Hong Kong Med J 2016 Jun;22(3):249–55 | Epub 6 May 2016
DOI: 10.12809/hkmj154659
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Diagnostic accuracy of spot urine protein-to-creatinine ratio for proteinuria and its association with adverse pregnancy outcomes in Chinese pregnant patients with pre-eclampsia
HC Cheung, MB, BS, MRCOG1;
KY Leung, FRCOG, FHKAM (Obstetrics and Gynaecology)1;
CH Choi, FHKCP, FHKAM (Medicine)2
1 Department of Obstetrics and Gynaecology, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Department of Medicine, Queen Elizabeth Hospital, Jordan, Hong Kong
Corresponding author: Dr HC Cheung (chc670@ha.org.hk)
Abstract
Introduction: International guidelines have
endorsed spot urine protein-to-creatinine ratio of
>30 mg protein/mmol creatinine as an alternative
to a 24-hour urine sample to represent significant
proteinuria. This study aimed to determine the
accuracy of spot urine protein-to-creatinine ratio
in predicting significant proteinuria and adverse
pregnancy outcome.
Methods: This case series was conducted in a
regional obstetric unit in Hong Kong. A total of
120 Chinese pregnant patients with pre-eclampsia
delivered at Queen Elizabeth Hospital from January
2011 to December 2013 were included. Relationship
of spot urine protein-to-creatinine ratio and 24-hour
proteinuria; accuracy of the ratio against 24-hour
urine protein at different cut-offs; and relationship
of such ratio and adverse pregnancy outcome were
studied.
Results: Spot urine protein-to-creatinine ratio was
correlated with 24-hour urine protein with Pearson
correlation coefficient of 0.914 (P<0.0001) when the
ratio was <200 mg/mmol. The optimal threshold of
spot urine protein-to-creatinine ratio for diagnosing
proteinuria in Chinese pregnant patients (33 mg/mmol) was similar
to that stated in the international literature (30 mg/mmol). A cut-off
of 20 mg/mmol provided a 100% sensitivity, and 52
mg/mmol provided a 100% specificity. There was
no significant difference in spot urine protein-to-creatinine
ratio between cases with and without
adverse pregnancy outcome.
Conclusions: Spot urine protein-to-creatinine ratio
had a positive and significant correlation with 24-hour
urine results in Chinese pre-eclamptic women when
the ratio was <200 mg/mmol. Nonetheless, this ratio
was not predictive of adverse pregnancy outcome.
New knowledge added by this study
- Spot urine protein-to-creatinine ratio (uPCR) had a positive and significant correlation with 24-hour urine results in Chinese pre-eclamptic women when uPCR was <200 mg/mmol.
- uPCR was not predictive of adverse pregnancy outcome in Chinese pre-eclamptic women.
- The optimal threshold for diagnosis of proteinuria in the local Chinese population was similar to the 30 mg/mmol suggested by international guidelines.
- When uPCR is <20 mg/mmol (significant proteinuria very unlikely) or >52 mg/mmol (significant proteinuria very likely), early clinical management can be facilitated without awaiting results of 24-hour urine protein.
- When uPCR is ≥200 mg/mmol, 24-hour urine is needed for an accurate quantification as correlation between these two tests is low above this level.
Introduction
Pre-eclampsia, or de-novo proteinuric hypertension
after 20 weeks of pregnancy,1 is a major cause of
maternal and perinatal morbidity and mortality
due to eclampsia, cerebrovascular events, preterm
delivery, and fetal growth restriction. In industrialised
countries, the incidence has been reported to be 3%
to 5% of pregnancies.2 3 In a territory-wide study in China, hypertensive disorders complicated 5.2% of
all pregnancies, with more than 50% of them being
pre-eclampsia.4
The gold standard for diagnosis of proteinuria
is the presence of >300 mg of protein in a 24-hour
urine sample.1 This test, however, is cumbersome
and time-consuming for women, has cost
implications, can cause a delay in diagnosis and
hence management because of its turnaround time,
and can lead to inaccurate results from incomplete
collection or varying use of assays.
International guidelines have endorsed spot
urine protein-to-creatinine ratio (uPCR) of >30
mg protein/mmol creatinine as an alternative to
a 24-hour urine sample to represent significant
proteinuria.1 5 6 Meta-analyses in 2012 and 2013
revealed that maternal uPCR showed promising
diagnostic value for significant proteinuria in
suspected pre-eclampsia.7 8 The optimal threshold to detect significant proteinuria varied from 0.30
to 0.35, and considerable heterogeneity existed in
the diagnostic accuracy at most thresholds across
studies.7 Moreover, there were few studies of the
diagnostic value of uPCR in a Chinese population.
Since Chinese women generally have a lower muscle
mass than their western counterparts, the former
may have a lower urinary creatinine excretion
that may alter their uPCR level9 and consequent
diagnostic accuracy of uPCR.
While some studies10 11 12 have suggested that
proteinuria is related to adverse pregnancy outcome,
others have not.1 13 In the 2012 meta-analysis, there was insufficient evidence that uPCR could
predict adverse pregnancy outcome.7 The latest
guidelines on hypertension in pregnancy from the
National Institute for Health and Care Excellence
have recommended research to identify diagnostic
thresholds of proteinuria that can accurately predict
clinically important outcomes.5
The aims of this study were to determine
the accuracy of uPCR in predicting significant
proteinuria in our local population, and adverse
maternal or neonatal outcomes. If uPCR can
accurately predict significant proteinuria and adverse
pregnancy outcomes, it will be a quick, acceptable,
and potentially cost-effective alternative to 24-hour
urine for protein analysis. The clinical management
of suspected proteinuric hypertension in pregnancy
can then be modified to facilitate an early diagnosis
or exclusion of pre-eclampsia.
Methods
All Chinese pregnant women with a diagnosis of
pre-eclampsia (new-onset proteinuric hypertension
after 20 weeks of gestation) and who delivered
at Queen Elizabeth Hospital in Hong Kong from
January 2011 to December 2013 (36 months) were
eligible for initial inclusion in this retrospective
study. Hypertension was defined as blood pressure of
≥140/90 mm Hg. Significant proteinuria was defined
as 24-hour urine total protein of ≥300 mg/day
or uPCR of ≥30 mg/mmol (local laboratory
reference) if the former was not available. Test of
uPCR has been available for a long period and has
been widely used in our department since January
2011. The diagnosis of proteinuria was mostly based
on 24-hour urine testing rather than uPCR before
January 2011. Women were excluded from the
study if they had pre-existing renal disease, chronic
hypertension, or co-existing urinary tract infection
(defined by a positive mid-stream urine culture).
The study was approved by the hospital research and
ethics committee as a registered study (Ref.: KC/KE-15-0025), with the requirement of patient informed
consent waived because of its retrospective nature.
In this study, uPCR was collected as a random
urine sample at any time of the day. It was collected
in the presence of a positive urine dipstick for protein
or in women who presented with hypertension (even
dipstick negative) to confirm or exclude proteinuria.
For 24-hour urine, women were provided with a
bottle and instructions to collect all urine within
a 24-hour period. All collections were sent to the
laboratory within 1 day of completion. Urine total
protein was measured using a turbidimetric method
based on benzethonium chloride reaction. Urine
creatinine was measured using a kinetic colorimetric
assay based on the Jaffé method. Both tests were
performed with a Roche/Hitachi cobas c501 analyser
(cobas 6000 system; Roche Diagnostics GmbH,
Mannheim, Germany). The imprecision (coefficient
of variation) of the urine protein assay was 3.7% at
0.18 g/L and 1.9% at 0.54 g/L. The imprecision of the
urine creatinine assay was 6.9% at 7.0 mmol/L and
2.2% at 20.8 mmol/L.
For the primary outcome analysis, women who
had both uPCR and adequate 24-hour urine results
collected within 24 hours were identified. 24-Hour
urine collection was often inaccurate (due to over- or
under-collection), even though patients have been
provided with a standard instruction. It has been
reported that 13% to 54% of 24-hour urine collections
were inaccurate, with 24.8% of patients having a
difference of ≥25% in the results between collections,
exceeding the analytical and biological variation.14
Completeness of a 24-hour urine collection was
assessed by urinary creatinine excretion. The normal
range for urinary creatinine excretion was 7 to 14
mmol/day in our laboratory as recommended by the
vendor of the test. Considering a mean body weight
of 70 kg during pregnancy and understanding that
urinary creatinine excretion remains unchanged
in pregnancy, this reference range was compatible
with general nephrology references of 133 to 177
µmol/kg/day of lean body mass.15 The two urine tests
should be collected within 1 day to avoid the effect
of day-to-day variation on the amount of protein in
urine.
For the secondary outcome analysis, adverse
maternal outcomes were represented by severe
hypertension (blood pressure ≥160/110 mm Hg),
raised liver enzyme (alanine aminotransferase
or aspartate aminotransferase ≥70 IU/L), renal
insufficiency (serum creatinine ≥80 µmol/L),
thrombocytopenia (platelet count <100 x 109 /L),
admission to intensive care unit (ICU), eclampsia,
or maternal mortality. Adverse neonatal outcomes
were represented by prematurity (delivery at <37
weeks’ gestation), low birth weight (<2500 g), small-for-gestational age based on local population data,
low Apgar score (<7) at 1 minute and 5 minutes of
birth, admission to the neonatal intensive care unit
(NICU), stillbirth, or early neonatal death. Data for
maternal and neonatal outcomes were obtained from
the hospital’s Clinical Data Analysis and Reporting
System and individual medical records. To minimise
the effect of multiple pregnancy on the clinical
outcome, only singleton pregnancies were included
for secondary outcome analysis. If more than one
sample of uPCR were collected during the pregnancy,
the first uPCR at the onset of proteinuria was used to
determine the association with adverse outcomes.
Data were analysed using the Statistical Package
for the Social Sciences (Windows version 22.0; SPSS
Inc, Chicago [IL], US). For the primary outcome
analysis, the relationship between uPCR and 24-hour
urine was assessed by Pearson correlation coefficient
after taking logarithm as the data distribution of
these two parameters were not nominal. Sensitivity,
specificity, and positive and negative predictive
values of uPCR were calculated. The sensitivity
and specificity of uPCR at different cut-offs were
analysed by receiver operating characteristics (ROC)
curve. For the secondary outcome analysis, Mann-Whitney U test was used to determine the difference
in proteinuria level between cases with or without an
adverse pregnancy outcome.
Results
Of 432 cases of pre-eclampsia identified during the 36-month study period, 175 (40.5%) had uPCR analysed after excluding cases without collection of uPCR
before delivery or ordering because of individual
clinician’s preference or because immediate delivery
was expected. Of these 175 cases, 55 (31.4%) were
excluded after review of medical records, including
28 non-Chinese patients, 24 cases with pre-existing
hypertension or pre-existing renal disease, one
woman with active urinary tract infection, one with
missing information, and one who did not
deliver at our hospital. Urine samples collected from
the remaining 120 women, who ranged from 24
weeks to 41 weeks of gestation, were analysed. The
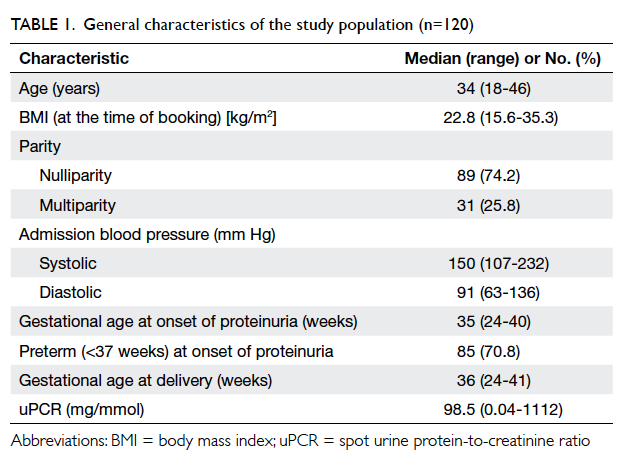
general characteristics of the study population are
shown in Table 1.
Spot urine protein-to-creatinine ratio and 24-hour urine protein
Of these 120 cases, 98 pairs of urine samples were
collected, of which 12 were inadequate and 20 were
collected more than 1 day apart. The remaining 66
pairs with both uPCR and adequate 24-hour urine
collection available within 1 day were used for the
primary outcome analysis. The median body weight
of these 66 women was 72.1 kg (range, 56.5-97.0 kg).
The two tests were correlated with a Pearson
correlation coefficient (r) of 0.914 (P<0.0001).
From Figure 1, it is clear that a positive and linear
correlation between uPCR and 24-hour urine
protein was evident up to a uPCR of 200 mg/mmol.
On subgroup analysis, the correlation coefficient was
high (0.875) and significant (P<0.0001) for uPCR of
<200 mg/mmol, but low (0.389) and non-significant
(P=0.152) for uPCR of ≥200 mg/mmol.
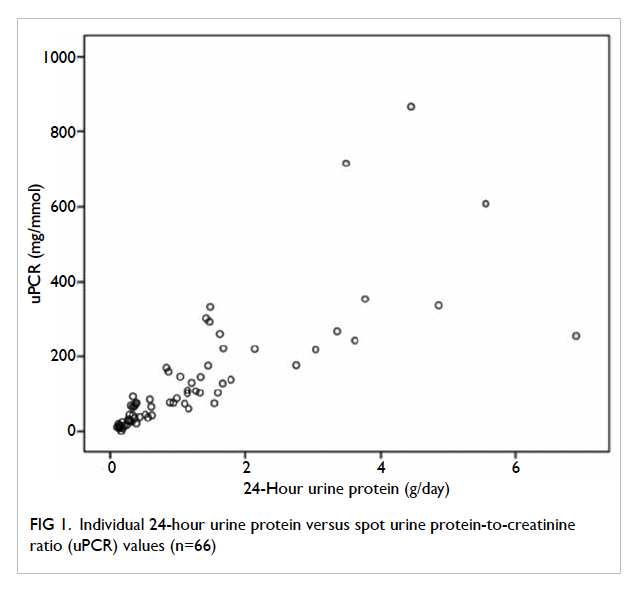
Figure 1. Individual 24-hour urine protein versus spot urine protein-to-creatinine ratio (uPCR) values (n=66)
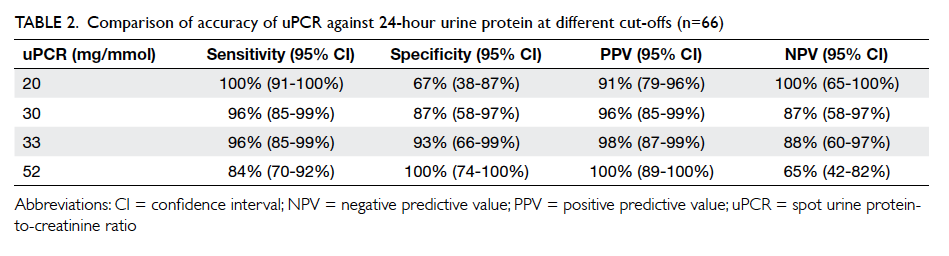
With the local laboratory reference of uPCR
set at 30 mg/mmol, as suggested by international
guidelines,1 5 6 the positive predictive value of
significant proteinuria (defined by 24-hour urine
total protein ≥300 mg/day) was 96%, sensitivity
96%, negative predictive value 87%, and specificity
87%. Two false-positive cases and two false-negative
cases were found. For the two false-positive cases,
the 24-hour urine results were 0.28 g/d and 0.26 g/d
and corresponding uPCR results were 44 mg/mmol
and 31 mg/mmol. Although these
24-hour urine results were negative, these women
subsequently developed proteinuria and were
confirmed to have pre-eclampsia. For the two false-negative
cases, their 24-hour urine results were 0.31
g/d and 0.38 g/d and corresponding uPCR results
were 26 mg/mmol and 21 mg/mmol.
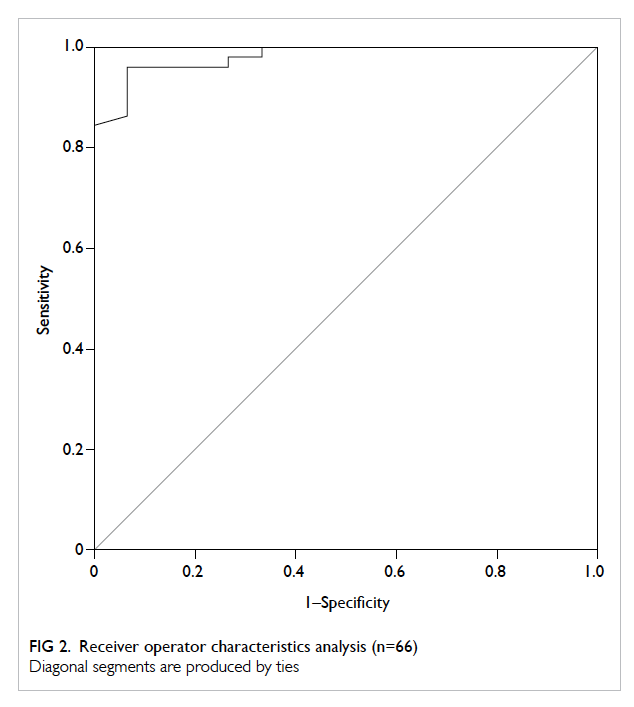
The area under ROC curve was 0.981 (95%
confidence interval [CI], 0.954-1.000; Fig 2). The
optimal threshold of uPCR for diagnosing proteinuria
was 33 mg/mmol. This gave the same sensitivity but
a slightly higher specificity when compared with
the suggested threshold of 30 mg/mmol,1 although
there was a large overlap of 95% CIs (Table 2). A
lower cut-off of 20 mg/mmol rather than the local
laboratory reference of 30 mg/mmol on the uPCR
would give 100% sensitivity. A higher cut-off of 52
mg/mmol would have 100% specificity (Table 2).
Spot urine protein-to-creatinine ratio and adverse pregnancy outcomes
We excluded 25 multiple pregnancies for this
secondary outcome analysis. Of the remaining
95 singleton pregnancies with pre-eclampsia, the
median gestation age of onset of proteinuria was
35 weeks and 64% were preterm (<37 weeks) at the
onset.
For maternal outcome, 47% of women
developed severe hypertension, 6% developed
raised liver enzymes, 6% renal insufficiency, and
2% thrombocytopenia. Admission to the ICU was
required by 16%. There was no case of eclampsia or
maternal mortality. Substantial differences in uPCR
were observed in cases with and without raised
liver enzymes and thrombocytopenia, although the
differences were not statistically significant due to
the small number of cases (Table 3).
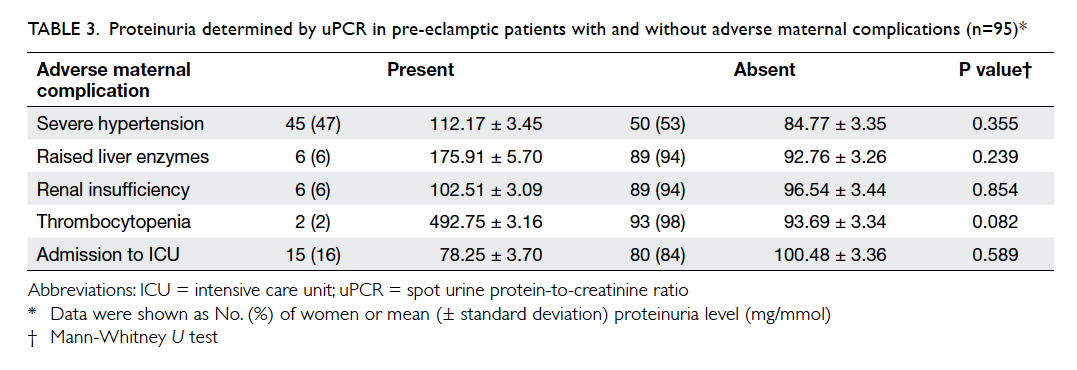
Table 3. Proteinuria determined by uPCR in pre-eclamptic patients with and without adverse maternal complications (n=95)
For the neonatal outcome of all singleton
pregnancies, 60% were born with low birth weight,
21% with low Apgar score at 1 minute, 6% with
low Apgar score at 5 minutes of birth, and 54%
required admission to the NICU. There was no case
of stillbirth or early neonatal death. On the other
hand, uPCR was significantly greater in newborns
who required admission to the NICU than in those
who did not. Nonetheless, if only women with onset
of proteinuria before 34 weeks were included, there
was no difference in uPCR between newborns with
and without neonatal complications (Table 4).
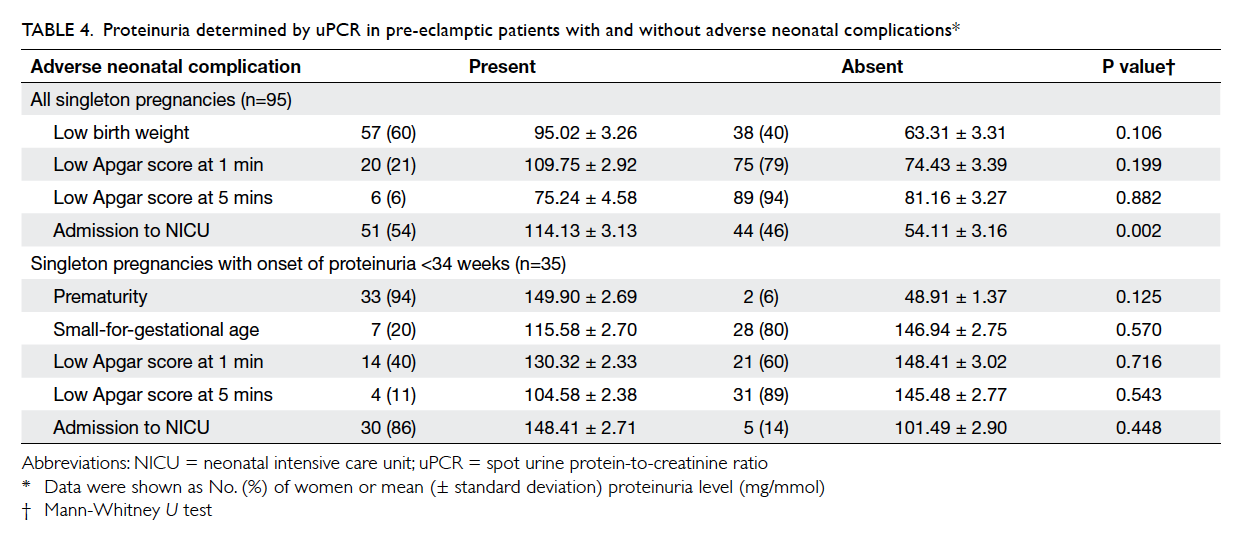
Table 4. Proteinuria determined by uPCR in pre-eclamptic patients with and without adverse neonatal complications
Discussion
Consistent with previous studies,7 8 our present study
has shown a positive and significant correlation of
uPCR with 24-hour urine result. This correlation was
low and insignificant if uPCR was ≥200 mg/mmol.
This is similar to the finding of another study that
reported a lower positive predictive value between
24-urine protein excretion and uPCR for the greater
degree of proteinuria (>1 g/day).16
In the present study, the optimal threshold of
uPCR for diagnosing proteinuria was 33 mg/mmol.
This gave a similar predictive value when compared
with the suggested threshold of 30 mg/mmol (Table 2).1 The area under ROC curve in the present study
was 0.981 and was comparable with the result of
a meta-analysis that included 24 trials with 3186
participants in which the area under summary
ROC curve was 0.90, with pooled sensitivities and
specificities of 91% and 86.3%, respectively.8
Using a cut-off of 20 and 52 mg/mmol had
100% sensitivity and 100% specificity, respectively
(Table 2). Similar findings for the cut-off value were
noted in a systematic review of seven studies with
1717 patients.17 Random uPCR determinations
are helpful primarily when they are <150 mg/g (17
mg/mmol) as ≥300 mg proteinuria is unlikely to be
below this threshold. Nonetheless, for uPCR >600
mg/g (67.8 mg/mmol), significant proteinuria could
be established.
In clinical practice, there are three scenarios.
First, if uPCR is >52 mg/mmol (66% of cases in the
present study), significant proteinuria will be highly
likely and the positive predictive value of a composite
adverse neonatal outcome will be high (78.7% in the
present study). Second, if uPCR is <20 mg/mmol
(13% in the present study), significant proteinuria
will be very unlikely. In either scenario, an earlier
clinical decision can be made without ordering or
completion of 24-hour urine collection or awaiting
results. 24-Hour urine collection can be omitted
in the majority of cases, therefore shortening the
time to diagnose or exclude pre-eclampsia. Third, if
uPCR is 20 to 52 mg/mmol (21% in the present study),
24-hour urine results will be required to confirm or
exclude significant proteinuria. Although 24-hour
urinary protein of ≥300 mg/day is the gold standard
for diagnosing abnormal proteinuria in pregnancy,
this is more a time-honoured value than one with
high scientific proof.1 Having said that, in cases of
gestational hypertension with proteinuria of <300
mg/day, attention should still be warranted if the
uPCR is >30 mg/mmol, particularly if it shows a rising
trend.
If uPCR is ≥200 mg/mmol, correlation with
24-hour urine analysis will be low. In such cases,
proteinuria should be confirmed with 24-hour
urine protein measurement. It is probable that
nephrotic range proteinuria exists above this
threshold, thus necessitating prophylaxis against
thromboembolism.1
It is controversial whether uPCR can predict
clinical outcome. Some studies10 11 12 have suggested
that proteinuria is related to adverse pregnancy
outcomes, for example, severe hypertension,
renal insufficiency, liver disease, preterm delivery,
small-for-gestational age, and transfer to NICU.
Nonetheless, the latest ISSHP (International Society
for the Study of Hypertension in Pregnancy) guideline
suggests that the degree of proteinuria provides very
little additional risk stratification in cases of pre-eclampsia,
and does not include it when defining the
severity of the disease.1 The recent multicentre PIERS
(Pre-eclampsia Integrated Estimate of Risk) study
demonstrated that neither uPCR nor 24-hour urine
protein output was predictive of adverse perinatal
outcome and hence concluded that the amount
of proteinuria should not be used in isolation for
decision making in women with pre-eclampsia.13
In the present study that focused on Chinese pre-eclamptic
women, uPCR was significantly greater
in cases that required admission to the NICU. This
difference was no longer evident if only cases with
early-onset proteinuria were included in the analysis.
This shows that the observed difference in uPCR
was related to the management strategy of preterm
delivery in cases with early-onset pre-eclampsia
rather than to the severity of proteinuria itself.
The study provides an insight into the accuracy
of uPCR and its relationship with pregnancy
outcomes in a local Chinese population. Nonetheless,
the present study was small and retrospective. Only
those with a valid spot urine sample were included in
the study and women with severe pre-eclampsia who
required immediate treatment and delivery were
excluded. The results would therefore be affected by
such selection bias. The precision of the ROC curve
is limited by the small sample size. Further research
by a prospective study with timed urine collection
and larger sample size is suggested to validate the
findings of the present study.
Conclusions
There was a positive and significant correlation of
uPCR with 24-hour urine protein result in Chinese
pre-eclamptic women when uPCR was <200
mg/mmol. Significant proteinuria can probably be
excluded in the presence of uPCR of <20 mg/mmol,
but it should be considered when uPCR >52 mg/mmol. Significant proteinuria should be
confirmed by 24-hour urine collection when uPCR
is 20 to 52 mg/mmol, and uPCR was not significant in
predicting adverse pregnancy outcomes.
Acknowledgements
The authors would like to thank Dr CC Shek from
the Department of Pathology of Queen Elizabeth
Hospital for his valuable assistance for providing
information on the laboratory tests. We would also
like to thank Ms Janice Yung for her kind clerical
assistance in the preparation of this manuscript.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Tranquilli AL, Dekker G, Magee L, et al. The classification,
diagnosis and management of the hypertensive disorders
of pregnancy: A revised statement from the ISSHP.
Pregnancy Hypertens 2014;4:97-104. Crossref
2. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology
of pre-eclampsia and the other hypertensive disorders
of pregnancy. Best Pract Res Clin Obstet Gynaecol
2011;25:391-403. Crossref
3. Tan KH, Kwek K, Yeo GS. Epidemiology of pre-eclampsia
and eclampsia at the KK Women’s and Children’s Hospital,
Singapore. Singapore Med J 2006;47:48-53.
4. Ye C, Ruan Y, Zou L, et al. The 2011 survey on hypertensive
disorders of pregnancy (HDP) in China: prevalence, risk
factors, complications, pregnancy and perinatal outcomes.
PLoS One 2014;9:e100180. Crossref
5. Hypertension in pregnancy: the management of
hypertensive disorders during pregnancy. NICE Clinical
Guidelines, No. 107. London: RCOG Press; 2010.
6. Lowe SA, Bowyer L, Lust K, et al. SOMANZ guidelines for
the management of hypertensive disorders of pregnancy
2014. Aust NZJ Obstet Gynaecol 2015;55:e1-e29. Crossref
7. Morris RK, Riley RD, Doug M, Deeks JJ, Kilby MD.
Diagnostic accuracy of spot urinary protein and albumin
to creatinine ratios for detection of significant proteinuria
or adverse pregnancy outcome in patients with suspected
pre-eclampsia: systematic review and meta-analysis. BMJ
2012;345:e4342. Crossref
8. Sanchez-Ramos L, Gillen G, Zamora J, Stenyakina A,
Kaunitz AM. The protein-to-creatinine ratio for the
prediction of significant proteinuria in patients at risk
for preeclampsia: a meta-analysis. Ann Clin Lab Sci
2013;43:211-20.
9. James GD, Sealey JE, Alderman M, et al. A longitudinal
study of urinary creatinine and creatinine clearance in
normal subjects. Race, sex, and age differences. Am J
Hypertens 1988;1:124-31. Crossref
10. Chan P, Brown M, Simpson JM, Davis G. Proteinuria in
pre-eclampsia: how much matters? BJOG 2005;112:280-5. Crossref
11. Thornton CE, Makris A, Ogle RF, Tooher JM, Hennessy
A. Role of proteinuria in defining pre-eclampsia: clinical
outcomes for women and babies. Clin Exp Pharmacol
Physiol 2010;37:466-70. Crossref
12. Bouzari Z, Javadiankutenai M, Darzi A, Barat S. Does
proteinuria in preeclampsia have enough value to
predict pregnancy outcome? Clin Exp Obstet Gynecol
2014;41:163-8.
13. Payne B, Magee LA, Côté AM, et al. PIERS proteinuria:
relationship with adverse maternal and perinatal outcome.
J Obstet Gynaecol Can 2011;33:588-97. Crossref
14. Côté AM, Firoz T, Mattman A, Lam EM, von Dadelszen
P, Magee LA. The 24-hour urine collection: gold standard
or historical practice? Am J Obstet Gynecol 2008;199:625.e1-6. Crossref
15. Perrone RD, Madias NE, Levey AS. Serum creatinine as
an index of renal function: new insights into old concepts.
Clin Chem 1992;38:1933-53.
16. Demirci O, Kumru P, Arınkan A, et al. Spot protein/creatinine ratio in preeclampsia as an alternative for
24-hour urine protein. Balkan Med J 2015;32:51-5. Crossref
17. Papanna R, Mann LK, Kouides RW, Glantz JC. Protein/creatinine ratio in preeclampsia: a systematic review.
Obstet Gynecol 2008;112:135-44. Crossref