Hong Kong Med J 2016 Aug;22(4):356–64 | Epub 17 Jun 2016
DOI: 10.12809/hkmj154667
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Therapeutic inertia in the management of hyperlipidaemia in type 2 diabetic patients: a
cross-sectional study in the primary care setting
FY Man, MB, BS, FHKCFP;
Catherine XR Chen, MRCP (UK), FHKAM (Family Medicine);
YY Lau, MB, BS, FHKAM (Family Medicine);
King Chan, FRACGP, FHKAM (Family Medicine)
Department of Family Medicine & General Outpatient Clinic, Queen
Elizabeth Hospital, Kowloon Central Cluster, Jordan, Hong Kong
Corresponding author: Dr FY Man (mfy252@ha.org.hk)
Abstract
Objectives: To study the prevalence of therapeutic
inertia in lipid management among type 2 diabetic
patients in the primary care setting and to explore
associated factors.
Methods: This was a cross-sectional study involving
type 2 diabetic patients with suboptimal lipid
control followed up in all general out-patient clinics
of Kowloon Central Cluster in Hong Kong from 1
October 2011 to 30 September 2013. Main outcome
measures included prevalence of therapeutic inertia
in low-density lipoprotein management among type
2 diabetic patients and its association with patient
and physician characteristics.
Results: Based on an agreed standard, lipid control
was suboptimal in 49.1% (n=9647) of type 2 diabetic
patients who attended for a regular annual check-up
(n=19 662). Among the sampled 369 type 2 diabetic
patients with suboptimal lipid control, therapeutic
inertia was found to be present in 244 cases, with a
prevalence rate of 66.1%. When the attending doctors’
profiles were compared, the mean duration of clinical
practice was significantly longer in the therapeutic
inertia group than the non–therapeutic inertia group.
Doctors without prior training in family medicine
were also found to have a higher rate of therapeutic
inertia. Patients in the therapeutic inertia group had
longer disease duration, a higher co-morbidity rate of
cardiovascular disease, and a closer-to-normal low-density
lipoprotein level. Logistic regression analysis
revealed that lack of family medicine training among
doctors was positively associated with the presence
of therapeutic inertia whereas patient’s low-density
lipoprotein level was inversely associated.
Conclusions: Therapeutic inertia was common in
the lipid management of patients with type 2 diabetes
in a primary care setting. Lack of family medicine
training among doctors and patient’s low-density
lipoprotein level were associated with the presence
of therapeutic inertia. Further study of the barriers
and strategies to overcome therapeutic inertia is
needed to improve patient outcome in this aspect of
chronic disease management.
New knowledge added by this study
- Lipid control among patients with type 2 diabetes mellitus (T2DM) was far from satisfactory, with nearly half being suboptimally controlled.
- Therapeutic inertia (TI) is common in the lipid management of T2DM patients in the primary care setting with a prevalence rate of 66.1%.
- Lack of family medicine training among doctors was positively associated with the presence of TI whereas patient’s low-density lipoprotein level was inversely associated.
- Comprehensive strategies should be devised to overcome TI so that long-term cardiovascular outcome of diabetic patients can be improved.
Introduction
Type 2 diabetes mellitus (T2DM) is one of the
most common chronic conditions encountered in
primary care, affecting up to 10% of the Hong Kong
population.1 It is also a leading cause of morbidity
and mortality due to diabetic complications.2
Optimal control of cardiovascular risk factors can
decrease the risk of developing diabetes-related
complications.3 4 5
Hyperlipidaemia is one of the most important
modifiable risk factors for cardiovascular disease
(CVD) prevention. Studies have shown that optimal
lipid control is associated with an improved
cardiovascular outcome.6 7 8 9 Low-density lipoprotein
(LDL) particles are considered more atherogenic
than other cholesterol components and therefore
stringent control of LDL is particularly important
for the prevention of CVD in high-risk patients.10
Despite this evidence, lipid control among
diabetic patients in the primary care setting, both
locally and internationally, has been inadequate.11
The most recent study performed in Hong Kong
found that 88.4% of diabetic patients had a suboptimal
lipid level.12 Studies in Europe and the US found that the LDL control rate ranged from 30% to 55%.13 14 15 16 17 Similarly, a study of dyslipidaemia management in South Asia including China,
South Korea, Malaysia, and Singapore revealed that only
48% of patients attained pre-defined low-density
lipoprotein–cholesterol goals.18
Similar to other chronic conditions, the reasons
for poor (lipid) control are multifactorial and may
include patient, physician, and health care delivery factors.
Among them, suboptimal medication augmentation
has been identified as an important physician factor.
This is known as therapeutic inertia (TI) and is said
to exist whenever the health care provider does not
initiate or intensify therapy appropriately when
therapeutic goals are not reached: “recognition of the
problem, but failure to act”.19 20 Such TI has become increasingly acknowledged as a major impediment
to CVD risk factor control. Studies have suggested
that TI is related to the management of diabetes and
hypertension (HT) and may contribute to up to 80%
of heart attacks and strokes.21 22
The prevalence of TI in chronic disease
management has not been explored in Hong Kong. In
this study, we specifically looked at the prevalence of
TI in hyperlipidaemia management among diabetic
patients. Internal statistical data (internal data from
Hospital Authority [HA] Head Office) revealed that
lipid control has been relatively poor in this cluster
when compared with blood pressure and glycaemic
control. Our study aimed to explore the prevalence
of TI in the management of hyperlipidaemia among
T2DM patients and to explore the underlying
factors. By overcoming the barriers to adequate and
appropriate treatment, it was expected that the long-term
cardiovascular outcome of T2DM patients
could be improved.
Methods
Subjects
Inclusion criteria
In this cross-sectional study, all T2DM patients with
International Classification of Primary Care code
T90 (Non-insulin Dependent Diabetes Mellitus),
who had been regularly followed up in all General
Outpatient Clinics (GOPCs) of Kowloon Central
Cluster (KCC) from 1 October 2011 to 30 September
2013, and had blood lipid levels checked at least once
during this period were recruited. In our clinics,
blood and urine check-ups are usually carried out
in patients with T2DM every 12 to 18 months.
This 2-year retrieval period was therefore likely to
cover all such patients regularly followed up in our
cluster. The diagnosis of diabetes was based on the
“Definition and description of diabetes mellitus”
from American Diabetes Association (ADA) in
2013.23
Exclusion criteria
The following patients were excluded: patients who
had been incorrectly diagnosed with diabetes, type
1 diabetic patients, diabetic patients who had no
regular blood or urine check-up during the study
period, diabetic patients followed up in a specialist
clinic, and patients who died during the study period.
Definition of treatment target and therapeutic inertia in lipid management among type 2 diabetic patients
Various studies and guidelines have recommended
targets in the treatment of hyperlipidaemia. In the
HA of Hong Kong, National Cholesterol Education
Program Adult Treatment Panel III Guidelines
(NCEP ATP III) and ADA guidelines were used
to set up the manual for the risk assessment and
management programme. In this study, we used
the same set of guidelines to define the level of
lipid control in T2DM patients. We focused on the
control of LDL as it is the most important risk factor
of the lipid profile.
According to NCEP ATP III 200224 and ADA
2013 Guidelines on Diabetes and Lipids,23 target
LDL should be <2.6 mmol/L in diabetic patients
without overt CVD and <1.8 mmol/L in diabetic
patients with overt CVD. In this study, CVD is
defined as established ischaemic heart disease (IHD),
cerebrovascular accident (CVA), or peripheral
vascular disease (PVD).
In this study, lipid control was defined as poor
and escalation of treatment indicated if the last LDL
level was ≥2.6 mmol/L in diabetic patients without
CVD and ≥1.8 mmol/L in diabetic patients with
established CVD. Consultation notes of the follow-up
immediately after the last available lipid profile
test were reviewed through the HA Clinical Management
System (CMS). Therapeutic inertia was considered
to be present when the attending doctor failed to
initiate or intensify treatment if target LDL level
was not achieved. If medical notes indicated a valid
reason for non-escalation of treatment despite
a clinical indication, it was not considered TI.
Common justifications included:
(1) Diet and lifestyle modification advice was
given to patients newly diagnosed with
hyperlipidaemia.
(2) Statin was started following the previous visit
and LDL level was improving.
(3) Patient was non-compliant with the existing
statin regimen and advice on regular drug
compliance was given.
(4) Patient refused to take a statin.
(5) Patient was unable to tolerate side-effects of
statin.
(6) Statin was contra-indicated, eg in patients
with deranged liver function.
Calculation of sample size and random sampling
According to the data drawn from Clinical Data
Analysis and Reporting System of the HA, a total
of 19 662 T2DM patients were attending GOPCs
of KCC for regular follow-up with checking of
blood lipid profile during the study period. Based
on the definitions mentioned above, 9647 of them
had suboptimal or poor LDL control. Using the
internet sample size calculator (Survey Software
from Creative Research System, http://www.surveysystem.com), a sample size of 369 would
provide 95% confidence level and 5% margin of error.
Thus, 400 patients were sampled to ensure adequate
statistical power and allow room for case exclusion.
A list of random numbers was then generated from
the research randomiser (http://www.randomizer.org/form.htm), from which 400 patients were
selected. Details of the visit with latest lipid profile
result seen were recorded. Data were derived
from the consultation notes in the CMS record of
selected patients and recorded on a standard data
collection form (Appendix). Data were collected by
the principal investigator and counter-checked by
another experienced doctor in the research team.
Determination of variables
Age and gender of all patients as well as smoking
status, body mass index (BMI), latest blood pressure,
haemoglobin A1c (HbA1c) level, serum creatinine
level, lipid profile, and urine albumin-to-creatinine
ratio were retrieved from the CMS. The most recent
blood or urine test was used for analysis if more
than one test had been performed during the study
period. The BMI was calculated as body weight/body height2 (kg/m2). The patient was considered a
smoker if he/she currently smoked or had stopped in
the last 6 months.25 The abbreviated Modification of
Diet in Renal Disease formula was used to calculate
the estimated glomerular filtration rate.26
The working profile of the attending doctors
was retrieved from the Central Office of Department
of Family Medicine (FM) and GOPC, KCC. Duration
of clinical practice was calculated as the number of
years from registration with the Medical Council of
Hong Kong. The training status of FM of doctors
was documented and categorised according to the
following criteria:
Statistical analysis
All data were entered and analysed using computer
software (Windows version 21.0; SPSS Inc, Chicago
[IL], US). Student’s t test and analysis of variance
were used to analyse continuous variables and
the Chi squared test for categorical data. Fisher’s
exact test was used if the sample size was less than
five. Multivariate stepwise logistic regression was
used to determine the association between TI and
the significant different variables from patient
characteristics and doctor characteristics. All
statistical tests were two-sided, and a P value of
<0.05 was considered statistically significant.
Ethical considerations
The study protocol was reviewed and approved by
the Research Ethics Committee of HA (Kowloon
Central/Kowloon East Cluster) [Reference number: KC/KE-13-0247/ER-1].
Results
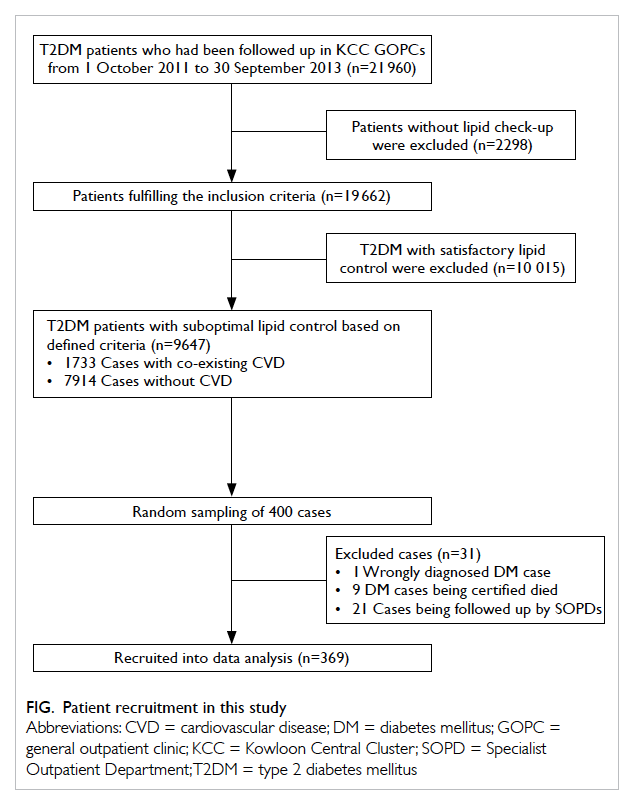
A total of 21 960 T2DM patients were identified
from the KCC GOPC Diabetes Mellitus registry
from 1 October 2011 to 30 September 2013. Among
them, 19 662 (89.5%) patients had their lipid profile
checked at least once during the study period; 9647
(49.1%) cases had suboptimal lipid control based on
the defined criteria above, including 1733 cases with
co-existing CVD and 7914 cases without CVD.
Among 400 randomly sampled diabetic
patients with suboptimal lipid control, 31 were
excluded including 21 who were being followed up
in other clinics for diabetic control, nine who died
during the study period, and one who was wrongly
diagnosed with diabetes. The remaining 369 cases
were recruited for data analysis (Fig).
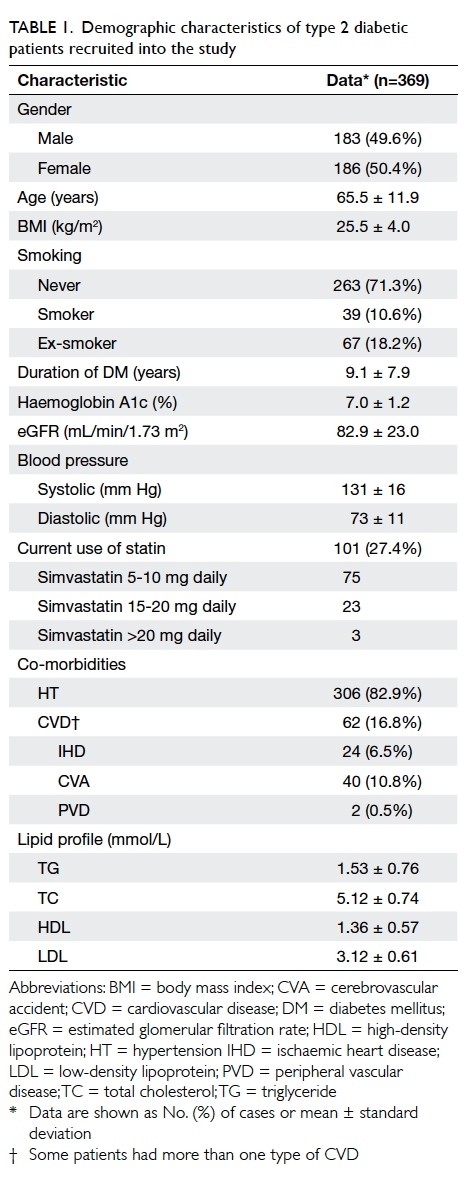
Table 1 summarises the demographic
characteristics of the recruited patients. The mean
(± standard deviation) age of the study population
was 65.5 ± 11.9 years and 186 (50.4%) were female.
The mean duration of diabetes was 9.1 ± 7.9 years.
With regard to their co-morbidities, 306 (82.9%)
patients had concomitant HT, 24 (6.5%) had IHD, 40
(10.8%) had CVA, and two (0.5%) had PVD. The mean LDL
level was 3.12 ± 0.61 mmol/L and only 101 (27.4%)
patients were prescribed a statin.
Table 2a summarises the demographic
characteristics of the attending doctors. A total of
56 doctors, among whom 19 (33.9%) were female,
attended the 369 diabetic patients. The mean
duration of clinical practice was 13.6 ± 9.6 years.
With regard to FM training status, 13 (23.2%)
doctors had received no FM training, 18 (32.1%)
received basic training or studied DFM, 13 (23.2%)
were intermediate FM fellows, and 12 (21.4%) were
FM specialists.
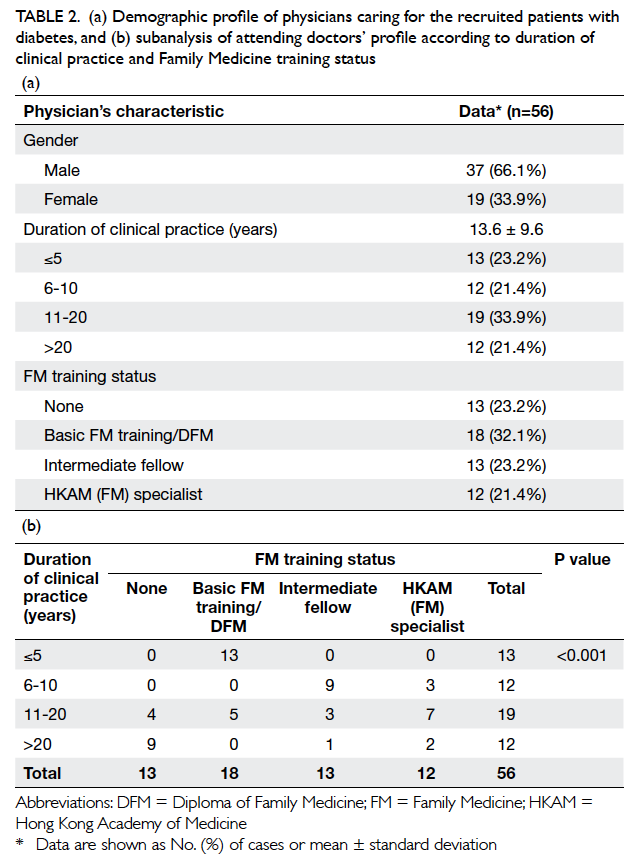
Table 2. (a) Demographic profile of physicians caring for the recruited patients with diabetes, and (b) subanalysis of attending doctors’ profile according to duration of clinical practice and Family Medicine training status
Subanalysis of attending doctors’ profile
according to their duration of clinical practice and
FM training status is shown in Table 2b. Training status of FM varied significantly with duration of
clinical practice (P<0.001). Among 13 doctors who
had worked for ≤5 years, all had been a basic FM
trainee or had obtained a DFM. On the other hand,
among 12 doctors who had worked for over 20 years,
most (n=9, 75%) had not received any formal FM
training.
Among the 369 recruited T2DM patients,
treatment was escalated in 47 (12.7%). Justification
for not intensifying treatment was provided in 78
(21.1%) cases. Justification was as follows: 19 patients
were given dietary advice on lifestyle modifications
as they were newly diagnosed with hyperlipidaemia;
in 13 patients, a statin had been newly commenced at
the previous visit and lipid level was lower compared
with pretreatment; five patients were non-compliant
with the existing treatment regimen and advice on
compliance was given; 28 patients refused to start
a statin despite medical advice; six patients had been
unable to tolerate side-effects of statin. Statin therapy
was contra-indicated in seven patients with impaired
liver function. In the remaining 244 cases, TI was
present with a prevalence rate of 66.1%.
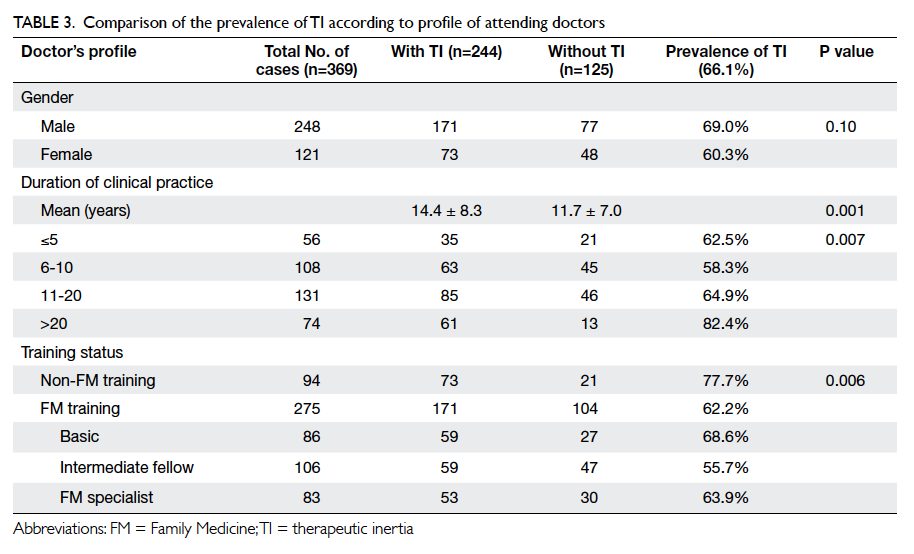
Table 3 shows the characteristics of physicians
in TI-positive and TI-negative patients. The
duration of clinical practice of attending doctors was
significantly longer in the TI group compared with
the non-TI group (P=0.001), with doctors working
for over 20 years having a particularly higher rate of
TI (82.4%). Doctors without any FM training also
had a higher rate of TI (77.7%; P=0.006).
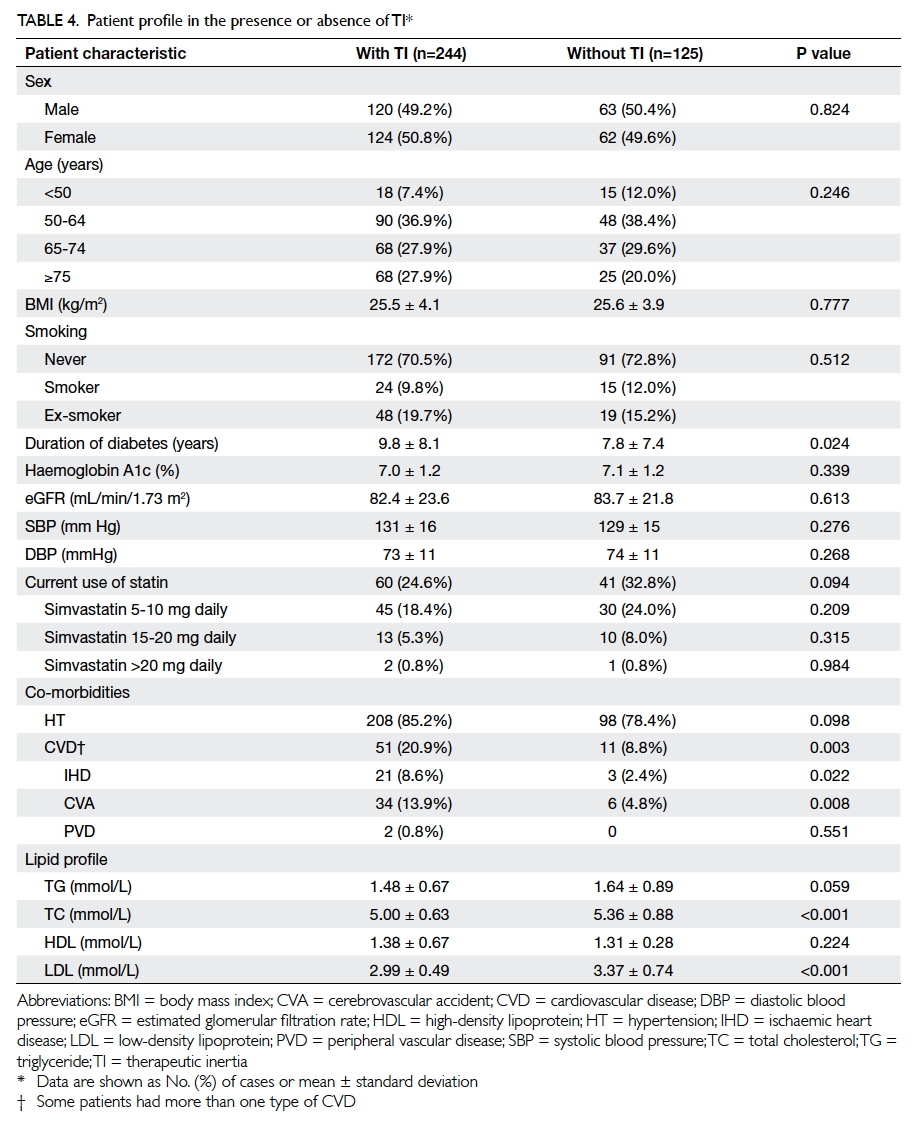
Table 4 summarises the characteristics of
T2DM patients in TI-positive and TI-negative
groups. Patients in the TI-positive group had a
longer duration of diabetes (9.8 ± 8.1 years in TI-positive
group vs 7.8 ± 7.4 years in TI-negative
group; P=0.024) and lower total cholesterol level
and LDL level (both P<0.001). The co-existence of
CVD (IHD, CVA, PVD) was more common in the
TI-positive group (P=0.003). Other characteristics
including patient gender, age, BMI, smoking status,
blood pressure, HbA1c level, and type and dose of
current statin use were comparable for both groups
(all P>0.05).
Based on the results from Tables 3 and 4,
multivariate stepwise logistic regression analysis was
performed to identify any factors that contributed to
TI (Table 5). Only variables that were significantly different in the univariate analysis were included
in the regression model. As the FM training status
varied significantly with the duration of clinical
practice (Table 2b, P<0.001) and these two factors were interrelated, only one of these two variables was
included in the logistic regression analysis. As the P
value of FM training status (P=0.006) was smaller
than that for years of clinical practice (P=0.007) in the
univariate analysis (Table 3), FM training status was entered into the logistic regression analysis. Lack of
FM training was positively associated with TI (odds
ratio [OR]=2.170; P=0.008), whereas patient’s LDL
level was inversely associated (OR=0.320; P=0.001).
Discussion
This was the first clinical analysis of TI in lipid
management among T2DM patients managed
locally in the primary care setting. It has provided
important background information about the
prevalence of TI in this group of patients. It also
explored possible underlying factors from both the
doctor’s and patient’s perspective.
Our study found that lipid control among
T2DM patients was far from satisfactory, with
49.1% suboptimally controlled. This is consistent
with reports that a high proportion of patients with
hyperlipidaemia do not achieve their LDL goal.27 28 It is important to note that TI was present in
66.1% of these cases, meaning that in over 60% of
diabetic patients with dyslipidaemia, appropriate
management including dietary advice or drug
treatment was not provided. This relatively high
TI rate should alert primary care physicians to the
importance of lipid control among T2DM patients as
greater TI leads to poorer clinical outcomes. A similar
study carried out by Whitford et al29 has shown
that TI was present in 80% of consultations when
lipid control was addressed among diabetic patients
managed in the primary care setting in Middle East
countries. This rate was much higher than the TI in
blood pressure control (68%) and glycaemic control
(29%). A similar study of lipid management in high-risk
patients at a large academic primary care practice
in the US has shown that statin dose was
augmented at only 16% of over 2000 patient visits
where the patient was suboptimally controlled.30
Among the sampled 369 poorly controlled T2DM
patients in this study, only 27.4% (n=101) were
treated with simvastatin, which is the only statin
available in Hong Kong GOPCs. In addition, most
(74.3%, 75/101) were treated with a lower dose (5-10
mg daily) that is considered inadequate according to
ATP-IV guidelines in which a moderate dose of statin,
such as simvastatin 20-40 mg daily, is recommended
for T2DM patients in order to achieve target LDL
level.31 Thus, the low statin prescription rate and the
inadequate dose of statin may together contribute to
the suboptimal lipid control among T2DM patients
in primary care.
A possible explanation for the TI in dose augmentation
of simvastatin is the potential drug-drug
interaction with calcium channel blockers (CCB)
such as amlodipine.32 The maximum recommended
dose for simvastatin in conjunction with amlodipine
use is 20 mg/day. Since 306 (82.9%) sampled diabetic
patients were found to have concomitant HT and
among them 122 (40%) were prescribed amlodipine
for blood pressure control, doctors might have
hesitated to increase the dose of simvastatin. In our
study, 10 diabetic patients in the TI-positive group
were prescribed amlodipine and simvastatin 20 mg
daily. In this scenario, either changing simvastatin to
an alternative statin such as atorvastatin or changing
amlodipine to an alternative CCB such as nifedipine
is recommended if lipid control remains suboptimal.
Failing to switch to another statin or CCB when
clinically indicated is also considered to be TI. A
more proactive approach to prescribing different
drug combinations is required in order to achieve
the target LDL in a timely manner.
Further studies of the physician profile relative
to the presence of TI have revealed that doctors with
longer duration of clinical practice have a higher
rate of TI that is even more prominent in those with
over 20 years’ clinical practice. These findings are
contrary to an overseas study where more experienced
doctors had a lower rate of TI33; nonetheless,
this study was performed in a secondary care
setting and involved cardiologists who managed
hyperlipidaemia in patients with IHD. In our study,
most doctors who had worked for over 20 years had
no formal FM training (9 [75%] of 12 doctors; Table 2b). In addition, when training status was compared, doctors with no FM training had a higher rate of TI
than those who had completed FM training (77.7% vs
60.0%; P=0.006). We postulate that doctors who have
worked for over 20 years may be less familiar with
the latest guidelines on lipid management, possibly
due to a lack of FM or related training. If physicians
lack appropriate training, there will be gaps in their
knowledge of latest clinical management guidelines.
This has been confirmed by review articles which
showed that TI could be attributed to insufficient
knowledge of guidelines.34
When patient’s profile was compared,
surprisingly, TI was present in 51 of 62 diabetic
patients with overt CVD, and only 11 cases were
properly managed (Table 4). This is a considerable concern since lipid control is particularly important
and as a secondary prevention strategy in this group
of patients. The target for LDL control is much more
stringent at <1.8 mmol/L in this group of patients,
and more difficult to achieve clinically. Some doctors
may not have been aware of this stricter/lower LDL
target and have been satisfied with LDL level of 1.8
to 2.6 mmol/L. This is supported by our finding that
among diabetic patients with overt CVD whose
lipid profile was inadequately controlled (n=62),
more than half (n=33, 53.2%) had LDL controlled
at 1.8 to 2.6 mmol/L. Physicians should take a more
proactive approach particularly in this high-risk
group of patients and adhere closely to the prevailing
management guidelines in CVD risk factor control.
Multiple variable logistic regression analysis
revealed that patients with lower LDL or LDL level
closer to normal was associated with TI (Table 5). This could be explained by the threshold effect, that is, the
closer the LDL level is to target level, the less likely
is the doctor to intensify treatment. This threshold
effect has been commonly observed in other similar
studies.30 35 Other factors that contribute to the threshold effect could be ‘overestimation of current
care’ or ‘complacency with borderline values’, leading
to the physician’s subjective misperception that the
care provided is sufficient.34
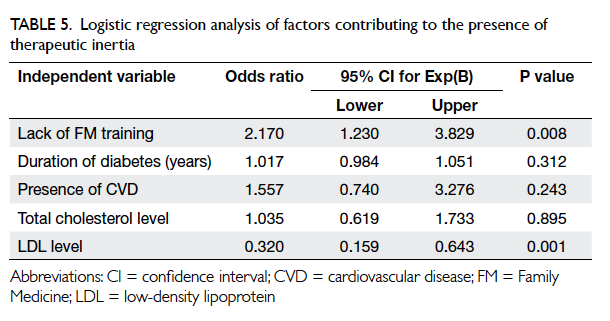
Table 5. Logistic regression analysis of factors contributing to the presence of therapeutic inertia
Implications for primary care
Our study found that TI was common in lipid
management among diabetic patients managed in the
GOPCs of KCC, with a prevalence of 66.1%. Doctors
with a longer duration of clinical practice and who
had not received formal FM training had a higher
rate of TI. Patients with a closer-to-target LDL were
more common in the TI group. Considering that a
large volume of diabetic patients are managed in
the primary care setting, comprehensive strategies
with a more proactive approach should be devised
to combat TI so that the cardiovascular outcome of
diabetic patients can be improved.
Strengths and limitations of the study
This is the first clinical analysis of TI in lipid
management among diabetic patients managed
locally in the primary care setting. It has provided
important background information about the
prevalence of TI in lipid management among diabetic
patients and explored the possible underlying factors
from both the doctor’s and patient’s perspective.
These findings will help improve strategies to
overcome TI in lipid control for these patients.
There are some limitations in this study.
First, the study was carried out in one single
cluster of HA and therefore selection bias might
exist. These results from the public primary health
care sector might not be applicable to the private
sector or secondary care. In addition, the number
of doctors with or without FM training was quite
discrepant in this study (43 vs 13) and may affect the
generalisation of findings. Nevertheless, the present
results may lay the groundwork for similar studies in
the future, both locally and internationally. Second,
patients with diabetes who had not had any blood
testing performed during the study period were
excluded (n=2298, 10.5% of all diabetic cases). The
lipid control status of this group of diabetic patients
remained unknown. This might bias the accurate
measurement of TI among our target population.
Third, only TI in LDL management was explored in
this study. Management of hypertriglyceridaemia
was not addressed in view of its less-important role
as a risk factor for CVD. Future studies exploring the
TI in hypertriglyceridaemia management are needed to
comprehensively assess lipid control among diabetic
patients. Lastly, this study relied heavily on review
of consultation notes to identify justification for
submaximal therapy and determine presence of TI.
Insufficient justification for a certain treatment may
have resulted in an overestimation of the prevalence
of TI.
Conclusions
This study found that TI was common in the lipid
management of diabetic patients managed in GOPCs
of KCC, with a prevalence rate of 66.1%. Doctors
without FM training and a closer-to-target LDL
level among T2DM patients were associated with
the presence of TI. Comprehensive strategies should
be devised to overcome TI so that the cardiovascular
outcome of diabetic patients can be improved.
Acknowledgements
We are indebted to Ms Katherine Chan,
statistical officer of Queen Elizabeth Hospital, for
her expert statistical support in the data analysis.
Appendix
Additional material related to this article can be
found on the HKMJ website. Please go to <http://www.hkmj.org>, and search for the article.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology,
risk factors, and pathophysiology. JAMA 2009;301:2129-40. Crossref
2. Leung GM, Lam KS. Diabetic complications and their
implications on health care in Asia. Hong Kong Med J
2000;6:61-8.
3. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary
prevention of cardiovascular disease with atorvastatin in
type 2 diabetes in the Collaborative Atorvastatin Diabetes
Study (CARDS): multicentre randomised placebo-controlled
trial. Lancet 2004;364:685-96. Crossref
4. Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart
Protection Study Collaborative Group. MRC/BHF Heart
Protection Study of cholesterol-lowering with simvastatin
in 5963 people with diabetes: a randomised placebo-controlled
trial. Lancet 2003;361:2005-16. Crossref
5. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH,
Pedersen O. Multifactorial intervention and cardiovascular
disease in patients with type 2 diabetes. N Engl J Med
2003;348:383-93. Crossref
6. Waters DD, Ku I. Early statin therapy in acute coronary
syndromes: the successful cycle of evidence, guidelines,
and implementation. J Am Coll Cardiol 2009;54:1434-7. Crossref
7. Ward S, Lloyd Jones M, Pandor A, et al. A systematic review
and economic evaluation of statins for the prevention of
coronary events. Health Technol Assess 2007;11:1-160,iii-iv. Crossref
8. O’Regan C, Wu P, Arora P, Perri D, Mills EJ. Statin therapy
in stroke prevention: a meta-analysis involving 121,000
patients. Am J Med 2008;121:24-33. Crossref
9. Vrecer M, Turk S, Drinovec J, Mrhar A. Use of statins
in primary and secondary prevention of coronary heart
disease and ischemic stroke. Meta-analysis of randomized
trials. Int J Clin Pharmacol Ther 2003;41:567-77. Crossref
10. Law MR, Wald NJ, Rudnicka AR. Quantifying effect of
statins on low density lipoprotein cholesterol, ischaemic
heart disease, and stroke: systematic review and meta-analysis.
BMJ 2003;326:1423. Crossref
11. Fung CS, Chin WY, Dai DS, et al. Evaluation of the quality
of care of a multi-disciplinary risk factor assessment and
management programme (RAMP) for diabetic patients.
BMC Fam Pract 2012;13:116. Crossref
12. Kung K, Chow KM, Hui EM, et al. Prevalence of complications
among Chinese diabetic patients in urban primary
care clinics: a cross-sectional study. BMC Fam Pract 2014;15:8. Crossref
13. Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä
K, Keil U; EUROASPIRE Study Group. Cardiovascular
prevention guidelines in daily practice: a comparison
of EUROASPIRE I, II, and III surveys in eight European
countries. Lancet 2009;373:929-40. Crossref
14. Toth PP, Zarotsky V, Sullivan JM, Laitinen D. Dyslipidemia
treatment of patients with diabetes mellitus in a US
managed care plan: a retrospective database analysis.
Cardiovasc Diabetol 2009;8:26. Crossref
15. Ferrières J, Gousse ET, Fabry C, Hermans MP; French
CEPHEUS Investigators. Assessment of lipid-lowering
treatment in France—the CEPHEUS study. Arch
Cardiovasc Dis 2008;101:557-63. Crossref
16. Kotseva K, Stagmo M, De Bacquer D, De Backer G, Wood
D; EUROASPIRE II Study Group. Treatment potential
for cholesterol management in patients with coronary
heart disease in 15 European countries: findings from the
EUROASPIRE II survey. Atherosclerosis 2008;197:710-7. Crossref
17. Santos RD, Waters DD, Tarasenko L, et al. Low- and
high-density lipoprotein cholesterol goal attainment in
dyslipidemic women: The Lipid Treatment Assessment
Project (L-TAP) 2. Am Heart J 2009;158:860-6. Crossref
18. Kim HS, Wu Y, Lin SJ, et al. Current status of cholesterol
goal attainment after statin therapy among patients with
hypercholesterolemia in Asian countries and region: the
Return on Expenditure Achieved for Lipid Therapy in Asia
(REALITY-Asia) study. Curr Med Res Opin 2008;24:1951-63. Crossref
19. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia.
Ann Intern Med 2001;135:825-34. Crossref
20. Okonofua EC, Simpson KN, Jesri A, Rehman SU, Durkalski
VL, Egan BM. Therapeutic inertia is an impediment to
achieving the Healthy People 2010 blood pressure control
goals. Hypertension 2006;47:345-51. Crossref
21. Andrade SE, Gurwitz JH, Field TS, et al. Hypertension
management: the care gap between clinical guidelines and
clinical practice. Am J Manag Care 2004;10:481-6.
22. O’Connor PJ, Sperl-Hillen JM, Johnson PE, Rush
WA, Biltz G. Clinical inertia and outpatient medical
errors. In: Henriksen K, Battles JB, Marks ES, Lewin
DI, editors. Advances in patient safety: from research
to implementation. Vol 2: Concepts and methodology.
Rockville (MD): Agency for Healthcare Research and
Quality (US); 2005.
23. American Diabetes Association. Standards of medical care
in diabetes—2013. Diabetes Care 2013;36 Suppl 1:S11-66. Crossref
24. National Cholesterol Education Program (NCEP) Expert
Panel on Detection, Evaluation, and Treatment of High
Blood Cholesterol in Adults (Adult Treatment Panel
III). Third Report of the National Cholesterol Education
Program (NCEP) Expert Panel on Detection, Evaluation,
and Treatment of High Blood Cholesterol in Adults
(Adult Treatment Panel III) final report. Circulation
2002;106:3143-421.
25. Centers for Disease Control and Prevention (CDC).
Smoking-attributable mortality, years of potential life
lost, and productivity losses—United States, 2000-2004.
MMWR Morb Mortal Wkly Rep 2008;57:1226-8.
26. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D.
A more accurate method to estimate glomerular filtration
rate from serum creatinine: a new prediction equation.
Modification of Diet in Renal Disease Study Group. Ann
Intern Med 1999;130:461-70. Crossref
27. Park JE, Chiang CE, Munawar M, et al. Lipid-lowering
treatment in hypercholesterolaemic patients: the CEPHEUS
Pan-Asian survey. Eur J Prev Cardiol 2012;19:781-94. Crossref
28. Khoo CM, Tan ML, Wu Y, et al. Prevalence and control of
hypercholesterolaemia as defined by NCEP-ATPIII guidelines
and predictors of LDL-C goal attainment in a multi-ethnic
Asian population. Ann Acad Med Singapore 2013;42:379-87.
29. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of
clinical inertia on cardiovascular risk factors in patients
with diabetes. Prim Care Diabetes 2014;8:133-8. Crossref
30. Goldberg KC, Melnyk SD, Simel DL. Overcoming inertia:
improvement in achieving target low-density lipoprotein
cholesterol. Am J Manag Care 2007;13:530-4.
31. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol
to Reduce Atherosclerotic Cardiovascular Risk in Adults,
Journal of the American College of Cardiology 2013.
Available from: https://www.joslin.org/docs/2013-ACC-AHA-Guideline-Treatment-of-Blood-Cholestero-_to-Reduce-Atherosclerotic-Cardiovascular-Risk-in-Adults.pdf. Accessed Mar 2016.
32. Simvastatin summary of product characteristics. Available from: http://www.medicines.org.uk/emc/medicine/1201/SPC. Accessed Mar 2016.
33. Aujoulat I, Jacquemin P, Rietzschel E, et al. Factors
associated with clinical inertia: an integrative review. Adv
Med Educ Pract 2014;5:141-7. Crossref
34. Byrnes PD. Why haven’t I changed that? Therapeutic inertia
in general practice. Aust Fam Physician 2011;40:24-8.
35. Berlowitz DR, Ash AS, Glickman M, et al. Developing a
quality measure for clinical inertia in diabetes care. Health
Serv Res 2005;40:1836-53. Crossref