Hong Kong Med J 2016 Feb;22(1):46–55 | Epub 15 Jan 2016
DOI: 10.12809/hkmj144529
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Acute carbon monoxide poisoning in a regional hospital in Hong Kong: historical cohort study
MY Chan, FHKCEM, FHKAM (Emergency Medicine)1;
Thomas TS Au, FHKCEM, FHKAM (Emergency Medicine)1;
KS Leung, FHKCEM, FHKAM (Emergency Medicine)1;
WW Yan, FHKAM (Medicine)2
1 Accident and Emergency Department, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 Department of Intensive Care Unit, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Corresponding author: Dr MY Chan (odin@ha.org.hk)
Abstract
Objectives: This study aimed to describe the
clinical profiles of all patients with carbon monoxide
poisoning admitted to a regional hospital in order to
enhance the vigilance of health care professionals for
delayed neurological sequelae associated with carbon
monoxide poisoning and to identify the prognostic
factors associated with their development. This study
also aimed to assess the impact of hyperbaric oxygen
therapy on the development of delayed neurological
sequelae in these patients.
Methods: This was a historical cohort study in which all patients with a diagnosis of carbon monoxide poisoning managed in a regional hospital in Hong Kong from 12 February 2003 to 8 November 2013 were recruited. Main outcome measures included delayed neurological sequelae.
Results: Of the clinical profiles of 93 patients
analysed, 24 patients received hyperbaric oxygen
therapy and did not develop delayed neurological
sequelae. Seven patients who did not receive
hyperbaric oxygen therapy developed delayed
neurological sequelae. Comparison of groups
with and without delayed neurological sequelae
(excluding hyperbaric oxygen therapy–treated
patients) revealed that loss of consciousness
(P=0.038), Glasgow Coma Scale score of 3 (P=0.012),
elevated troponin level (P<0.001), higher creatine
kinase level (P=0.008), and intubation requirement
(P=0.007) were possible prognostic factors for the
development of delayed neurological sequelae.
Conclusion: Although not statistically significant,
this study showed a 100% protective effect of
hyperbaric oxygen therapy against development
of severe delayed neurological sequelae in patients
with severe carbon monoxide poisoning. Further
study with better study design is warranted. Loss
of consciousness, low Glasgow Coma Scale score,
intubation requirement, elevated troponin and
higher creatine kinase levels were possible prognostic
factors for development of delayed neurological
sequelae in patients with severe carbon monoxide
poisoning. A well-defined treatment protocol,
appropriate follow-up duration and neuropsychiatric
tests together with a hospital-based hyperbaric
chamber are recommended for management of
patients with severe carbon monoxide poisoning.
New knowledge added by this study
- Loss of consciousness, low Glasgow Coma Scale score, intubation requirement, and elevated troponin and creatine kinase levels were possible prognostic factors for development of delayed neurological sequelae in patients with severe carbon monoxide poisoning.
- Presentation of neurological sequelae can be delayed from a few months to a year.
- A hospital-based hyperbaric oxygen chamber is recommended to decrease the burden of off-site therapy for patients with severe carbon monoxide poisoning and to facilitate timely treatment in a safe environment in Hong Kong.
- A well-defined treatment protocol with adequate follow-up and neuropsychiatric tests are recommended for patients with severe carbon monoxide poisoning.
Introduction
Carbon monoxide (CO) poisoning was not common
in Hong Kong prior to 1998. The first reported case
of CO poisoning from suicidal charcoal burning
occurred in 1998 in Hong Kong. A middle-aged
woman who was a chemical engineer invented
this method of suicide that became the third most
common method in Hong Kong within 2 months
of her death and the associated publicity.1 To date,
suicidal charcoal burning remains the top cause of
suicidal death in this crowded and stressful city.
Victims of CO poisoning are sent to either the
mortuary or public hospitals in Hong Kong. Most
patients who are admitted to hospitals eventually
survive with supportive management but there is no
standard treatment protocol. Treatment regimens
varied in different hospitals at different times. Cases
of delayed neurological sequelae (DNS) secondary to
CO poisoning are well reported in the literature.2 3 4 5 6 In a Cochrane review in 2011, it was stated “It is
possible that some patients, particularly those with
more severe poisoning, may derive benefit from
[hyperbaric oxygen] treatment, but this remains
unproven.”7 Hyperbaric oxygen therapy (HBOT) is
now a standard treatment option in many developed
countries and China for selected patients with severe
CO poisoning.
According to Lam et al in 2006,8 the incidence
of DNS in Hong Kong was 3.4%, which was much
lower than other reported rates of 10% to 30%.2 3 4 5 6 7 The overall incidence of DNS is likely to have been
underdiagnosed and under-reported in Hong
Kong because of a lack of detailed neurological
examination and neuropsychiatric tests during acute
management and follow-up sessions.
Pamela Youde Nethersole Eastern Hospital
(PYNEH) has been one of the pioneer hospitals to
support HBOT for CO poisoning in Hong Kong. The
lack of a hospital-based hyperbaric oxygen chamber
in Hospital Authority (HA) hospitals in Hong
Kong has limited the number of HBOT sessions
administered to patients with CO poisoning,
particularly those with severe poisoning, because
of the risks associated with patient management at
a remote site deprived of medical support. Special
arrangements would currently be required to send
a patient to Ngong Shuen Chau for HBOT. To date,
PYNEH has been the principal advocator of HBOT
for CO poisoning patients in Hong Kong and has
treated the largest number of severe cases.
This 10-year retrospective study aimed to
describe the clinical profile of all CO poisoning
patients admitted to PYNEH with the aim of
improving vigilance of health care professionals for
DNS associated with CO poisoning and identifying
the prognostic factors for their development. The
study also aimed to assess the impact of HBOT on
the development of DNS in these patients.
Methods
Data collection
Patients with a diagnosis of CO poisoning
documented in the Clinical Management System
(CMS) of the HA and being managed at PYNEH
between 12 February 2003 and 8 November 2013 were
included. This entailed recourse to the Clinical Data
Analysis and Reporting System (CDARS) of the HA.
Relevant accident and emergency notes, radiological
reports, laboratory results, and discharge summaries
were retrieved. The following were recorded where
available: age, sex, systolic and diastolic blood
pressure, heart rate, temperature, electrocardiogram
(ECG), Glasgow Coma Scale (GCS) score at
presentation, endotracheal intubation, history of or
presence of loss of consciousness (LOC), blood tests
for carboxyhaemoglobin (COHb) level, creatine
kinase (CK) level, troponin (Tn) level, HBOT,
complications from HBOT, development of DNS,
co-ingestion, suicidal methods and intention.
Case selection of hyperbaric oxygen therapy in our hospital
The use of HBOT for severe CO poisoning was
advocated in the Intensive Care Unit of PYNEH after
2008. The need for HBOT in patients who presented
with CO poisoning was judged on a case-by-case
basis. The indications for and contra-indications
to HBOT for patients with severe CO poisoning
are summarised in Table 1. The treatment protocol
of HBOT for CO poisoning at Ngong Shuen Chau
is shown in the Figure. Most patients were given
three sessions of HBOT over 3 consecutive days.
Exceptions included refusal by patients or their
relatives, or operational difficulties.
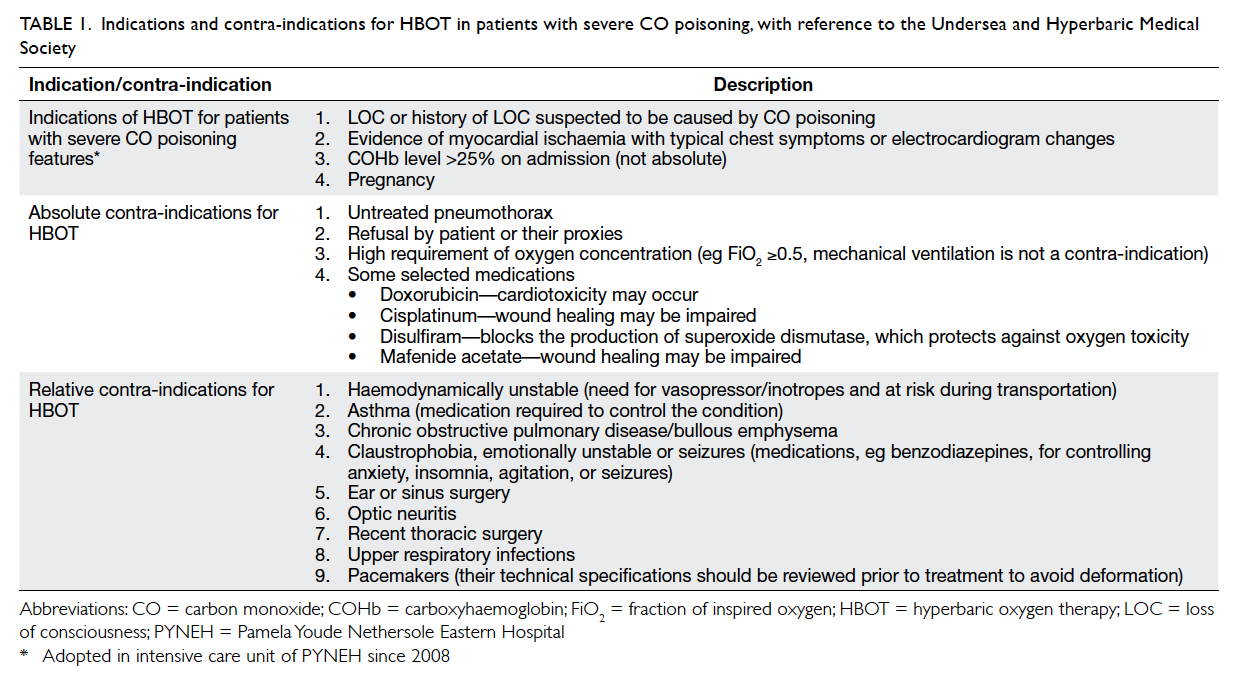
Table 1. Indications and contra-indications for HBOT in patients with severe CO poisoning, with reference to the Undersea and Hyperbaric Medical Society
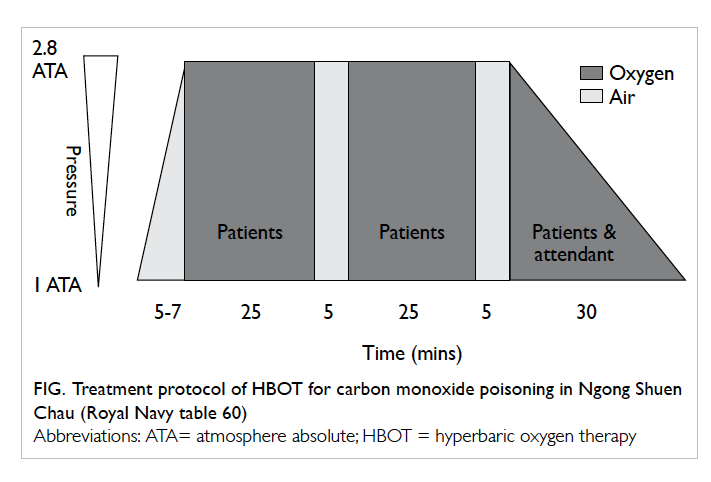
Figure. Treatment protocol of HBOT for carbon monoxide poisoning in Ngong Shuen Chau (Royal Navy table 60)
Statistical analyses
Univariate analysis for the development of DNS
was done by the Fisher’s exact test for dichotomous
variables and the Mann-Whitney U test for
continuous variables, with respect to the following
factors: age, sex, source of CO, cause of CO
poisoning, systolic and diastolic blood pressure,
heart rate, temperature, GCS, GCS=3, LOC, COHb
level, COHb level >25%, Tn level, Tn level >0.03 ng/mL,
CK level, CK level >200 IU/L, ECG ischaemic changes,
endotracheal intubation, co-ingestion, and HBOT.
The distribution of Tn and CK levels showed positive
skewness, thus the parameters were expressed in
terms of means and standard deviations, as well
as medians and interquartile ranges. All statistical
analyses were performed using the Statistical
Package for the Social Sciences (Windows version
22.0; SPSS Inc, Chicago [IL], US). The level of
statistical significance was set at 0.05.
Results
A total of 95 cases were diagnosed with CO
poisoning during the study period. One case was
excluded because the patient presented with cardiac
arrest and succumbed shortly after admission
before any blood tests were performed. Another
case of DNS that resulted in convulsion, dysphasia,
and double incontinence was excluded as the
acute CO poisoning event occurred in Manila. The
remaining 93 cases were recruited for analysis. All
the demographic data and blood test results are
shown in Table 2. Among the 93 patients analysed,
24 received HBOT; DNS had not developed in this
group of patients. Nonetheless DNS had developed
in seven patients who did not undergo HBOT.
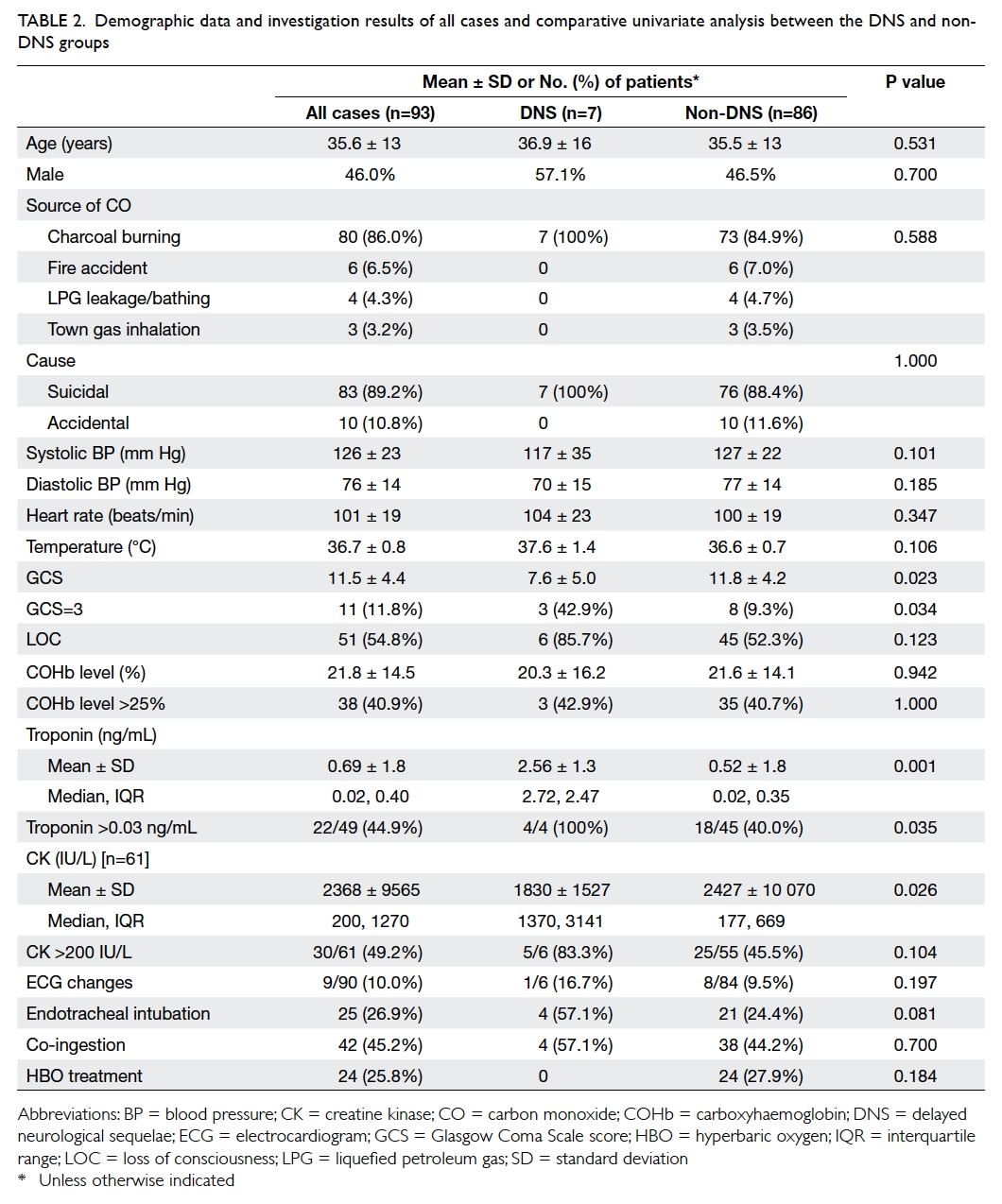
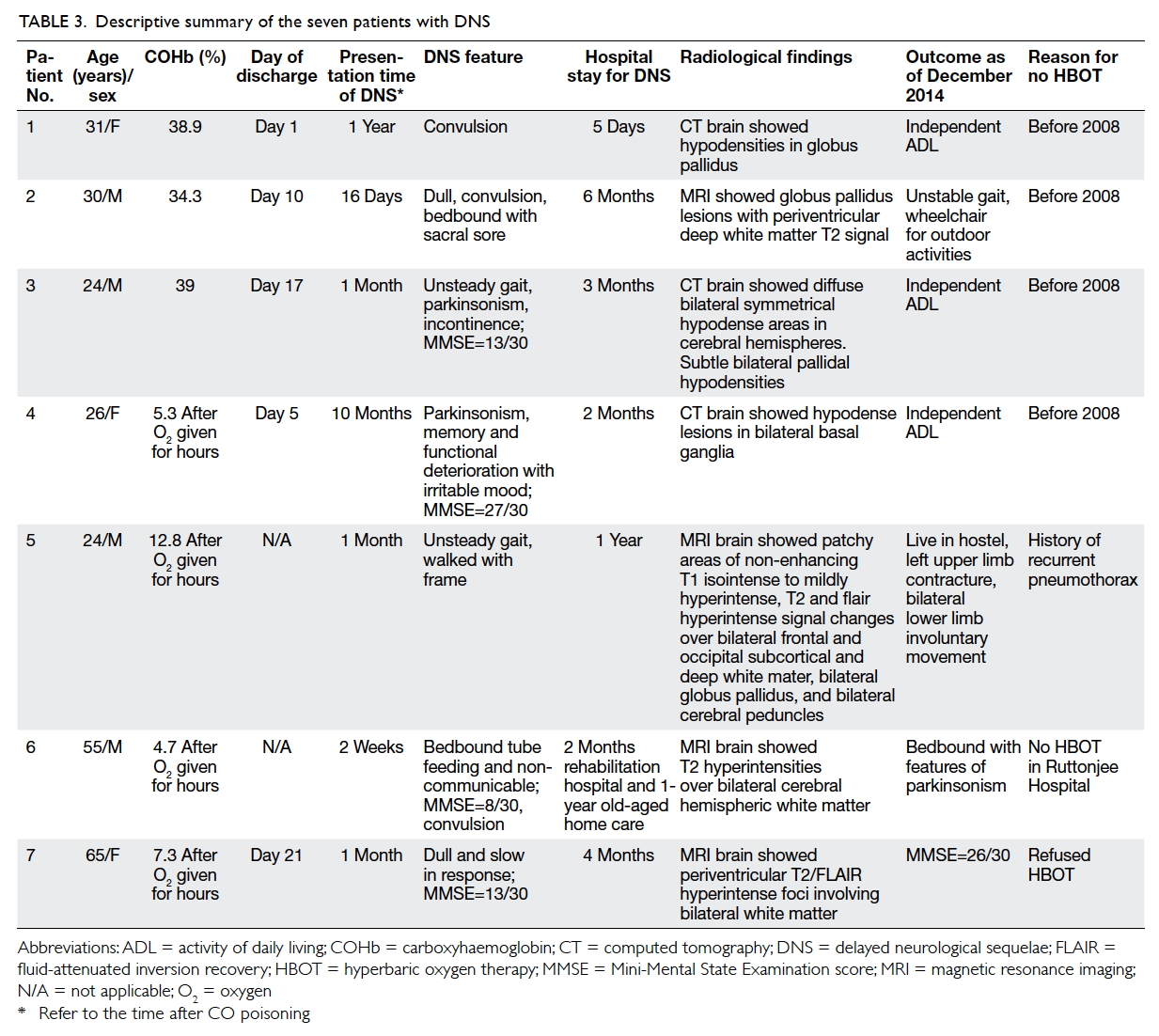
Table 2. Demographic data and investigation results of all cases and comparative univariate analysis between the DNS and non-DNS groups
Patients with delayed neurological sequelae
Among the seven cases of DNS where patients
did not undergo HBOT, no formal follow-up had
been arranged to detect DNS associated with CO
poisoning. Nonetheless, DNS was diagnosed in these
cases because the neurological symptoms became
evident during the same episode of hospitalisation,
or the neurological symptoms were detected by
their caretakers after the initial hospitalisation.
The clinical profiles of these DNS patients with
radiological confirmation are summarised in Table
3.
Hyperbaric oxygen therapy
There were 24 patients treated with HBOT. Their
mean age was 36.3 (range, 19-61) years and the
male-to-female ratio was 1:1. Of these 24 patients,
the source of CO poisoning in 23 (96%) was charcoal
burning and one (4%) patient had accidental CO
poisoning due to leakage of a liquid petroleum
gas combustion system while bathing. In 21 (87.5%)
patients, LOC developed prior to admission. There
were four (17%) patients with GCS score of 15/15
and 20 (83%) patients with GCS score of 3-14/15. The
mean COHb level was 29.2% (range, 3.3%-48.7%); Tn level
was elevated in 15 (78.9%) of 19 cases; CK level was elevated in 13
(65%) of 20 cases; ECG showed acute ischaemic changes
in five (21%) cases. No DNS developed in patients
treated with HBOT.
Complications of hyperbaric oxygen therapy
Complications secondary to HBOT developed in
three patients: perforated tympanic membrane in
one patient, left otitis media related to grommet
insertion before HBOT in one, and barotrauma with
left ear pain due to blockage of the myringotomy
site in another who required repeated myringotomy.
Nonetheless all patients completed the whole course
of HBOT without long-term sequelae.
Patients with carbon monoxide poisoning and acute ischaemic electrocardiographic changes
The most common ischaemic change on ECG was
ST segment depression. Acute ischaemic changes
were evident in nine patients. Their mean age was
42 (range, 27-61) years, and the male-to-female
ratio was 5:4. Eight (89%) cases committed suicide
by charcoal burning and one (11%) patient had CO
poisoning due to an accidental fire. In seven (78%)
patients, LOC developed prior to admission. There
was one (11%) patient with GCS score of 15/15,
and eight (89%) patients with score of 3-14/15. The
mean COHb level was 24.3% (range, 3.9%-48.7%);
Tn level was elevated in seven (78%) patients and CK level was
elevated in four (44%). The HBOT was offered to
five (56%) patients with ischaemic ECG changes and
DNS developed in one (11%) patient.
Detailed descriptive analysis of the GCS of all
patients showed a bimodal distribution with one
mode at GCS of 3 points and the other at GCS of
15 points. Thus, GCS of 3 points was used as an
indicator of severe CO poisoning. One of the clinical
indications for HBOT was COHb level of >25% and
served as another indicator of severe CO poisoning.
Thus, GCS of 3 and COHb level of >25% were tested as
possible prognostic factors for DNS.
Due to variations in the clinical management,
blood tests for Tn and CK were not carried out in all
patients. Elevated Tn was defined as serum Tn level
of >0.03 ng/mL on admission. Level of Tn was not
checked throughout the clinical course in 44 patients
but was elevated in 22 and normal in 27. Elevated CK
was defined as serum CK level of >200 IU/L. This
value was used for simplicity as the upper limit of
normal is 180 IU/L in our laboratory. Level of CK
was not checked throughout the clinical course in 32
patients but was raised in 30 and normal in 31.
Comparative statistical analysis showed that
the DNS group had significantly lower GCS score, a
greater proportion of patients with GCS of 3 points,
and higher levels of Tn (Table 2). However, CK levels
were significantly higher in the non-DNS than in the
DNS group, although the proportions of elevated CK
were not statistically significant. The higher CK level in the non-DNS
group can be explained by one single case of severe
CO poisoning with prolonged LOC, pressure sores,
and compartment syndrome leading to a supremely
high CK level of 73 560 IU/L on admission. The result
is better illustrated by comparison of the median
and interquartile range as shown in Table 2. The
association between the possible benefit of HBOT
and DNS was not statistically significant (P=0.184).
In order to identify possible prognostic factors
for DNS development and to eliminate the possible
effect of HBOT, a second comparative analysis was
performed between the DNS group and non-DNS
group after excluding those patients treated by
HBOT (Table 4). This second analysis showed that when compared with the non-DNS group, the DNS
group had a significantly greater proportion of
patients with LOC and GCS score of 3, lower GCS score, higher levels of Tn and CK, higher proportion of
patients with elevated Tn, and higher tendency to
have been intubated.
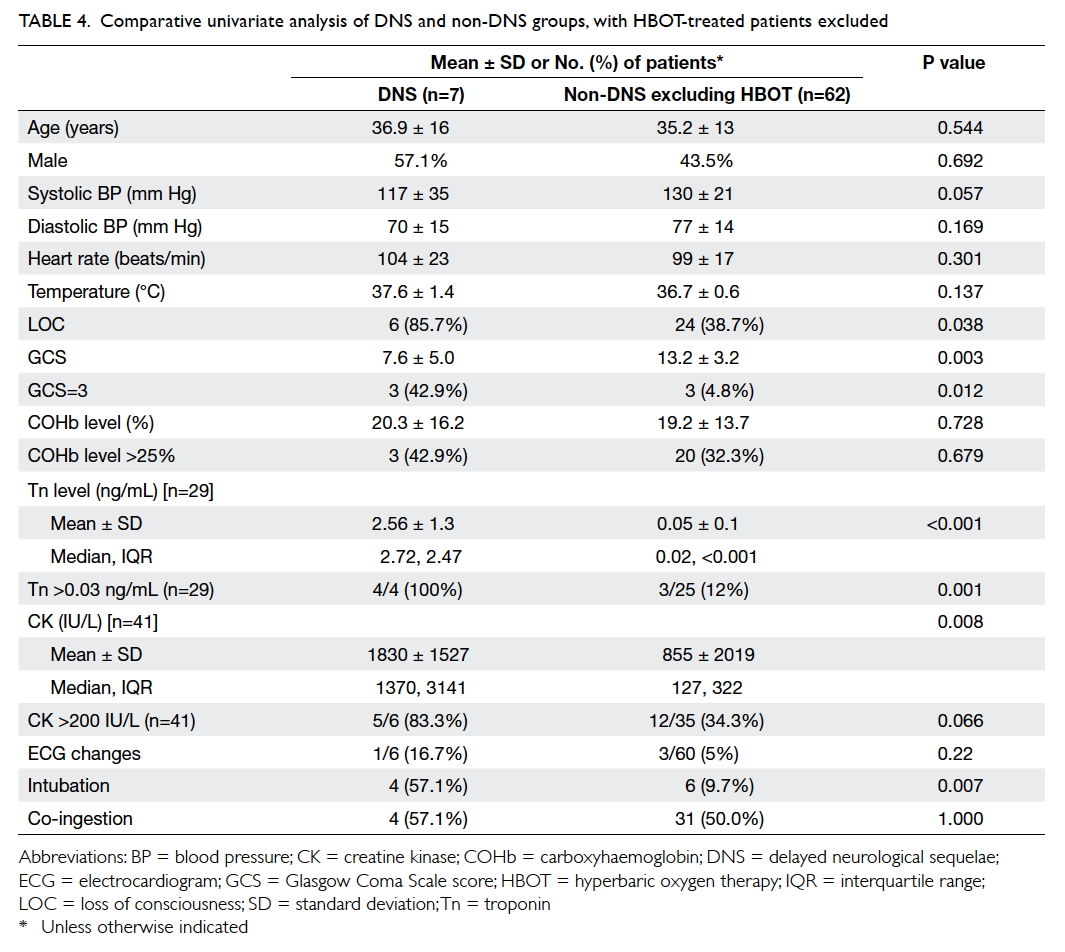
Table 4. Comparative univariate analysis of DNS and non-DNS groups, with HBOT-treated patients excluded
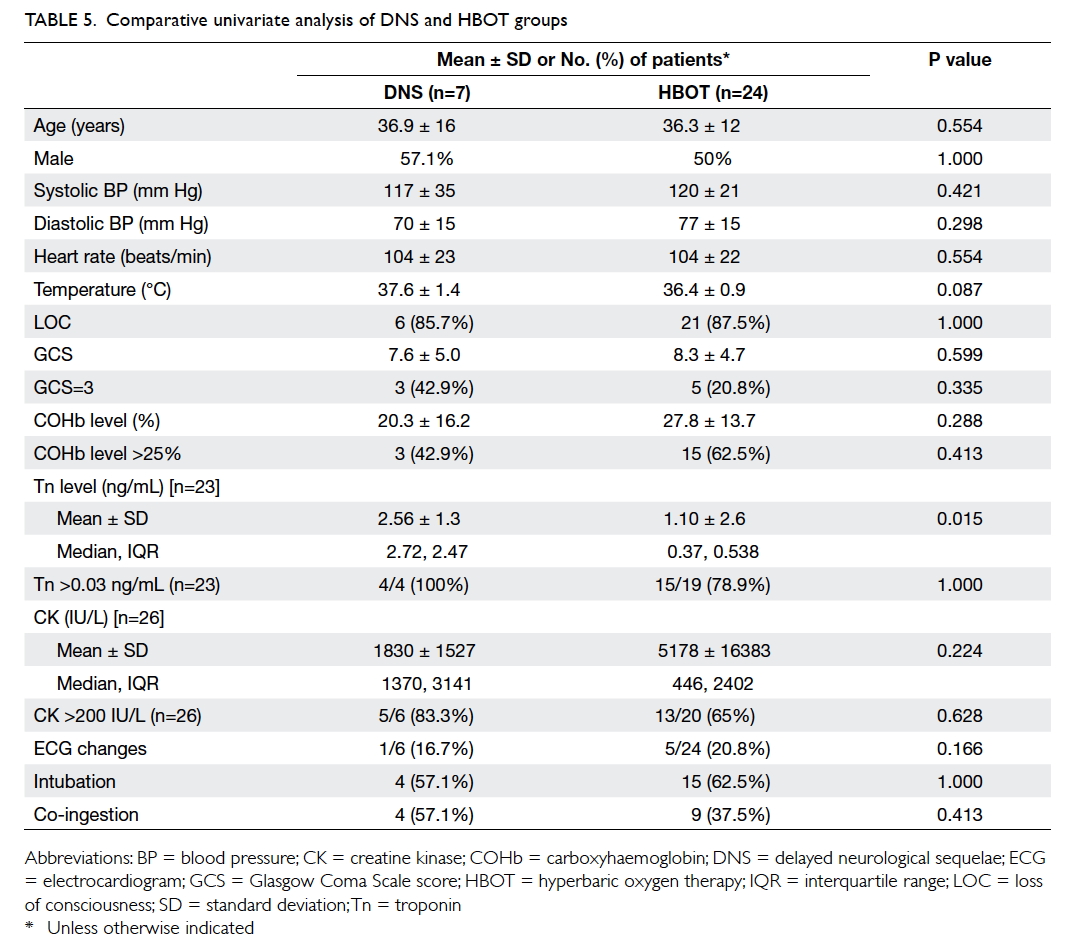
Since the HBOT group did not develop DNS
and the DNS group did not receive HBOT, another
univariate analysis was performed to detect any
significant difference between these two groups
(Table 5). This showed that the DNS and HBOT
groups were similar for all the above tested variables,
except for the Tn level that was significantly higher
in the DNS group. The proportion of patients with
elevated Tn was similar for the two groups, however.
Discussion
Pathophysiology of carbon monoxide poisoning
Carbon monoxide is a colourless, odourless, non-irritating
but highly toxic gas produced during
incomplete carbon combustion. A small amount
of CO is produced after degradation of heme
physiologically in human body.9 Poisoning of CO
develops only after the dose exceeds the elimination
capacity. The elimination half-life of COHb in
humans is approximately 208 to 358 minutes in
room air,10 74 minutes in normobaric oxygen
therapy,11 and 20 minutes in 3 atmosphere absolute pressure of HBOT.12 Carbon monoxide binds to haemoglobin, myoglobin, and
cytochrome oxidase with an affinity of 200 to 300
times, 30 to 60 times, and 9 times more than oxygen,
respectively. The decrease in oxygen carrying and
delivery capacity of blood results in tissue hypoxia.13
Cellular hypoxia causes the release of free radicals
that bind with nitric oxide (NO) from heme to
produce peroxynitrite (ONOO-), further inhibiting
cytochrome oxidase and resulting in DNA damage
and apoptosis.14 15 16
The clinical presentation of ‘cherry-like’ skin
discolouration is secondary to the red colour of
COHb and CO-induced vasodilation. Headache from
CO poisoning is likely mediated by extracerebral
and intracerebral vasodilation with displacement of
NO from heme by CO.15 17 On the other hand, DNS is likely to be caused by the combination of COHb,
mitochondrial oxidative stress, NO, ONOO-, oxygen
free radicals, apoptosis, immune-mediated injury,
inflammatory response, brain lipid peroxidation,
and other unknown mechanisms.13 14 15 16 17 18 19 20 21 22
Delayed neurological sequelae
There is no universal agreed definition of DNS
following CO poisoning. It is typically preceded
by a lucid period of 2 to 40 days after the initial
poisoning.20 Clinical manifestations range from
impairment of concentration, attention, learning,
memory, language and motor function, as well as
psychiatric functions such as depression, dementia,
psychosis and mutism, to neurological dysfunction
such as paralysis, convulsion, urine or faecal
incontinence, gait disturbance, and Parkinson-like
syndrome.
According to the uniqueness of the health
care system in Hong Kong, there is more than a 90%
chance that patients with severe DNS will rely on the
public sector for further management. In addition,
all cases were analysed for detection of DNS until
December 2014 in this study. All cases were followed up by
CMS record for more than 1 year, thus most patients
in this cohort who developed severe DNS should
have been captured. Contrary to the current belief
that onset of DNS development ranges typically
from a few days to a few weeks,23 this study showed
that DNS might present as late as 6 months to 1 year
after the index CO poisoning. Regular follow-up is
required to detect the onset of DNS. Further studies
are necessary to determine the optimum follow-up
duration for DNS.
All seven DNS cases illustrated that DNS
secondary to CO poisoning can be very debilitating
to both patients and their caretakers. Of 93 patients,
only 55 (59.1%) were followed up in our psychiatric
unit. None of the 93 patients with CO poisoning
were followed up in our medical unit. No mild-to-moderate
case of DNS was reported in this study,
probably due to the absence of a standardised
treatment protocol and formal neuropsychiatric tests
for detection of DNS secondary to CO poisoning.
It is therefore likely that cases of mild-to-moderate
DNS are underdiagnosed and under-reported in
Hong Kong. In order to diagnose DNS secondary
to CO poisoning, a standardised treatment protocol
with adequate follow-up and neuropsychiatric tests
is recommended.
We identified the following prognostic factors
associated with DNS development in patients with
severe CO poisoning: LOC, lower GCS score, GCS
score of 3, intubation requirement, elevated Tn level,
and higher levels of Tn and CK. Other investigational
prognostic markers reported worldwide include
S100B protein,24 low Mini-Mental State Examination
score,25 positive computed tomography of brain,25 26 and plasma copeptin.27
The results of this study reveal that severe
DNS did not develop in any patient with severe CO
poisoning who was treated with HBOT at PYNEH
between 2008 and 2013. Although the results did not
reach statistical significance due to limited sample
size, this 100% protective effect indicates a potential
clinical benefit of HBOT to prevent severe DNS in
patients with severe CO poisoning. In order to look
for potential selection bias between the DNS group
and HBOT group, comparative univariate analysis
between the DNS and HBOT groups was performed
(Table 5). The DNS groups and HBOT groups were
similar in terms of the initial presentation. Although
the magnitude of Tn level in the DNS group (2.56
± 1.3 ng/mL) was statistically greater than that of
HBOT group (1.10 ± 2.6 ng/mL) with a P value of
0.015, it was not clinically significant in terms of
patient management. The sole different factor was
the treatment of HBOT. It is strongly suggested that
HBOT prevents DNS development in severe CO
poisoning.
The role of HBOT in the management of
patients with CO poisoning remains controversial
with conflicting results from large randomised
controlled trials. All trials have been criticised
for bias, thus there has been a pledge for a better-designed
trial with multicentre participation.
Nonetheless ethical, financial, and practical issues
associated with most clinical toxicology studies
make such a trial unlikely in the near future. The
results of this study did show clinical significance
despite a statistically insignificant result. Current
literature supports the potential of HBOT to prevent
or treat DNS resulting from CO poisoning.2 4 5 6 7 22 On
balance, HBOT should be considered for all patients
at risk of development of neurological sequelae.22
In Hong Kong, HBOT has been underutilised
in public hospitals because of the unavailability of
hospital-based hyperbaric chambers. Most patients
in Hong Kong with severe CO poisoning do not receive
HBOT because of the risks of transporting a critically
ill patient to Ngong Shuen Chau, the inadequacy
of intensive care support in the hyperbaric chamber
of Ngong Shuen Chau, occupational hazards, beliefs
of individual health care providers, and availability
of expertise. A hospital-based hyperbaric oxygen
chamber is essential to decrease the burden of HBOT
and provide timely treatment in a safe environment
for patients with severe CO poisoning.
Records retrieved from CDARS for the
period 2003 to 2013 revealed 1451 patients with
CO poisoning who presented to HA hospitals.
This indicates that approximately two patients with
CO poisoning presented to hospitals every 5 days
over the last 10 years. According to the indications
for HBOT adopted in this study, the percentage
of patients in whom HBOT was indicated was
67.7% (63/93). Assuming three sessions of HBOT
would have been required for each patient and the
percentage of patients requiring HBOT over the
last 10 years was 67.7%, it can be estimated that
295 sessions of HBOT would have been required
by patients treated in HA hospitals (1451 patients x
67.7% x 3 sessions / 10 years = 295). If a hyperbaric
oxygen chamber is available in a public hospital,
more patients with CO poisoning can be treated
in a safe and controlled environment. Suppose the
incidence of DNS in Hong Kong is similar to that
worldwide (10%-30%), then 145 to 435 instances of
DNS may have been potentially prevented in these
10 years. In addition, the length of stay in hospital
may have been significantly decreased.
Limitations
First, selection bias existed in this study although
diagnosis coding entry has been compulsory in the
accident and emergency department of PYNEH
since 2008. It is possible that some patients with
CO poisoning were admitted with another principal
diagnosis and discharged without coding of CO
poisoning. If this is the case, then not all patients
with CO poisoning during the study period were
retrieved in this study. This was a single-centre study
and results might not be applicable to all patients
with CO poisoning in Hong Kong. Second, there was
information bias due to its retrospective nature. Not
all patients were investigated with Tn, CK, and ECG
leading to potential bias. The time of investigations
and oxygen treatment given to patients were not
standardised giving rise to difficulty in interpretation
of a relatively low COHb level at presentation. In
addition, no neuropsychiatric tests were performed
to detect any DNS after CO poisoning, resulting in
underdiagnosis of DNS. The strength in information
bias is the outcome measurement of DNS and
laboratory results that provide an objective measure,
unlike a questionnaire. Third, confounding bias was
inevitable as 45.2% of patients had co-ingestion of
medications or alcohol. This might have influenced
the initial clinical presentation as well as the results.
Subgroup analysis of different medications was not
shown because of the diversities and complexity
without significant results. Lastly, despite the length
of the study period, the sample size was inadequate
to provide statistically significant results.
Conclusion
Although not statistically significant, this study
showed 100% protective effect of HBOT against
development of severe DNS in patients with severe
CO poisoning. Further study with better study
design is warranted. This study revealed that LOC,
low GCS score, intubation requirement, elevated Tn and
higher CK levels were possible prognostic factors for
development of DNS. As there was no standardised
treatment protocol and no formal follow-up arranged
for detection of DNS in patients with severe CO
poisoning, mild-to-moderate DNS was probably
underdiagnosed and under-reported in Hong Kong.
A well-defined treatment protocol, appropriate
follow-up duration, and neuropsychiatric tests
together with a hospital-based hyperbaric chamber
are recommended for management of patients with
severe CO poisoning.
Declaration
No conflicts of interest were declared by the authors.
References
1. Chan KP, Yip PS, Au J, Lee DT. Charcoal-burning suicide
in post-transition Hong Kong. Br J Psychiatry 2005;186:67-73. Crossref
2. Raphael JC, Elkharrat D, Jars-Guincestre MC, et al. Trial
of normobaric and hyperbaric oxygen for acute carbon
monoxide intoxication. Lancet 1989;2:414-9. Crossref
3. Hu H, Pan X, Wan Y, Zhang Q, Liang W. Factors affecting
the prognosis of patients with delayed encephalopathy
after acute carbon monoxide poisoning. Am J Emerg Med
2011;29:261-4. Crossref
4. Scheinkestel CD, Bailey M, Myles PS, et al. Hyperbaric or
normobaric oxygen for acute carbon monoxide poisoning:
a randomised controlled clinical trial. Med J Aust
1999;170:203-10.
5. Thom SR, Taber RL, Mendiguren II, Clark JM, Hardy
KR, Fisher AB. Delayed neuropsychologic sequelae after
carbon monoxide poisoning: prevention by treatment with
hyperbaric oxygen. Ann Emerg Med 1995;25:474-80. Crossref
6. Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric
oxygen for acute carbon monoxide poisoning. N Engl J
Med 2002;347:1057-67. Crossref
7. Buckley NA, Juurlink DN, Isbister G, Bennet MH, Lavonas
EJ. Hyperbaric oxygen for carbon monoxide poisoning.
Cochrane Database Syst Rev 2011;(4):CD002041. Crossref
8. Lam KK, Fung HT, Kam CW. The severity and prognostic
markers of 148 cases of carbon monoxide poisoning by
burning charcoal. Hong Kong J Emerg Med 2006;13:6-16.
9. Owens EO. Endogenous carbon monoxide production in
disease. Clin Biochem 2010;43:1183-8. Crossref
10. Bruce EN, Bruce MC. A multicompartment model of
carboxyhemoglobin and carboxymyoglobin responses
to inhalation of carbon monoxide. J Appl Physiol (1985)
2003;95:1235-47. Crossref
11. Weaver LK, Howe S, Hopkins R, Chan KJ.
Carboxyhemoglobin half-life in carbon monoxide-poisoned
patients treated with 100% oxygen at atmospheric
pressure. Chest 2000;117:801-8. Crossref
12. Peterson JE, Stewart RD. Absorption and elimination of
carbon monoxide by inactive young men. Arch Environ
Health 1970;21:165-71. Crossref
13. Coburn RF, Mayers LB. Myoglobin oxygen tension
determines from measurements of carboxyhemoglobin in
skeletal muscle. Am J Physiol 1971;220:66-74.
14. Zhang J, Piantadosi CA. Mitochondrial oxidative stress
after carbon monoxide hypoxia in the rat brain. J Clin
Invest 1992;90:1193-9. Crossref
15. Thom SR, Ohnishi TS, Ischiropoulos H. Nitric oxide
release by platelets inhibits neutrophil B2 integrin function
following acute carbon monoxide poisoning. Toxicol Appl
Pharmacol 1994;128:105-10. Crossref
16. Hardy KR, Thom SR. Pathophysiology and treatment
of carbon monoxide poisoning. J Toxicol Clin Toxicol
1994;32:613-29. Crossref
17. Thom SR, Fisher D, Xu YA, Garner S, Ischiropoulos H. Role
of nitric oxide-derived oxidants in vascular injury from
carbon monoxide in the rat. Am J Physiol 1999;276:H984-92.
18. Thom SR. Carbon monoxide-mediated brain lipid
peroxidation in the rat. J Appl Physiol (1985) 1990;68:997-1003.
19. Thom SR. Dehydrogenase conversion to oxidase and lipid
peroxidation in brain after carbon monoxide poisoning. J
Appl Physiol (1985) 1992;73:1584-9.
20. Thom SR. Antagonism of carbon monoxide–mediated
brain lipid peroxidation by hyperbaric oxygen. Toxicol
Appl Pharmacol 1990;105:340-4. Crossref
21. Thom SR, Bhopale VM, Fisher D, Zhang J, Gimotty
P. Delayed neuropathology after carbon monoxide
poisoning is immune-mediated. Proc Natl Acad Sci USA
2004;101:13660-5. Crossref
22. Weaver LK. Clinical practice: carbon monoxide poisoning.
N Engl J Med 2009;369:1217-25. Crossref
23. Choi IS. Delayed neurological sequelae in carbon monoxide
intoxication. Arch Neurol 1983;40:433-5. Crossref
24. Park E, Ahn J, Min YG, et al. The usefulness of the serum
s100b protein for predicting delayed neurological sequelae
in acute carbon monoxide poisoning. Clin Toxicol (Phila)
2012;50:183-8. Crossref
25. Ku HL, Yang KC, Lee YC, Lee MB, Chou YH. Predictors
of carbon monoxide poisoning–induced delayed
neuropsychological sequelae. Gen Hosp Psychiatry
2010;32:310-4. Crossref
26. Kudo K, Otsuka K, Yagi J, et al. Predictors for delayed
encephalopathy following acute carbon monoxide
poisoning. BMC Emerg Med 2014;14:3. Crossref
27. Pang L, Wang HL, Wang ZH, et al. Plasma copeptin as a
predictor of intoxication severity and delayed neurological
sequelae in acute carbon monoxide poisoning. Peptides
2014;59:89-93. Crossref