom of N atra, the
surgical intervention rate remains high.1 8 The main
objective of this study was to investigate the clinical
presentations and predictors for surgery in patients
with N atra envenomation. Due to high wound
infection rates, the isolated bacteria from surgical
wounds and the antimicrobial susceptibility were
also analysed.
Methods
Study design and patient population
The Buddhist Tzu Chi General Hospital is the only
tertiary care centre in eastern Taiwan. There are 1000
beds and the emergency department (ED) has more
than 55 000 patient visits per year. This hospital is also
the toxicant, drug information, and antidote control
centre for eastern Taiwan. A retrospective study was
conducted to analyse data from patients admitted to
the ED with N atra envenomation between 1 January
2008 and 30 September 2014.
Data collection, processing, and categorisation
A medical assistant was responsible for collecting the
medical records of patients admitted with snakebite
during the study period by using the computerised
chart system and International Classification
of Diseases, 9th Revision, Clinical Modification codes 989.5, E905.0, E905.9, E906.2,
and E906.5. Two physicians (the first and fifth authors)
independently reviewed the charts and categorised
these patients as having venomous or non-venomous
snakebites based on the patient’s presentation with
or without toxic effects. For venomous snakebites,
classification of the snake species was based on the
identification of the snake brought in by the patient
or identification by the patient from a picture. All
the included patients had a compatible presentation
and consistent antivenin use as recorded in the
patient chart. Patients who were initially recognised
as having venomous snakebites but did not receive
antivenin treatment were excluded from the study
because of the high probability of a dry bite or
misidentification of the snake species. Patients with
a toxic presentation who could not identify the
snake species or who received more than one type
of antivenin were recorded as having an unknown
poisonous snakebite.
Here we only report patients who were bitten
by N atra. To identify the early clinical predictors
of surgery, we categorised the patients into surgical
and non-surgical groups. All surgical interventions
were performed after surgical consultation in the
ED or after admission when patients presented
with progressive signs suggesting tissue necrosis,
necrotising fasciitis, or suspected compartment
syndrome. The final diagnoses of necrotising
fasciitis and compartment syndrome were made
according to surgical pathological findings and
intracompartmental pressure measurement,
respectively. The surgical procedures included
debridement, fasciotomy, skin graft, and digit or
limb amputation. The potential clinical predictors
of surgery in N atra envenomation included the
patient’s age, gender, season of snakebite, co-morbidities,
details of envenomation, site of
snakebite, initial vital signs on arriving at the ED,
clinical presentation, laboratory data, treatment,
timing of initial antivenin therapy, and total dose of
antivenin.
For the laboratory analyses, the initial data
obtained in the ED were collected, including
haematology, biochemistry, and coagulation
profiles. In regard to clinical presentation, the
local signs and symptoms, local complications,
and systemic manifestations and complications
were classified. Local signs and symptoms included
swelling, ecchymosis, necrosis, numbness, and bulla
formation. Local complications included necrotising
fasciitis and suspected compartment syndrome.
Systemic manifestations and complications included
neurological symptoms, including ptosis, blurred
vision, drooling, and paralysis of facial, limb, or
respiratory muscles; leukocytosis, defined as a white
blood cell count of >11.0 x 109 /L; thrombocytopenia,
defined as a platelet count of <150 x 103 /mm3;2
prothrombin time (PT) prolongation, defined as PT
of >11.6 seconds; activated partial thromboplastin
time (aPTT) prolongation, defined as aPTT of >34.9
seconds (prolonged PT and aPTT were defined
according to our clinical laboratory reference range);
fibrinogen consumption, defined as a fibrinogen
level of <1.8 g/L; elevated D-dimer level, defined
as a D-dimer level of >500 µg/L; acute renal
impairment, defined as a creatinine level of >123.8
µmol/L9; and rhabdomyolysis, defined as a creatine
kinase level of >1000 U/L.10 Two physicians reviewed
the charts of the enrolled patients and rechecked the
accuracy of the data collection. If the patient’s initial
vital signs were not measured or laboratory tests
were not performed in the ED, this was recorded
as a missing value in the database. Any discrepancy
regarding the collected data was resolved through
discussion with the third physician on the research
team. The study protocol was approved by the
institutional review board of the Buddhist Tzu Chi
General Hospital (IRB102-38). All patient records
and information were anonymised and de-identified
prior to analysis.
Statistical analyses
To identify significant early clinical presentation and
laboratory data associated with surgery in patients
with N atra envenomation, the Student’s t test or the
Mann-Whitney U test for continuous variables and
Chi squared test for categorical variables were used
to perform univariate analysis. A P value of <0.05 was
considered statistically significant, and all statistical
tests were two-tailed. For multivariate analysis, the
categorical variables with a P value of <0.05 in the
initial univariate analysis were selected and entered
into a logistic regression forward stepwise Wald test
to calculate the odds ratios (ORs). The Statistical
Package for the Social Sciences (Windows version
12.0; SPSS Inc, Chicago [IL], US) was used to
perform the statistical analyses.
Results
Epidemiology and surgical intervention rate for snake envenomation
Between 1 January 2008 and 30 September 2014,
a total of 245 patients with venomous snakebites
were recorded. Among these, 64 (26.1%) patients
had P mucrosquamatus envenomation, 56 (22.9%)
had T stejnegeri envenomation, 28 (11.4%) had N atra envenomation, five (2.0%) had B multicinctus
envenomation, six (2.4%) had D acutus envenomation,
seven (2.9%) had D r siamensis envenomation, and
79 (32.2%) had unknown poisonous snake
envenomation.
The snakebites associated with the highest
surgical intervention rates were N atra (60.7%),
followed by D acutus (33.3%), and P mucrosquamatus
(12.5%).
Characteristics and clinical status of patients with Naja atra envenomation
Of the 28 patients with a N atra bite, 20 (71.4%)
were male. The mean (± standard deviation) age of
patients was 52.3 ± 3.2 years. Of the patients, 22
(78.6%) were bitten in the summer or fall; 17 (60.7%)
were bitten on an upper limb; and 17 (60.7%) with
N atra envenomation received surgical treatment.
These patients had a significantly longer duration
of hospitalisation than non-surgical patients (27.5
± 10.2 days vs 2.7 ± 3.1 days; P<0.001). The main
operative diagnosis was necrotising fasciitis (n=13,
76.5%) with confirmation by histopathology. The
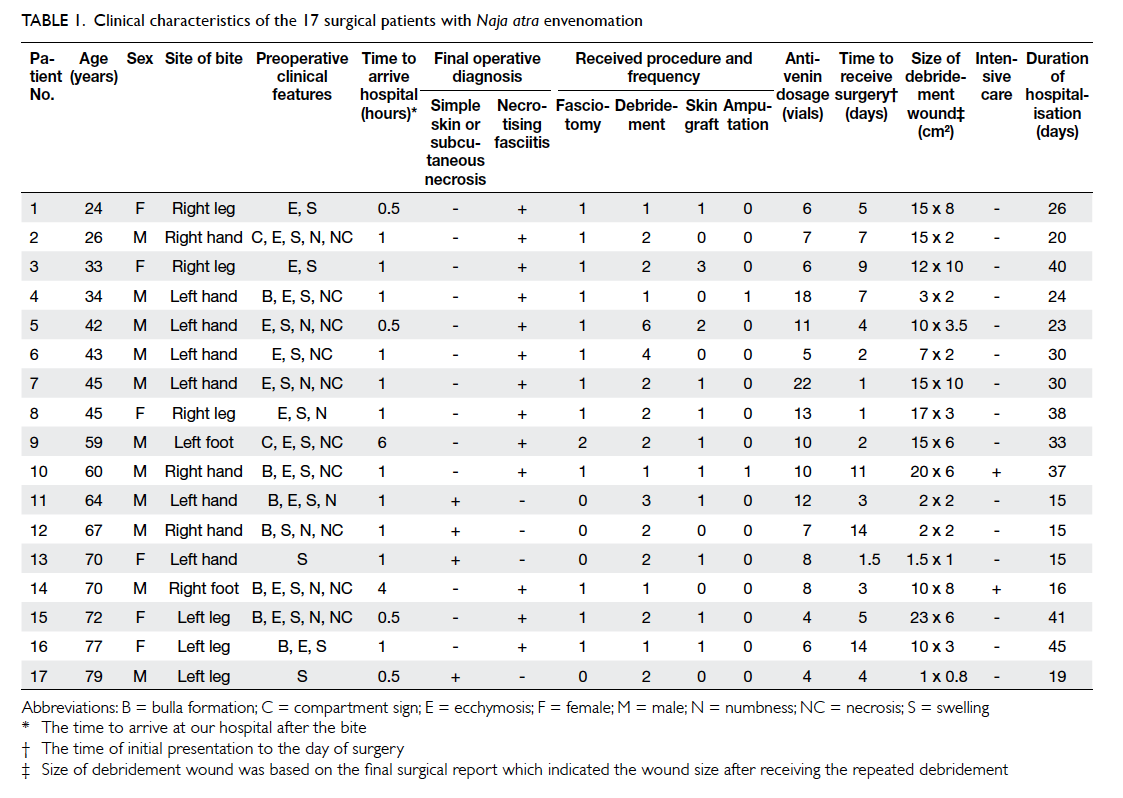
clinical characteristics of the 17 surgical patients are
shown in Table 1. The mean duration from the time of initial presentation to the day of surgery was 5.5
± 4.3 days. All 13 patients with necrotising fasciitis
underwent emergency fasciotomy and debridement,
and two required limb or digit amputation. The other
four surgical patients without necrotising fasciitis
only received local debridement with or without
skin graft due to local tissue necrosis. Therefore, a
smaller surgical wound and a shorter duration of
hospitalisation were observed for these patients
(Table 1). Nearly all surgical patients presented with local swelling and ecchymosis on the bite wound. Only
one non-surgical patient presented with ecchymosis
on a finger and was discharged from the ED 1 day
later after four vials of antivenin were administered.
The Figure shows the initial ecchymosis and necrosis of a N atra bite wound, the development of extensive
tissue necrosis, and the postoperative wounds of a
surgical patient (patient No. 9 in Table 1).
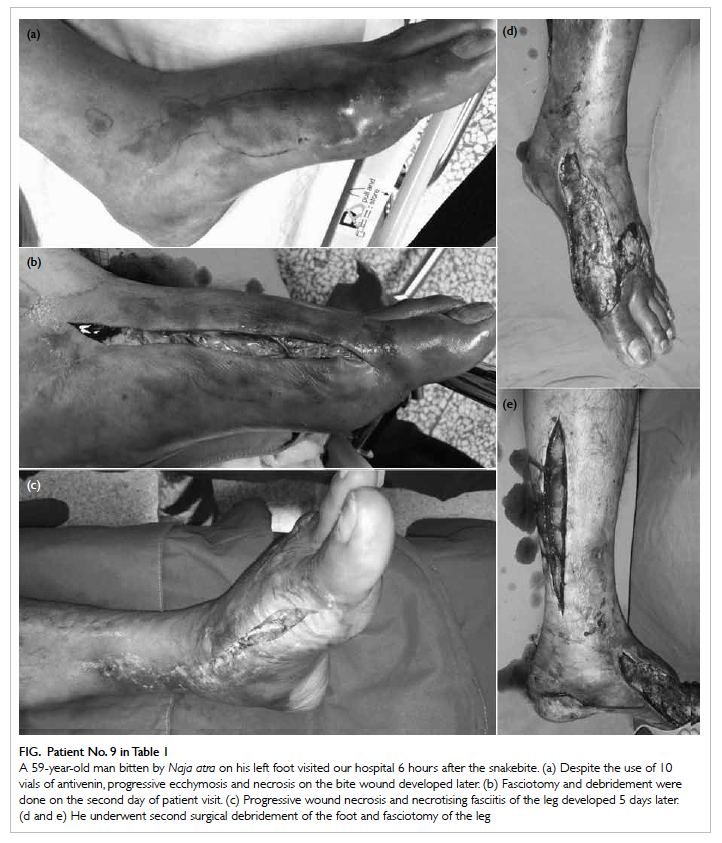
Figure. Patient No. 9 in Table 1
A 59-year-old man bitten by Naja atra on his left foot visited our hospital 6 hours after the snakebite. (a) Despite the use of 10 vials of antivenin, progressive ecchymosis and necrosis on the bite wound developed later. (b) Fasciotomy and debridement were done on the second day of patient visit. (c) Progressive wound necrosis and necrotising fasciitis of the leg developed 5 days later. (d and e) He underwent second surgical debridement of the foot and fasciotomy of the leg
Demographic and clinical characteristics associated with surgical treatment in patients with Naja atra envenomation
The demographic and clinical characteristics were
compared between the surgical and non-surgical
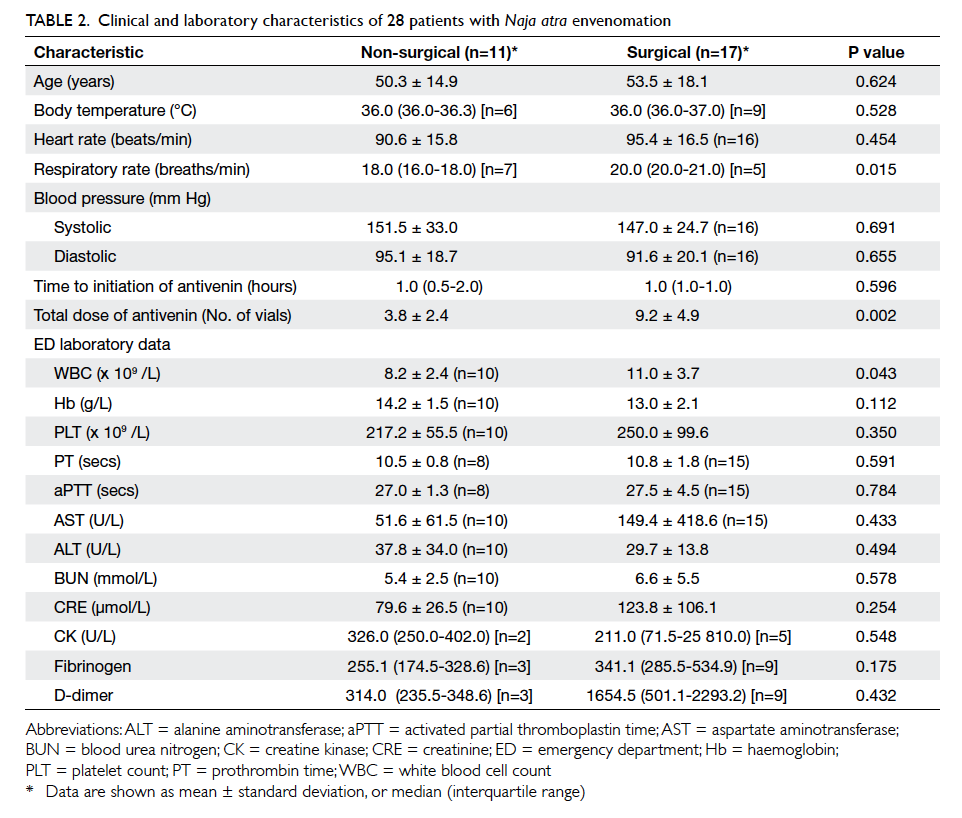
patients with N atra envenomation (Tables 2 and 3). Overall, the surgical patients received significantly
higher doses of antivenin (9.2 ± 4.9 vials vs 3.8 ± 2.4
vials; P=0.002) and had significantly higher white
blood cell counts (11.0 ± 3.7 x 109 /L vs 8.2 ± 2.4 x 109 /L; P=0.043). A higher respiratory rate was also evident in surgical patients (median [interquartile
range]: 20 [20-21] vs 18 [16-18] breaths/min; P=0.015), but the
incidence of missing data in both groups for this factor
was high (Table 2). A significantly higher proportion of surgical patients received six or more vials of antivenin in
total compared with non-surgical patients (82.4% vs
18.2%; P=0.001) [Table 3]. For local signs, symptoms and complications, a significantly higher proportion
of surgical patients presented with local swelling
(100% vs 72.7%; P=0.05), ecchymosis (82.4% vs 9.1%;
P<0.001), necrosis (58.8% vs 0%, P=0.002), bulla
formation (41.2% vs 0%; P=0.023), and necrotising
fasciitis (76.5% vs 0%; P<0.001) [Table 3]. Age, season and site of snakebite, co-morbidity with
diabetes, allergy to antivenin, and other systemic
manifestations were not found to be significantly
different between surgical and non-surgical patients.
None of the patients with N atra envenomation
presented with neurological symptoms. One patient
with a small area of ecchymosis on the bite wound
of his left hand did not receive surgical intervention,
because the condition of the local wound improved
and healed after administration of four vials of
antivenin and intravenous antibiotics.
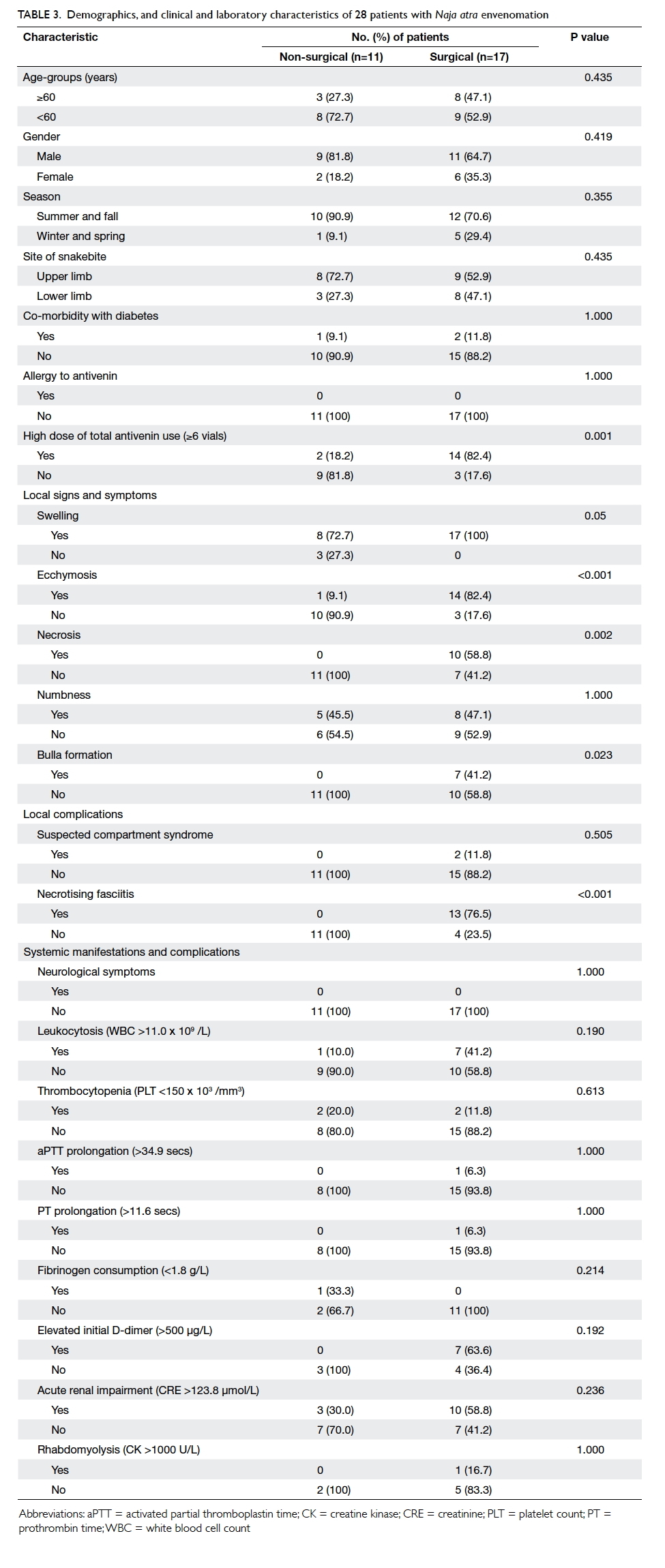
Table 3. Demographics, and clinical and laboratory characteristics of 28 patients with Naja atra envenomation
Independent predictors of surgery in patients with Naja atra envenomation
To determine clinical predictors of surgery, a
multivariate logistic regression analysis was
conducted for the significant variables derived from
the univariate analysis. Necrotising fasciitis was not
included in the multivariate analysis because it was
a surgical finding and not an early sign that could be
identified in the ED. The results showed that local
ecchymosis (OR=34.36; 95% confidence interval
[CI], 2.20-536.08; P=0.012) and a high total dose of
antivenin (≥6 vials; OR=14.59; 95% CI, 1.10-192.72;
P=0.042) were the most significant clinical predictors
of surgery in patients with N atra envenomation.
Bacterial isolates identified from the snakebite wounds of surgical patients with Naja atra envenomation, and bacterial susceptibility to common antibiotics
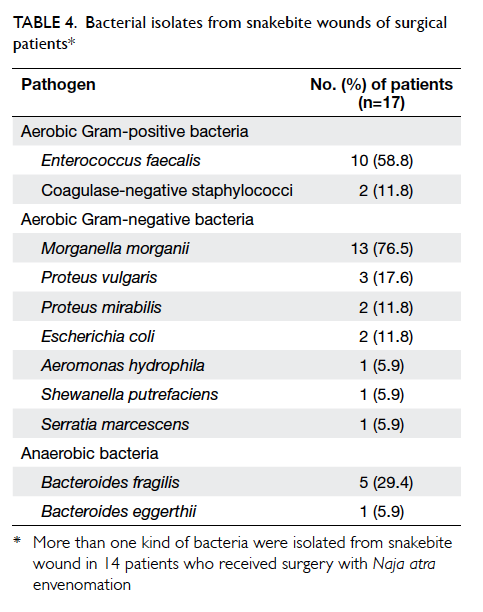
To analyse the cause of necrotising fasciitis in
surgical patients, the bacterial isolates identified
from snakebite wounds were further analysed in
surgical patients. The positive culture rate was
88.2% (n=15). More than one type of bacteria were
isolated from the snakebite wound in 14 (82.4%)
surgical patients. The isolated pathogens included
aerobic Gram-positive and Gram-negative bacteria,
as well as anaerobic bacteria. The most commonly
identified pathogen was Morganella morganii
(76.5%), followed by Enterococcus faecalis (58.8%)
and Bacteroides fragilis (29.4%) [Table 4].
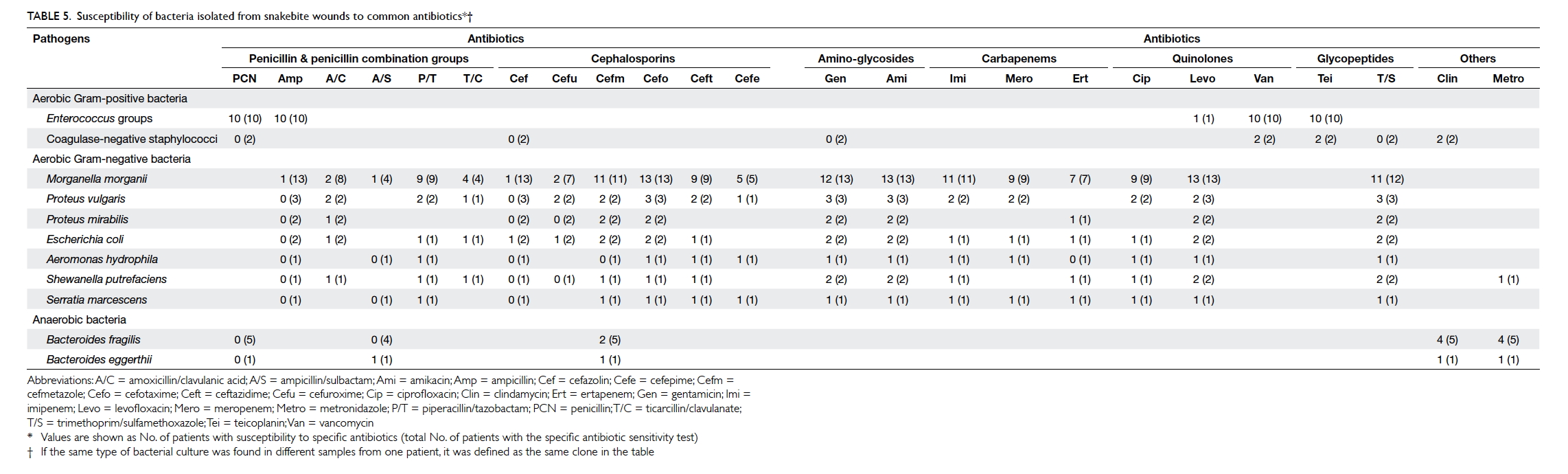
The susceptibility of the bacteria to common
antibiotics was analysed (Table 5). All Gram-positive bacteria were susceptible to vancomycin
and teicoplanin. All Gram-negative bacteria were
susceptible to cefotaxime and amikacin. Cefmetazole,
gentamicin, levofloxacin, and trimethoprim/sulfamethoxazole were also effective against
the isolated Gram-negative bacteria. Nearly all
anaerobic bacteria were susceptible to clindamycin
and metronidazole (Table 5).
Discussion
In our study, skin change of ecchymosis on the bite
wound was a good clinical predictor of surgery for N atra envenomation. The majority of N atra venom
is cytotoxic, not haemorrhagic. The cardiotoxin
and phospholipase A2 in N atra venom are direct
cytotoxic polypeptides and cause degradation of cell
membranes. They induce cell death by activating
calcium-dependent proteases, and inhibit mitochondrial
respiration. Hyaluronidase in N atra venom destroys
interstitial constituents and precipitates the
spreading of venom.11 A histopathological study of
N atra bite wounds demonstrated thrombotic and
fibrinoid deposits in superficial and deep dermal
vessels, and leukocytoclastic vasculitis.12 Hence, both
the cytotoxic and ischaemic effects of N atra venom
may lead to blood extravasation from the destroyed
subcutaneous vessels or capillaries and result in the
characteristic ecchymosis on the bite wound. This
finding may be a potentially important clinical sign
of irreversible subcutaneous tissue necrosis due to
development of tissue ischaemia.3 If management
at this stage is inadequate, tissue destruction may
progress to involve the fascia rapidly and extensively
with ultimate development of necrotising fasciitis.13
In our patients, extensive tissue destruction beyond
the original bite site was evident once necrotising
fasciitis developed. Further study is required to verify
whether early surgical intervention can prevent the
development of necrotising fasciitis, reduce the size
of surgical wound, or shorten the length of hospital
stay. Nonetheless, surgical assessment may be
needed in patients with N atra bite who present with
local ecchymosis on the bite wound.
Traditionally, immediate injection of antivenin
to neutralise N atra venom was the only efficient
management.14 A study using an enzyme-linked
immunosorbent assay to detect the amount of
N atra venom revealed that two to eight vials
of antivenin are sufficient to eliminate systemic
circulating venom if presentation is early.6 The
efficacy of systemically administrated antivenin to
diminish local tissue destruction is still controversial,
however, and needs further study.3 In an animal
study, the cytotoxic venom of N atra was shown
to bind with high affinity to tissues leading to high
levels of local tissue destruction.15 This finding may
explain the difficulties associated with neutralisation
of local venom toxicity, especially in cases of delayed
presentation. Thus, the adequate dose of antivenin
for preventing advanced tissue destruction remains
unknown. In our study, nearly all patients presented
within 1 hour following envenoming. Intravenous
injection of antivenin was administered as soon as
clinically possible following identification of cobra
envenoming. Interestingly, the use of higher doses
of antivenin in patients with N atra envenomation
did not decrease surgical rates even in cases of
early presentation. More than half of the patients
underwent surgery and the majority were diagnosed
with necrotising fasciitis. Surgical intervention
appears to be crucial for the management of N atra
envenomation. Hence, the identification of clinical
predictors of surgical need and sufficient evidence
to support surgeons’ decisions to carry out early
surgical intervention are important issues in N atra
management.
High bacterial isolation rates and the growth
of mixed spectrums of bacteria from bite wounds
indicate bacterial infection (which may be another
cause of necrotising fasciitis in N atra envenomation),
bacterial colonisation, or both. Morganella morganii
and Enterococcus species were the most common
pathogens cultured from N atra bite wounds in this
study. This finding is consistent with the bacterial
cultures taken from oral swabs of N atra in Hong
Kong.16 Similar results were also described in a
previous study in western Taiwan.17 Hence, the
use of adequate antibiotics is important in N atra
envenomation management. In accordance with the
results of our tests of the antibiotic susceptibility of
the isolated bacteria, treatment with glycopeptide
antibiotics (vancomycin or teicoplanin) combined
with a third-generation cephalosporin (cefotaxime)
with or without anti-anaerobic antibiotics
(clindamycin or metronidazole) is recommended.
Limitations
There are several limitations in our study. First,
this was a retrospective chart review comparative
study. Non-uniform description of symptoms and
signs documented by different providers may have
influenced the validity of the statistics. Second,
the small sample size may limit the statistical
power in the multivariate analysis. Third, there
are no definitive guidelines for the management
of venomous snakebites in Taiwan, and various
treatment strategies were employed; this may
have influenced the final outcome. A large-scale
prospective study is warranted to verify the risk
factors we have identified to provide more accurate
data for early risk stratification, treatment, and
management of these patients.
Conclusions
Of the six common venomous snakes in eastern
Taiwan, bites by N atra most frequently lead to
surgical intervention. Severe tissue necrosis and
necrotising fasciitis were the main findings during
surgery. Patients who present with ecchymosis
on the bite wound or who require higher doses of
antivenin may have a higher probability of surgical
intervention. In addition to early and adequate
antivenin treatment, combined broad-spectrum
antibiotics and surgical intervention may be needed
in the management of N atra snakebites.
Acknowledgement
This work was supported by Buddhist Tzu Chi
General Hospital Grants TCRD103-53 (to the first author).
Declaration
All authors have disclosed no conflicts of interest.
References
1. Liau MY, Huang RJ. Toxoids and antivenoms of venomous
snakes in Taiwan. Toxin Rev 1997;16:163-75.
2. Hung DZ. Taiwan’s venomous snakebite: epidemiological,
evolution and geographic differences. Trans R Soc Trop
Med Hyg 2004;98:96-101. Crossref
3. Wong OF, Lam TS, Fung HT, Choy CH. Five-year
experience with Chinese cobra (naja atra)–related injuries
in two acute hospitals in Hong Kong. Hong Kong Med J
2010;16:36-43.
4. Li S, Wang J, Zhang X, et al. Proteomic characterization of
two snake venoms: Naja naja atra and Agkistrodon halys.
Biochem J 2004;384:119-27. Crossref
5. Watt G, Padre L, Tuazon L, Theakston RD, Laughlin L.
Bites by the Philippine cobra (Naja naja philippinensis):
prominent neurotoxicity with minimal local signs. Am J
Trop Med Hyg 1988;39:306-11.
6. Hung DZ, Liau MY, Lin-Shiau SY. The clinical significance
of venom detection in patients of cobra snakebite. Toxicon
2003;41:409-15. Crossref
7. Wang W, Chen QF, Yin RX, et al. Clinical features and
treatment experience: A review of 292 Chinese cobra
snakebites. Environ Toxicol Pharmacol 2014;37:648-55. Crossref
8. Huang LW, Wang JD, Huang JA, Hu SY, Wang LM, Tsan
YT. Wound infections secondary to snakebite in central
Taiwan. J Venom Anim Toxins Incl Trop Dis 2012;18:272-6. Crossref
9. Hung DZ, Wu ML, Deng JF, Lin-Shiau SY. Russell’s
viper snakebite in Taiwan: differences from other Asian
countries. Toxicon 2002;40:1291-8. Crossref
10. Chen YW, Chen MH, Chen YC, et al. Differences in clinical
profiles of patients with Protobothrops mucrosquamatus
and Viridovipera stejnegeri envenoming in Taiwan. Am J
Trop Med Hyg 2009;80:28-32.
11. Harris JB. Myotoxic phospholipases A2 and the
regeneration of skeletal muscles. Toxicon 2003;42:933-45. Crossref
12. Pongprasit P, Mitrakul C, Noppakun N. Histopathology
and microbiological study of cobra bite wounds. J Med
Assoc Thai 1988;71:475-80.
13. Gozal D, Ziser A, Shupak A, Ariel A, Melamed Y.
Necrotizing fasciitis. Arch Surg 1986;121:233-5. Crossref
14. Russell FE. Snake venom immunology: historical and
practical considerations. Toxin Rev 1988;7:1-82. Crossref
15. Guo MP, Wang QC, Liu GF. Pharmacokinetics of cytotoxin
from Chinese cobra (Naja naja atra) venom. Toxicon
1993;31:339-43. Crossref
16. Lam KK, Crow P, Ng KH, et al. A cross-sectional survey
of snake oral bacterial flora from Hong Kong, SAR, China.
Emerg Med J 2011;28:107-14. Crossref
17. Chen CM, Wu KG, Chen CJ, Wang CM. Bacterial infection
in association with snakebite: a 10-year experience in a
northern Taiwan medical center. J Microbiol Immunol
Infect 2011;44:456-60. Crossref