Hong Kong Med J 2016 Aug;22(4):306–13 | Epub 3 Jun 2016
DOI: 10.12809/hkmj154737
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Validity and reliability of the Chinese version of the Insulin Treatment Appraisal Scale among primary care patients in Hong Kong
KP Lee, FRACGP, MSc Mental Health (CUHK)
Department of Family Medicine and Public Health Unit, Kowloon West Cluster, Hospital Authority, 118 Shatin Pass Road, Hong Kong
This paper was presented at the Hospital Authority Convention, 18-19 May
2015, Hong Kong.
Corresponding author: Dr KP Lee (ineric_2000@yahoo.com.hk)
Abstract
Introduction: Patients with diabetes mellitus
often delay insulin initiation and titration due to
psychological factors. This phenomenon is known
as ‘psychological insulin resistance’. Tools that
identify psychological insulin resistance are valuable
for detecting its causes and can lead to appropriate
counselling. The Insulin Treatment Appraisal Scale
was initially developed for western populations
and has been translated and validated to measure
psychological insulin resistance in Taiwan (Chinese
version of the Insulin Treatment Appraisal Scale, C-ITAS).
The current study examined the prevalence
of psychological insulin resistance and the validity of
the C-ITAS in a local population.
Methods: This cross-sectional study involved 360
patients with diabetes mellitus from a government-funded
general out-patient clinic who completed the
C-ITAS questionnaire. The total C-ITAS score was
compared for patients with psychological insulin
resistance and those without, and the internal
consistency and test-retest reliability of the C-ITAS
were calculated. An exploratory factor analysis was
used to identify factors within the C-ITAS.
Results: The prevalence of psychological insulin
resistance was 44.9%. The internal consistency of
the scale was high (Cronbach’s alpha=0.78). The
test-retest reliability was positive with all C-ITAS
questions (0.294-0.725). The mean C-ITAS
score was significantly higher among patients with
psychological insulin resistance than those without
(42.42 vs 35.78; P<0.001). The exploratory factor
analysis, however, failed to identify the two clear
factors identified in the original validation study.
Conclusions: The C-ITAS appears to be a
feasible and potentially useful tool for identifying
psychological insulin resistance, but additional
validation or translation is required before it can be widely used clinically.
New knowledge added by this study
- The Chinese version of the Insulin Treatment Appraisal Scale (C-ITAS) is a potentially useful and reliable tool to understand patients’ underlying reasons for psychological insulin resistance (PIR).
- Further validation of C-ITAS is needed.
- Understanding patients’ PIR can lead to appropriate and patient-centred counselling.
- Validation of C-ITAS can facilitate a comparison of local PIR studies with those in other countries.
Introduction
Type 2 diabetes mellitus (DM) is a prevalent and
increasingly common disease worldwide.1 It is
estimated to affect 10% of the Hong Kong (HK)
population (approximately 700 000 people).2
Achieving satisfactory DM control during the
early disease course can reduce DM-induced
microvascular and macrovascular complications (ie
the ‘legacy effect’).3 4 These benefits were maintained
in patients in a tight DM-control group even though
their glycosylated haemoglobin (HbA1c) level
became similar to those in the control group after
the end of the United Kingdom Prospective Diabetes
Study.4 It was proposed that a ‘reverse legacy effect’
also persists: “intensive glycaemic intervention
started late in the natural course of diabetes seems
disappointingly ineffective in limiting cardiovascular
events”.5 6 Very tight control may even result in mortality.7 8 Therefore, achieving tight HbA1c control early via lifestyle changes and the use of
medications including insulin is important.
Because of the progressive nature of DM, most
patients eventually require insulin.9 Despite robust
evidence of the benefits of early strict HbA1c control,
patients often delay insulin initiation and titration.
In a UK study, 50% of patients with DM delayed
insulin initiation despite suboptimal control for 5
years, regardless of the presence of complications.10
Their reluctance to initiate insulin use10 11 12 and its
subsequent titration13 is known as ‘psychological
insulin resistance’ (PIR). The prevalence of PIR has
been estimated to be higher in Singapore (70.6%)11
than in western countries (approximately 20%-40%).12 A HK survey of 97 participants found a similarly high prevalence of PIR (72.1%).14 Previous
studies conducted in western countries have
identified several factors that can lead to PIR.11 12 13
These reasons might differ in Asian countries,
however.15 16 Recently, a local primary care research group developed a scale, Chinese Attitudes to
Starting Insulin questionnaire, to identify barriers
to insulin initiation in insulin-naïve patients with
DM.16 These investigators found that Asian patients
might be more affected by the availability of social
support and that cultural differences might also
play a role. For example, Chinese patients are more
likely to combine western medical treatments with
traditional Chinese medicine17 and might believe
that hypoglycaemic agents cause renal toxicity.18
Doctors, particularly primary care physicians,
can be insensitive to patients’ psychological needs;
physicians often fail to recognise psychological
needs19 and might incorrectly identify the
reasons for a patient’s PIR.20 21 Identifying one’s
psychological needs might be hindered in HK due
to short consultation times (lasting an average of
5-7 minutes per consultation). A limited number of
longer sessions may be offered to DM patients with
difficult glycaemic control, but the time limit would
be 10 to 14 minutes. Therefore, a quick tool to help
identify PIR and its underlying causes might help
general practice physicians optimise care for their
patients with DM.12 The Insulin Treatment Appraisal
Scale (ITAS) was developed for this purpose.22 The
Chinese version of the ITAS (C-ITAS) was validated
in Taiwan,23 and has been used in Taiwan15 to investigate the underlying causes of PIR. Validating
C-ITAS scores might enable direct comparisons of data between local and international studies. The
C-ITAS might also be used to help local primary
care clinicians identify PIR and offer appropriate
counselling. The ITAS is sensitive to changes in PIR
throughout the course of DM.24
This study is the first to be conducted in HK to
examine the prevalence of PIR and the validity and
reliability of the C-ITAS in our local population.
Methods
This research has been approved by the Research
Ethics Committee at Kowloon West Cluster, Hospital
Authority.
Participants
Participants were recruited from a government-funded
primary care general out-patient clinic in HK
from July to September 2013. Written consent was
obtained when the participants were approached
by the research assistant. The investigator’s contact
information was given to each participant if they
had concerns after the administration of the
questionnaire. Patients who fulfilled the following
criteria were recruited: (1) diagnosed with type 2 DM
as defined by the World Health Organization25 for
≥6 months; (2) aged 30 years or above; (3) of Chinese
ethnicity; (4) able to communicate effectively in
Cantonese or Mandarin; and (5) had the mental
capacity to provide informed written consent. The
exclusion criteria were severe sensory deficits,
severe mental illness (eg dementia, psychosis, or
mental retardation), or any other health condition
that compromised the ability to comprehend and
complete the questionnaire. The required sample size
was calculated from the estimated prevalence rate of
PIR in the primary care setting. To achieve a 95%
confidence interval with a margin of error of 5% and
an estimated 70% prevalence of PIR among patients
with DM in public primary care,11 14 the required sample size was estimated to be 312 patients. To
compensate for the predicted 20% refusal rate, at
least 390 patients were recruited.
A list of DM patients who would attend the
clinic the next day was obtained daily. From that list,
40 patients were randomly selected by computer (25
in the morning and 15 in the afternoon). A reminder
was set in the clinical computer system such that
clinic staff were alerted once the patient attended
his or her appointment. The procedure was repeated
until the number of patients recruited exceeded 390,
which was checked at clinic closing time.
Patients were encouraged to complete the
questionnaire unaided because the C-ITAS is self-administered.
Because the majority of patients who
attend public primary care clinics are of lower socio-economic
status and education level, those who had
difficulty completing the questionnaire were assisted
by research assistants who were trained by the
principal investigator.
Each patient was asked whether he or she
was willing to have insulin started or titrated upon
his or her case doctor’s suggestion. The response
options included “strongly unwilling”, “unwilling”,
“might consider it”, “willing”, and “very willing”.
Demographic data were collected, and clinical data
(eg the presence of DM complications, insulin use,
and control of DM and lipid levels) were retrieved
from a computer database.
Insulin Treatment Appraisal Scale
The ITAS is a 20-item instrument that contains 16
negative and four positive statements that appraise
insulin treatment. Each statement is ranked using
a 5-point Likert-type scale from 1 to 5. Positive
scores are reversed to allow for summation. The
total possible score ranges from 0 to 80. A higher
score signifies a more negative appraisal of insulin.
The ITAS was developed for clinical use to measure
PIR.22 No cut-off score is used to diagnose PIR. Of
those who completed the clinical interview, 26 were
selected for phone interview 2 to 4 weeks later to
examine test-retest reliability. Because of the lack
of a written language difference between Taiwan
and HK, the validated C-ITAS was used with the
permission of the Taiwan research group.
Statistical analyses
The C-ITAS was examined for its internal reliability
using Cronbach’s alpha, the test-retest reliability
was assessed using Pearson’s correlation of test
scores and retest scores, and construct validity
was assessed using an exploratory factor analysis
(EFA) [using Oblimin rotation as this was used in
the original development study of ITAS22]. Patients
who answered “strongly unwilling” or “unwilling”
to the question “Would you agree to start or titrate
insulin treatment if advised by your case doctor?”
were classified as having PIR. Descriptive statistics
were used to describe the prevalence of PIR. Each
C-ITAS item was dichotomised as “unwilling”
(scores of 1 and 2) or “neutral/willing” (scores of
3 to 5); this dichotomy was created to assess the
difference between patients with and without PIR.
The responses of the patients with or without PIR
were compared using a Chi squared test.
Results
Participants
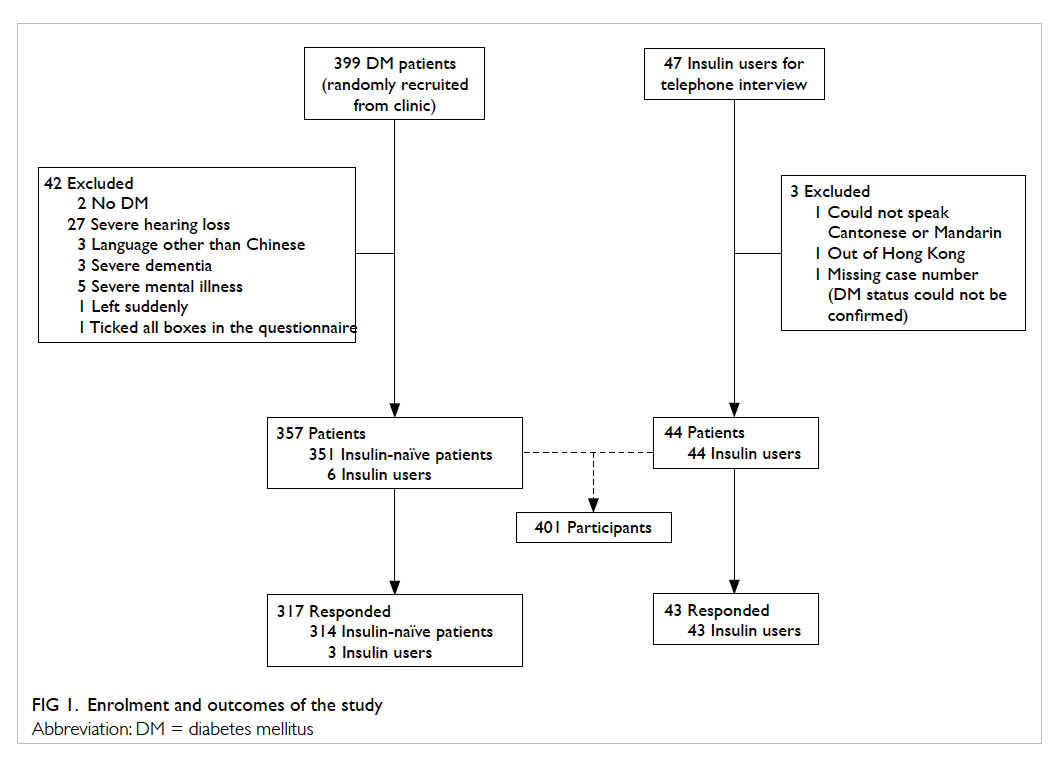
A total of 399 patients with DM were randomly
selected from the clinical database and approached
by the research team (Fig 1). Of them, 42 patients
were excluded due to the following circumstances: two
patients were incorrectly diagnosed with DM; 27 had
severely impaired hearing not compensated for with
the use of hearing aids; three spoke languages other than
Cantonese or Mandarin; eight had severe psychiatric
illness such as dementia, psychosis, or mental
retardation; one left at the beginning of the interview
when called into a consultation room; and one was
excluded for checking all boxes of the questionnaire.
In addition to the insulin-naïve patients with
DM who were recruited as outlined above, all of
the current insulin users who were not interviewed
during the above period (47 patients) were invited to
participate in this study and were interviewed over
the phone; of whom three
were excluded for the following reasons: one could
not speak Cantonese or Mandarin, one was out of HK
during the interview period, and one questionnaire was
invalid due to a missing subject case number.
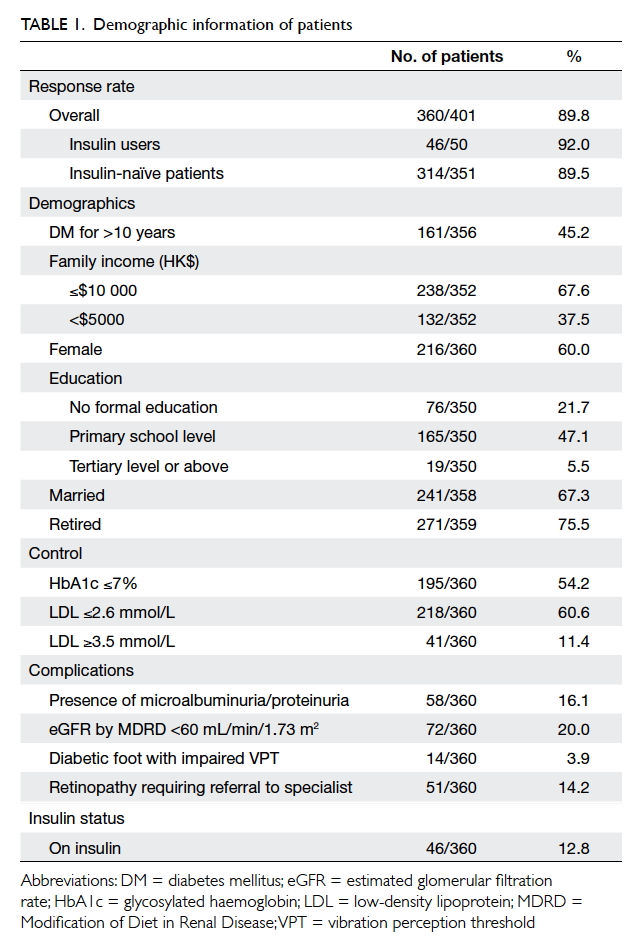
The overall response rate was 89.8% (n=360):
89.5% (n=314) for the insulin-naïve patients
and 92.0% (n=46) for the insulin users. Other
demographic data are shown in Table 1.
A total of 12.8% (n=46/360) of participants
were insulin users. Patients with HbA1c ≥7% (≥53
mmol/mol; 21.6%) were more likely to be on insulin
than those with HbA1c <7% (<53 mmol/mol; 2.9%;
P<0.001). The HbA1c level was not significantly
associated with the presence of DM complications in
the current study. Of all participants, 96.3% received
DM complication screening within 2 years, which
was a nurse-led clinical service to screen for DM
complications and provide counselling.
Non-respondents were significantly older
(mean age=72.32 vs 67.17 years, t test: P<0.001),
less likely to agree to titrate insulin (for current
insulin users), and less educated (91.7% educated
up to primary level vs 68.9%; Chi squared test;
P=0.004). The differences with regard to the other
demographics, including DM complication rate,
insulin use status, marriage, work, family income,
and gender were not significant.
The prevalence of PIR was 44.9% (141/314; 95%
confidence interval [CI], 39.4% to 50.4%) in insulin-naïve
patients; in contrast, the PIR rate was 6.8%
(3/44; 95% CI, -0.64% to 14.24%) in current insulin
users.
The questionnaire
The internal consistency of the C-ITAS questionnaire
was high, with Cronbach’s alpha of 0.78. The original
ITAS was designed to have 16 negative and four
positive statements. Cronbach’s alpha was calculated
separately for the negative and positive statements,
yielding values of 0.812 and 0.738, respectively.
Within the negative statement scale, removing two
negatively stated questions individually, including
Q1, “Insulin signifies failure with pre-insulin
therapy”, and Q18, “Taking insulin causes family/friends to be more concerned” improved the overall Cronbach’s alpha to 0.819 and 0.825, respectively.
Of the 20 individual questions within the
C-ITAS, answers to 17 questions were significantly
different in the expected direction between patients
with PIR and those without. Importantly, Q18,
“Taking insulin causes family/friends to be
more concerned” was originally designed to detect
a negative view towards insulin use; however, more
insulin-accepting patients agreed with the statement
(Table 2).
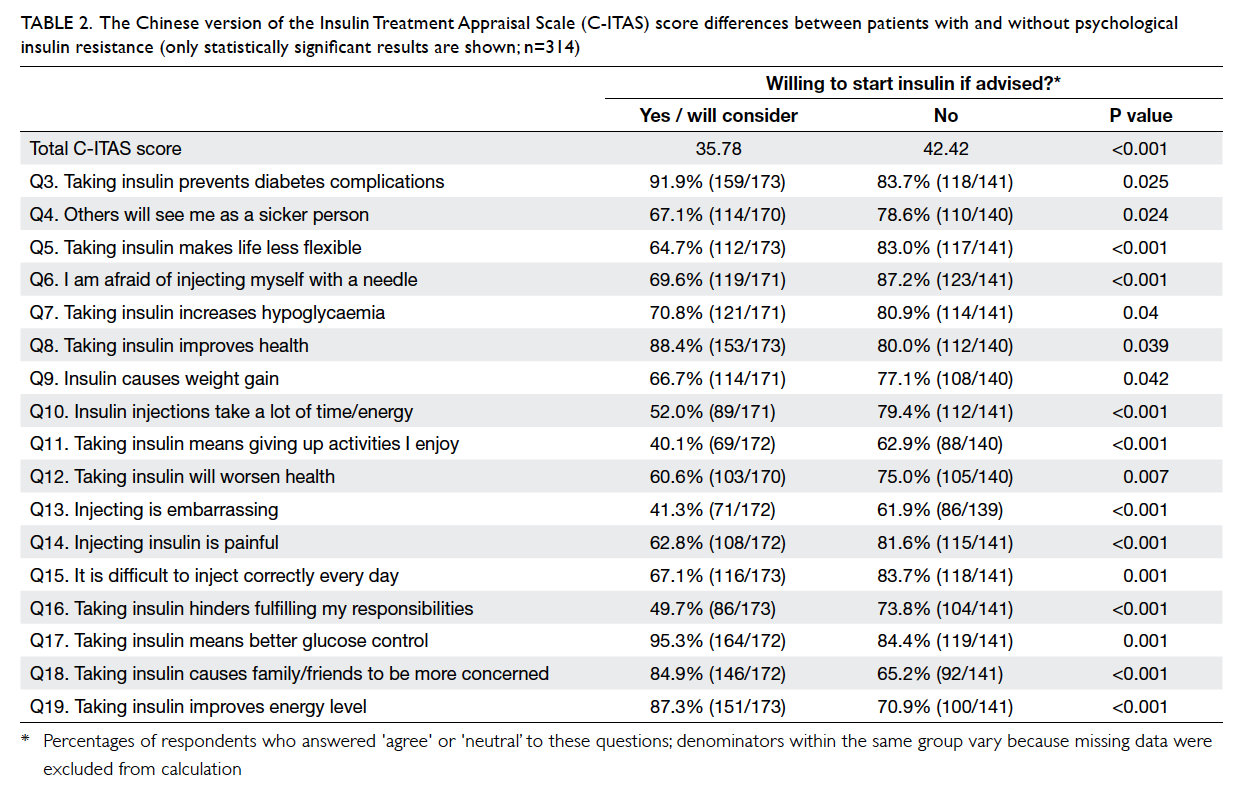
Table 2. The Chinese version of the Insulin Treatment Appraisal Scale (C-ITAS) score differences between patients with and without psychological insulin resistance (only statistically significant results are shown; n=314)
The total C-ITAS scores, as described above,
were higher among participants who refused insulin
initiation (42.42 vs 35.78; t test, P<0.001). The test-retest
reliability for each question ranged from 0.294
to 0.725, and 13 questions were significant (P<0.05).
The test-retest reliability of the overall scores as
defined above was 0.571 (P=0.002).
The EFA identified five factors with an eigenvalue of >1. Nonetheless, the scree plot correctly identified two factors within the questionnaire.
When two factors were extracted using an Oblimin
rotation, a few negative statements including Q18
were significantly associated with the other positive
statements (Table 3). The three-, four-, and five-factor solutions were calculated as suggested by the eigenvalue, which did not provide better representation of
the latent structure of ITAS.
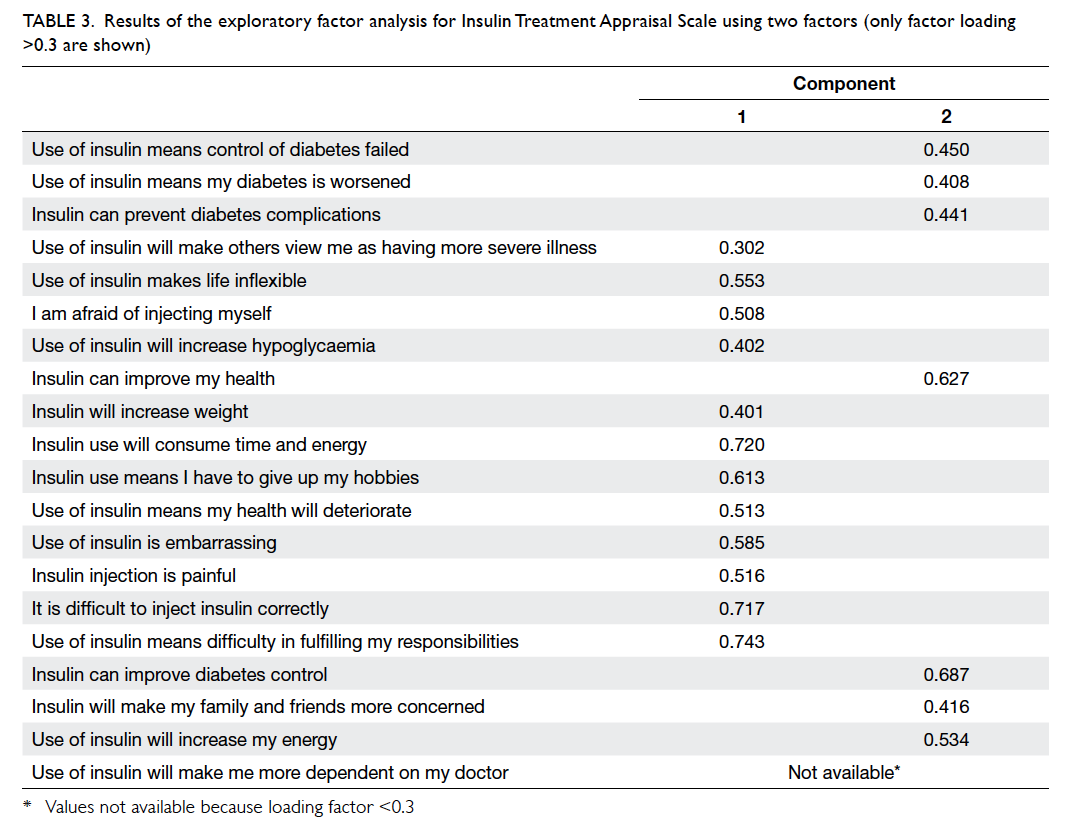
Table 3. Results of the exploratory factor analysis for Insulin Treatment Appraisal Scale using two factors (only factor loading >0.3 are shown)
In the EFA, the Kaiser-Meyer-Olkin measure
of sampling adequacy was 0.834 and Bartlett’s test
of sphericity was significant (P<0.001), and signified
adequate sample size for the test.
Discussion
Because the participants were old and not well
educated, difficulties in answering the C-ITAS were
expected. This assumption was further supported by
the fact that the non-respondents were less educated
and were older than the respondents. Nevertheless
a high proportion of participants (89.8%) were able
to complete the entire questionnaire. Additional
research might be necessary to assess the response
rate if the questionnaire is self-administered because
the staffing at our public out-patient clinics was
limited. The use of ITAS might be limited if it cannot
be self-administered because it was developed as a
self-administered tool.
Prevalence of psychological insulin resistance
It is surprising that the prevalence of PIR was not
as high as reported by previous studies.11 14 More than 50% of patients were willing to consider or accept
insulin if suggested by their primary doctor. This
finding might be because of differences in the patient
cohorts or the improvements made to the PIR over
the years due to patient education. Only 53 patients
with DM out of the thousands of patients followed
up in our clinic were started on insulin. Alternative
reasons might explain the low rates of insulin use (eg
physician beliefs and competencies regarding the
use of insulin), and might merit additional research.
Validity and reliability of the questionnaire
The C-ITAS was reliable because it yielded high
Cronbach’s alpha scores (0.738-0.812) and correctly
provided a higher score for patients who resisted
insulin use. It identified many different attitudes
towards insulin use; in the current study, answers to
17 out of 20 of the C-ITAS items significantly differed
between patients who resisted insulin and those who
did not, whereas a previous study showed that only
four questions were able to make this distinction.12
This may be because individual patients had multiple
concerns and many different attitudes towards
insulin use.
Although the test-retest reliability value of
all ITAS items was positive, the values were low,
ranging from 0.294 to 0.725 for individual C-ITAS
questions. In the present study, the C-ITAS was
completed either via a personal interview with a
research assistant or by self-administration. Retests
were administered via telephone interviews by either
the research assistant or the principal investigator.
Therefore, the low test-retest reliability scores might
be because of the different means of administration
or due to the different interviewers. Conversely,
this difference might reflect the actual low test-retest
reliability of the current C-ITAS that requires
additional validation.
Question 18, “Taking insulin causes family/friends to be more concerned”,
merits additional discussion. Originally designed
as a negative statement, it is expected that patient
resistance to insulin would positively predict the
score. The reverse was true, however, in the current
study (Table 2). When the statement was reviewed
by six family physicians and one psychiatrist, the
word “concerned” (關心) was translated into a word
in Chinese that can also mean “caring” (使用胰島素使家人和朋友對我更關心). It is likely that patients
understood the question as, “Taking insulin causes
my family and friends to be more caring toward me”.
Because Q18 was meant to be a negative statement,
it is more appropriate to translate its meaning to
“worry”. This supposition is supported by both the
Cronbach’s alpha analysis, in which exclusion of
Q18 improved the value of Cronbach’s alpha,
and the factor analysis, where Q18 was regarded as a
factor with the other positive statements. The factor
analysis did not show a two-factor structure within
the ITAS, as in the previous study.22 As the factor
analysis table notes (Table 3), when set as a two-factor
construct, no trend can be drawn for these
two groups. The factor analyses of the first study
on the development of the ITAS22 and the validation
study in Taiwan23 both showed a two-factor construct,
with the two factors being positive statements and
negative statements. This finding might reflect the
previously noted translation problem; alternatively,
our local community might have had a different
set of causes for PIR. This finding suggests that a
dialectic or cultural difference remains between HK
and Taiwan, despite a shared written language.26 27 Additional validation of the C-ITAS in our local
population is likely necessary.
Strengths and weaknesses
The strengths of our study include its large sample
size, the use of random sampling, and the high
response rate. The use of an internationally validated
questionnaire might aid comparison with results
from other countries. The C-ITAS, however, might
require additional validation as noted above.
The statement proposing the use of insulin to
patients was hypothetical. For example, estimated
PIR rates might be lower when patients perceive
their disease as having deteriorated so that additional
intervention is necessary.
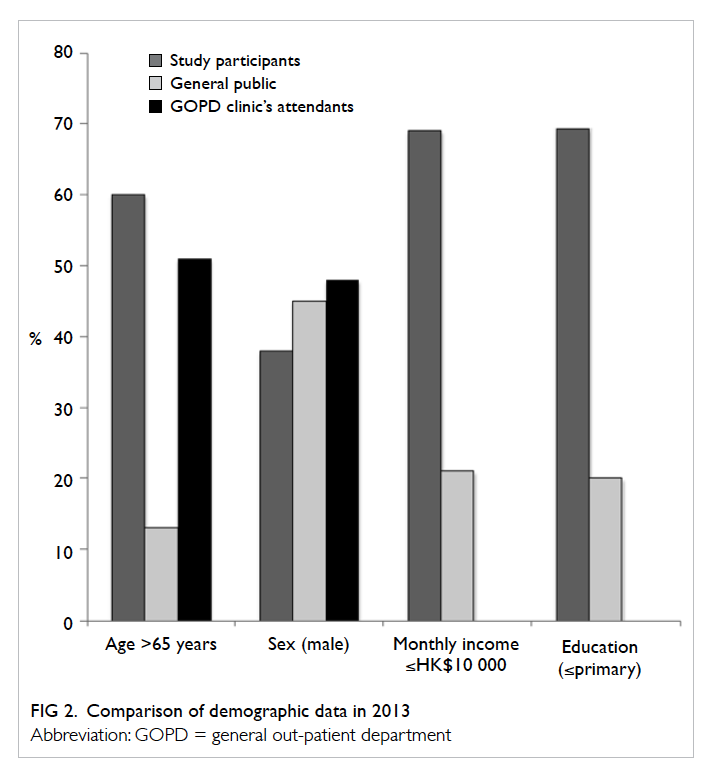
This study was conducted in a major
government-funded clinic in Hong Kong, and the
demographics of the participants were more similar
to those of other government clinics than to the
general population (Fig 2). The extent to which the
results can be generalised to other countries and to
other social classes (eg wealthy patients attending
private primary clinics) is not known.
A majority of the patients in the current study
were insulin-naïve. Despite including all available
insulin users in the clinic, the number of insulin
users was small, and limits the potential applicability
of this study’s results to secondary or tertiary care
where many patients may be on insulin.
The study also did not distinguish between
questionnaires that were completed with the help of
research staff and those that were self-administered.
The influence of different administration methods
on the outcome has not been previously described.
For example, when participants did not understand
a statement, the trained research assistant may
use her own words to elaborate and explain it to
the participant and thus may alter the statement’s
original sentence structure or intended meaning.
Another weakness was that data on
macrovascular complications were not collected.
Microvascular complications were well documented
during the DM complication screening and were
easily traceable. The tracing of macrovascular
complications, however, was difficult because
diagnostic coding needed to be entered or the
complication needed to be mentioned in the latest
case record by the respective doctors, and missed
coding for macrovascular complications was not
uncommon.
Conclusions
The prevalence of PIR was 44.9% in our population,
which is less than that previously estimated. Tools such as
the C-ITAS can improve physician’s understanding
of patient views on insulin and might help physicians
to appropriately counsel their patients. The C-ITAS
may provide clues to patients’ knowledge about
insulin use, eg the risk of hypoglycaemia or the
side-effects of obesity. Despite good psychometric
properties such as high internal consistency,
there is a translation issue in at least one of the 20
statements. Health care professionals who wish to
use the C-ITAS clinically should be aware of the
instrument’s limitations.
Acknowledgements
The author expresses gratitude to Prof Sandra
Chan for her teaching and guidance regarding this
research; to Prof Samuel Wong for his kind and
timely advice; and to Drs YK Yiu and SN Fu and the
Department of Family Medicine, Kowloon West
Cluster, HK for their research support. The author
would like to thank Ms Man-ping Chang and her
team for the development of the C-ITAS and for
allowing the use of the C-ITAS in the current study.
Declaration
The author has disclosed no conflicts of interest.
References
1. International Diabetes Federation. IDF diabetes atlas
update 2012. Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf. Accessed 1 Apr
2013.
2. Hong Kong Department of Health. Hong Kong reference
framework for diabetes care for adults in primary care
settings. Available from: http://www.pco.gov.hk/english/resource/professionals_diabetes_pdf.html. Accessed 1
Apr 2013.
3. Genuth S, Eastman R, Kahn R, et al. Implications of the
United Kingdom prospective diabetes study. Diabetes Care
2003;26 Suppl 1:S28-32. Crossref
4. Davis TM, Colagiuri S, United Kingdom Prospective
Diabetes Study. The continuing legacy of the United
Kingdom Prospective Diabetes Study. Med J Aust
2004;180:104-5.
5. ADVANCE Collaborative Group, Patel A, MacMahon
S, et al. Intensive blood glucose control and vascular
outcomes in patients with type 2 diabetes. N Engl J Med
2008;358:2560-72. Crossref
6. Riddle MC, Yuen KC. Reevaluating goals of insulin therapy:
perspectives from large clinical trials. Endocrinol Metab
Clin North Am 2012;41:41-56. Crossref
7. Action to Control Cardiovascular Risk in Diabetes Study
Group, Gerstein HC, Miller ME, et al. Effects of intensive
glucose lowering in type 2 diabetes. N Engl J Med
2008;358:2545-59. Crossref
8. ACCORD Study Group, Gerstein HC, Miller ME, et
al. Long-term effects of intensive glucose lowering on
cardiovascular outcomes. N Engl J Med 2011;364:818-28. Crossref
9. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control
with diet, sulfonylurea, metformin, or insulin in patients
with type 2 diabetes mellitus: progressive requirement for
multiple therapies (UKPDS 49). UK Prospective Diabetes
Study (UKPDS) Group. JAMA 1999;281:2005-12. Crossref
10. Rubino A, McQuay LJ, Gough SC, Kvasz M, Tennis P.
Delayed initiation of subcutaneous insulin therapy after
failure of oral glucose-lowering agents in patients with
type 2 diabetes: a population-based analysis in the UK.
Diabet Med 2007;24:1412-8. Crossref
11. Wong S, Lee J, Ko Y, Chong MF, Lam CK, Tang WE.
Perceptions of insulin therapy amongst Asian patients with
diabetes in Singapore. Diabet Med 2011;28:206-11. Crossref
12. Woudenberg YJ, Lucas C, Latour C, Scholte op Reimer WJ.
Acceptance of insulin therapy: a long shot? Psychological
insulin resistance in primary care. Diabet Med 2012;29:796-802. Crossref
13. Jenkins N, Hallowell N, Farmer AJ, Holman RR, Lawton
J. Participants’ experiences of intensifying insulin therapy
during the Treating to Target in Type 2 diabetes (4-T) trial:
qualitative interview study. Diabet Med 2011;28:543-8. Crossref
14. Yiu MP, Cheung KL, Chan KW, et al. A questionnaire study
to analyze the reasons of insulin refusal of DM patients
on maximum dose of oral hypoglycemic agents (OHA)
among 3 GOPCs in Kowloon West Cluster. 2010. Available
from: http://www.ha.org.hk/haconvention/hac2010/proceedings/pdf/Poster/spp-p5-38.pdf. Accessed 1 Apr
2013.
15. Chen CC, Chang MP, Hsieh MH, Huang CY, Liao LN, Li
TC. Evaluation of perception of insulin therapy among
Chinese patients with type 2 diabetes mellitus. Diabetes
Metab 2011;37:389-94. Crossref
16. Fu SN, Chin WY, Wong CK, et al. Development and
validation of the Chinese Attitudes to Starting Insulin
questionnaire (Ch-ASIQ) for primary care patients with
type 2 diabetes. PLoS One 2013;8:e78933. Crossref
17. Ma GX. Between two worlds: the use of traditional
and western health services by Chinese immigrants. J
Community Health 1999;24:421-37. Crossref
18. Lai WA, Lew-Ting CY, Chie WC. How diabetic patients
think about and manage their illness in Taiwan. Diabet
Med 2005;22:286-92. Crossref
19. Martin A, Rief W, Klaiberg A, Braehler E. Validity of the
Brief Patient Health Questionnaire Mood Scale (PHQ-9) in
the general population. Gen Hosp Psychiatry 2006;28:71-7. Crossref
20. Peyrot M, Rubin RR, Khunti K. Addressing barriers to
initiation of insulin in patients with type 2 diabetes. Prim
Care Diabetes 2010;4 Suppl 1:S11-8. Crossref
21. Brod M, Kongsø JH, Lessard S, Christensen TL.
Psychological insulin resistance: patient beliefs and
implications for diabetes management. Qual Life Res
2009;18:23-32. Crossref
22. Snoek FJ, Skovlund SE, Pouwer F. Development and
validation of the insulin treatment appraisal scale (ITAS) in
patients with type 2 diabetes. Health Qual Life Outcomes
2007;5:69. Crossref
23. Chang MP, Huang CY, Li TC, Liao LN, Chen CC. Validation
of the Chinese version of the insulin treatment appraisal
scale. J Diabetes Investig 2010;1(Suppl 1):88.
24. Hermanns N, Mahr M, Kulzer B, Skovlund SE, Haak T.
Barriers towards insulin therapy in type 2 diabetic patients:
results of an observational longitudinal study. Health Qual
Life Outcomes 2010;8:113. Crossref
25. World Health Organization. About diabetes 2013. Available
from: http://www.who.int/diabetes/action_online/basics/en/index1.html. Accessed 1 Feb 2013.
26. Chow KM, Chan CW, Choi KC, et al. Psychometric
properties of the Chinese version of Sexual Function After
Gynecologic Illness Scale (SFAGIS). Support Care Cancer
2013;21:3079-84. Crossref
27. Tang DY, Liu AC, Leung MH, Siu BW. Antisocial
Personality Disorder Subscale (Chinese version) of the
Structured Clinical Interview for the DSM-IV Axis II
disorders: validation study in Cantonese-speaking Hong
Kong Chinese. East Asian Arch Psychiatry 2013;23:37-44.