Hong Kong Med J 2015 Dec;21(6):490–8 | Epub 29 Sep 2015
DOI: 10.12809/hkmj144445
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Outcome of elderly patients who receive intensive care at a regional hospital in Hong Kong
HP Shum, FHKCP, FHKAM (Medicine)1;
KC Chan, FHKCA, FHKAM (Anaesthesiology)2;
HY Wong, BSN, MSN1;
WW Yan, FHKCP, FHKAM (Medicine)1
1 Department of Intensive Care, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 Department of Anaesthesia and Intensive Care, Tuen Mun Hospital, Tuen Mun, Hong Kong
Corresponding author: Dr HP Shum (shumhp@ha.org.hk)
Abstract
Objective: To evaluate the clinical outcome (180-day mortality) of very elderly critically ill patients
(age ≥80 years) and compare with those aged 60 to
79 years.
Design: Historical cohort study.
Setting: Regional hospital, Hong Kong.
Patients: Patients aged ≥60 years admitted between
1 January 2009 and 31 December 2013 to the
Intensive Care Unit of the hospital.
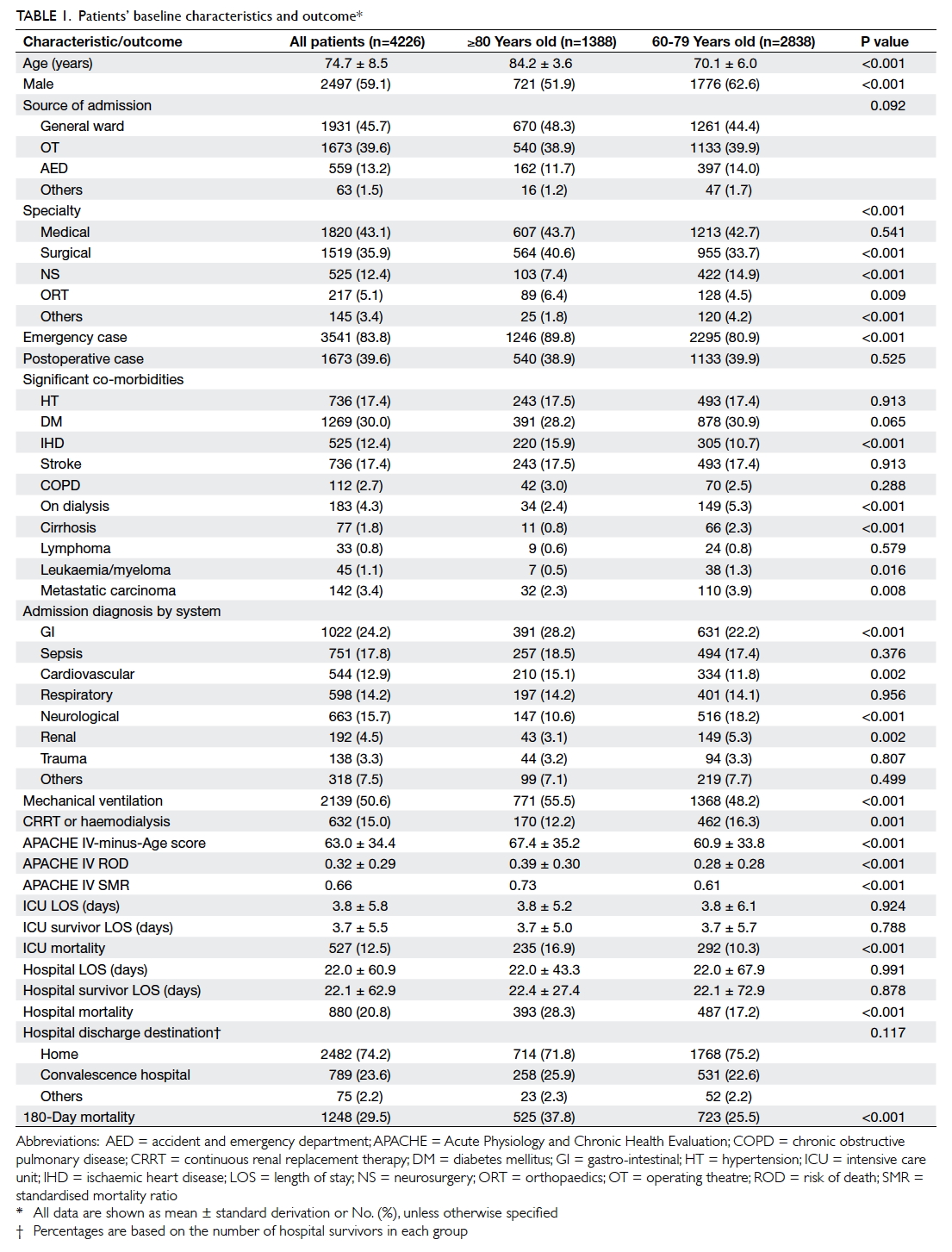
Results: Over 5 years, 4226 patients aged ≥60 years
were admitted (55.5% total intensive care unit
admissions), of whom 32.8% were aged ≥80 years. The
proportion of patients aged ≥80 years increased over
5 years. As expected, those aged ≥80 years carried
more significant co-morbidities and a higher disease
severity compared with those aged 60 to 79 years.
They required more mechanical ventilatory support,
were less likely to receive renal replacement therapy,
and had a higher intensive care unit/hospital/180-day mortality compared with those aged 60 to 79
years. Nonetheless, 71.8% were discharged home and
62.2% survived >180 days following intensive care
unit admission. Cox regression analysis revealed
that Acute Physiology and Chronic Health Evaluation IV-minus-Age score, emergency
admission, intensive care unit admission due
to cardiovascular problem, neurosurgical cases,
presence of significant co-morbidities (diabetes
mellitus, metastatic carcinoma, leukaemia, or
myeloma), and requirement for mechanical
ventilation independently predicted 180-day
mortality.
Conclusions: The proportion of critically ill patients
aged ≥80 years increased over a 5-year period.
Despite having more significant co-morbidities,
greater disease severity, and higher intensive care
unit/hospital/180-day mortality rate compared
with those aged 60 to 79 years, 71.8% of those ≥80
years could be discharged home and 62.2% survived
>180 days following intensive care unit admission.
Disease severity, presence of co-morbidities,
requirement for mechanical ventilation, emergency
cases, and admission diagnosis independently
predicted 180-day mortality.
New knowledge added by this study
- This study provides up-to-date information on the outcome for critically ill elderly patients. It is currently the largest study focused on the local population.
- Despite having more significant co-morbidities, greater disease severity, and a higher intensive care unit (ICU)/hospital/180-day mortality rate compared with those aged 60 to 79 years, our study showed that >70% of critically ill patients aged ≥80 years could be discharged home and their 180-day survival rate was >60%. Such information supports ICU admission for those aged ≥80 years. We recommend further studies to explore the long-term functional outcome of those critically ill elderly patients and the potential health economic impact associated with increased ICU admission for those aged ≥80 years.
Introduction
According to the Hong Kong Population Projections
2012-2041 report, the proportion of Hong Kong
population aged ≥80 years is projected to increase
markedly from 273 000 (3.9%) to 957 000 (11.3%)
by the year 2041.1 Improvements to health-care
provision and environmental factors are responsible
for this change. The very elderly patients consume
a higher proportion of health-care resources due to
the presence of significant co-morbidities.2 Similar
to most other specialties, intensive care units
(ICUs) face an increasing demand for care by elderly
patients. A large multicentre cohort study conducted
in Australia and New Zealand reported that the ICU
admission rate for those aged ≥80 years increased by
5.6% per year.3 An Austrian group noted a similar
trend.4 Intensive care unit is an expensive and limited
resource. In theory, the decision to admit or decline
a patient from ICU care should not depend solely
on the patient’s age, although some earlier studies
hinted at such practice.5 6 The debate on the role
of advanced age, as opposed to severity of illness,
on clinical outcome of these critically ill elderly
patients remains unresolved. Commonly used ICU
prognostic scores, eg Acute Physiology and Chronic
Health Evaluation (APACHE) score and simplified
acute physiology score (SAPS), include ‘age’ as one
of the components of a mortality risk prediction
model. Although some studies have highlighted the
importance of age in patient outcome,3 7 8 others
have concluded that age was not predictive of a
poor prognosis in ICU.9 10 They suggest that severity
of illness or premorbid functional status are more
important determinants of ICU outcome.9 10 Hong
Kong, which has the longest life expectancy in the
world, has few data focused on the outcome for
critically ill elderly patients.11 Our primary objective
of this study was to evaluate the clinical outcome
(180-day mortality) of very elderly patients (≥80
years old) and compare it with that of patients
aged 60 to 79 years. The secondary objective was
to determine factors associated with the survival of
elderly patients (aged ≥60 years) who require ICU
care.
Methods
This study was approved by the hospital Ethics
Committee and written informed consent was
waived. This study was a retrospective, single-centre,
cohort study conducted at the ICU
of Pamela Youde Nethersole Eastern Hospital
(PYNEH), a 2000-bed acute care regional hospital
that provides comprehensive services except
cardiothoracic surgery, transplant surgery, and
burns. The ICU is a 22-bed closed mixed medical-surgical
unit with an average admission of 1400
patients per year. All patients who were admitted to
the ICU between 1 January 2009 and 31 December 2013
were evaluated. During the study period, there were
no changes to ICU operation guidelines or major
clinical decision makers. Patients aged ≥60 years
were recruited for this study. The cutoff value of
60 years was adopted based on the United Nations
definition of an older or elderly person.12 Those for
whom there were insufficient data for analysis or
who remained in the ICU for <4 hours were
excluded. Admissions that involved the same patient
for different hospitalisation episodes were treated
independently.
The following data were collected: demographics,
significant co-morbidities (hypertension,
congestive heart failure, diabetes mellitus, ischaemic
heart disease, ischaemic or haemorrhagic
stroke, chronic respiratory failure, end-stage renal
failure requiring dialytic support, liver cirrhosis
or liver failure, haematological malignancy, or
immunosuppressed status), admission diagnosis,
emergency or elective cases, ICU and hospital length
of stay, and ICU and hospital outcomes. Mortality in
ICU was defined as PYNEH ICU death within the
index admission. Hospital mortality was defined
as PYNEH death within the index admission. The
180-day mortality was defined as death within 180
days, calculated from ICU admission.
Patient’s severity of illness was quantified using
the APACHE IV system.13 This is a severity-adjusted
methodology that predicts outcome for critically ill
adult patients and comprises the following major
components: (1) acute physiology score focused on
cardiopulmonary parameters and laboratory data
retrieved as the worst value within the first 24 hours
of ICU admission, (2) significant co-morbidities, (3)
age, (4) ICU admission disease classification, and (5)
patient’s length of stay in the hospital prior to ICU
admission. All patient data were collected from the
hospital’s information system and an ICU clinical
information system (IntelliVue Clinical Information
Portfolio, Philips Medical Systems, Amsterdam, The
Netherlands). Patients were followed up until death
or 180 days from ICU admission, whichever was the
earlier. The most updated mortality and survival
data were obtained from the Clinical Management
System.
Statistical analyses
Data were reported as frequencies, percentages,
means, and standard deviations. Univariate analyses
were performed using Chi squared test, Fisher’s
exact test, and Student’s t test where appropriate.
Cox regression analysis using a forward stepwise
strategy was performed (on factors with P<0.1 in
univariate analyses) to determine the independent
predictors of 180-day mortality. The interpretation
of multivariable Cox regression analyses may carry
significant problems in the presence of collinear
variables such as age together with APACHE
IV score, in which age is one of the prognostic
components. In order to examine the effect of age
per se and to avoid collinearity, age points were
deducted from the total APACHE IV score to
generate the APACHE IV-minus-Age score. Trend
analysis was performed using Chi squared test for
trend in proportions. All analyses were performed
using the Statistical Package for the Social Sciences
(Windows version 16.0; SPSS Inc, Chicago [IL], US)
and R statistical program version 3.2 (R Foundation,
http://www.r-project.org/). A P value of <0.05 was
considered statistically significant and all statistical
tests were two-tailed. The APACHE IV standardised
mortality ratio (SMR) was calculated by dividing the
observed mortality by the predicted mortality based
on the APACHE IV score. An SMR of <1 indicated
better performance than expected and >1 indicated
suboptimal performance.13
Results
All patients aged ≥60 years
Over the 5-year period, 4247 patients aged ≥60 years
were admitted to the ICU. After exclusion of 21
patients who had insufficient data for analysis (due
to incomplete APACHE form data entry) or who
remained in the ICU for <4 hours, 4226 patients
were recruited. They represented 55.5% of total ICU
admissions. This proportion was similar across 5 years
(57.4% in 2009, 55.9% in 2010, 51.9% in 2011, 56.6%
in 2012, and 55.9% in 2013; P value not significant).
Emergency admission accounted for 83.8% of cases
and 39.6% were for postoperative care. The mean
APACHE IV predicted risk of death was 32%. The
overall observed ICU mortality was 12.5% and the
hospital mortality was 20.8% that translated into an
APACHE IV SMR of 0.66. The ICU mortality (13.8%
in 2009, 12.9% in 2010, 10.8% in 2011, 11.7% in 2012,
and 13.2% in 2013) and hospital mortality (21.5% in
2009, 21.0% in 2010, 17.4% in 2011, 22.3% in 2012,
and 21.6% in 2013) did not change significantly over
5 years. The overall 180-day mortality was 29.5% and
likewise showed no significant change over 5 years.
The outcome for all patients was successfully traced
from the Clinical Management System.
Difference between patients aged 60 to 79 years
and those ≥80 years
Among those ≥60 years old (4226 patients), 32.8%
were aged ≥80 years, representing 18.2% of total ICU
admissions during the study period. The proportion
of patients aged ≥80 years increased over 5 years
(16.2% in 2009, 18.9% in 2010, 16.0% in 2011, 20.3%
in 2012, and 19.4% in 2013; P=0.006). Compared with
patients aged 60 to 79 years, those ≥80 years old were
more commonly female, admitted as an emergency,
had more co-morbidities (had more ischaemic heart disease, but less likely to have renal failure on dialysis, cirrhosis, or malignancy), and
had greater disease severity as assessed by APACHE
IV-minus-Age score (Table 1). With regard to clinical
outcome, those ≥80 years required more mechanical
ventilatory support (55.5% for ≥80 years vs 48.2% for
60-79 years; P<0.001) and were less likely to receive
renal replacement therapy (12.2% for ≥80 years
vs 16.3% for 60-79 years; P=0.001). They also had
higher ICU mortality (16.9% for ≥80 years vs 10.3%
for 60-79 years; P<0.001), hospital mortality (28.3%
for ≥80 years vs 17.2% for 60-79 years; P<0.001), and
180-day mortality (37.8% for ≥80 years vs 25.5% for
60-79 years; P<0.001). Their ICU and hospital length
of stay were nonetheless similar. Despite having more
significant co-morbidities, greater disease severity,
and higher ICU/hospital/180-day mortality rate
than those aged 60 to 79 years, 71.8% of those aged ≥80
years could be discharged home and 62.2% survived
>180 days from ICU admission. Patients were
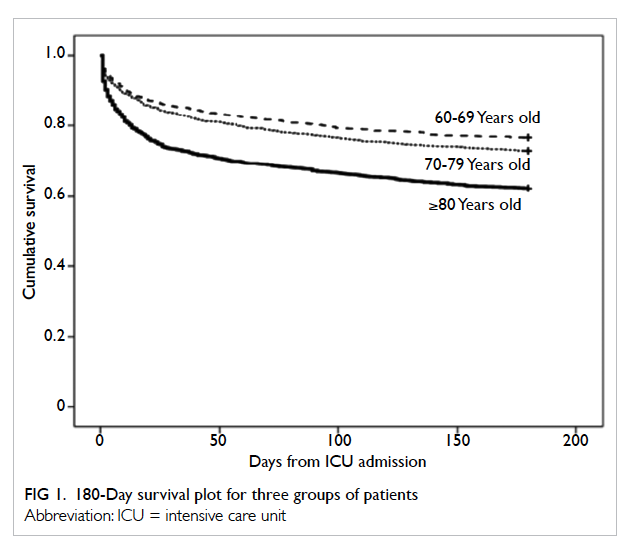
divided into three age-groups namely 60-69, 70-79,
and ≥80 years. Kaplan-Meier survival plot indicated
a significant survival difference between the groups (log
rank test P<0.001 for both ≥80 vs 60-69 and ≥80 vs
70-79 years; Fig 1). Half of all deaths occurred within
the first 15 days from ICU admission. The ratio of
hospital death versus ICU death was the same across
the three groups of patients (1.67 for all three groups
of patients).
Independent predictors of 180-day mortality
For those aged ≥80 years (Table 2), Cox regression
analysis revealed that APACHE IV-minus-Age
score, emergency admission, ICU admission due to
cardiovascular cause, neurosurgical cases, presence
of significant co-morbidities (diabetes mellitus,
metastatic carcinoma, leukaemia, or myeloma),
and requirement for mechanical ventilation
independently predicted 180-day mortality. The
findings of Cox regression analysis for those aged
≥60 are shown in Table 3. Age, APACHE IV-minus-Age score, emergency admission, ICU admission
due to cardiovascular or renal cause, neurosurgical
cases, presence of significant co-morbidities
(diabetes mellitus, metastatic carcinoma, leukaemia, or myeloma), and requirement for mechanical
ventilation or renal replacement therapy were
likewise independent predictors of 180-day mortality
for elderly patients ≥60 years old who received
intensive care.
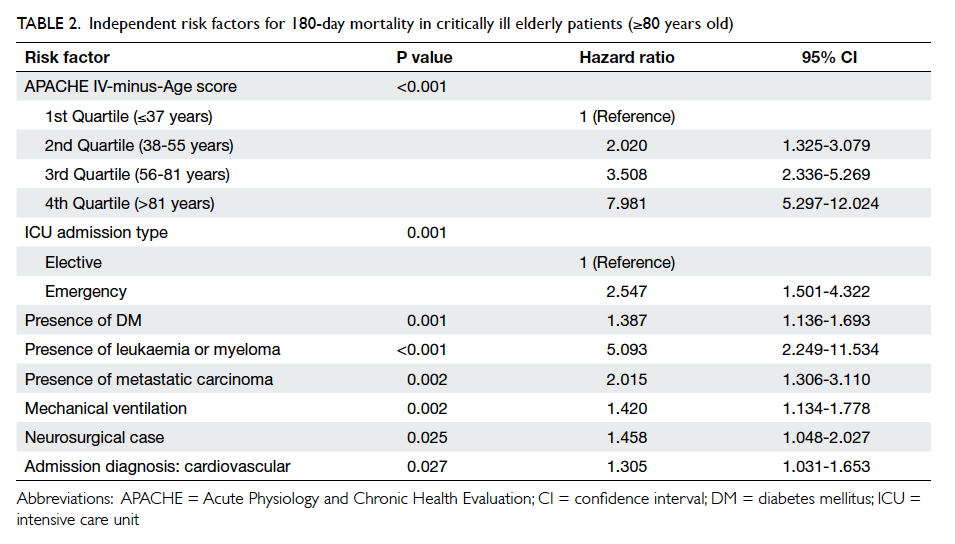
Table 2. Independent risk factors for 180-day mortality in critically ill elderly patients (≥80 years old)
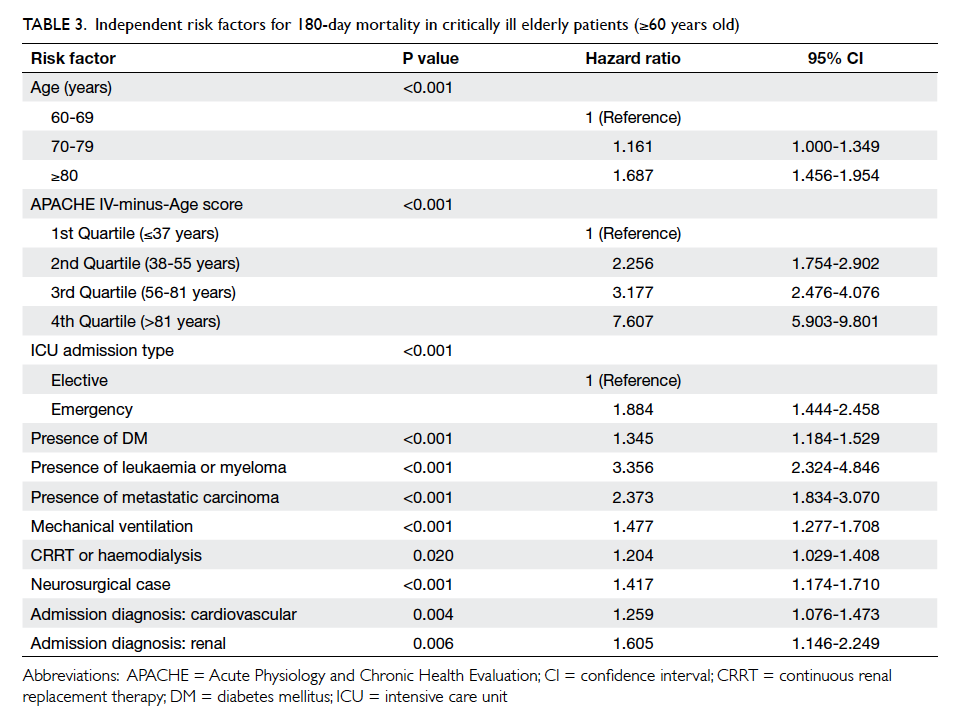
Table 3. Independent risk factors for 180-day mortality in critically ill elderly patients (≥60 years old)
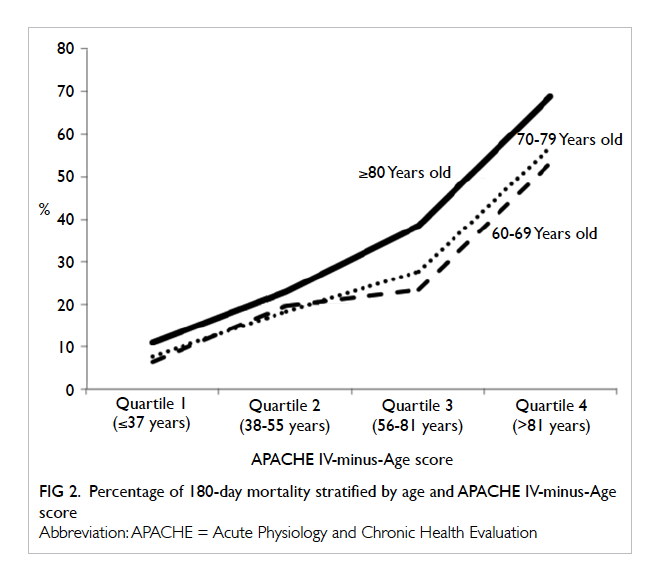
Relationship between age, disease severity, and 180-day mortality
Patient disease severity was stratified into four
groups (quartiles) according to APACHE IV-minus-Age score (1st quartile ≤37, 2nd quartile 38-55, 3rd
quartile 56-81, 4th quartile >81 years). In general, the
180-day mortality rate increased with disease severity
(Fig 2). The mortality rates were quite similar (with
<5% difference) for those ≥80 years and those aged
60 to 79 years with low disease severity (quartiles 1 and
2) but the gap widened (with 10%-15% difference)
with higher disease severity (quartiles 3 and 4).
Discussion
Our results show that the proportion of patients
aged ≥80 years who required ICU care increased
over 5 years (16.2% in 2009, 18.9% in 2010, 16.0%
in 2011, 20.3% in 2012 and 19.4% in 2013; P=0.006).
This is similar to the population growth in Hong
Kong of this age-group (3.4% in 2009, 3.6% in 2010,
3.8% in 2011, 4.0% in 2012 and 4.4% in 2013).14
They usually have more co-morbidity, are admitted
to ICU as an emergency, and have higher disease
severity. Their 180-day mortality rate was 1.7-fold that of 60-69
years old. The 180-day mortality rate also increased
with disease severity (Fig 2). The mortality rates
were quite similar (with <5% difference) for those
aged ≥80 years and those aged 60 to 79 years with low
disease severity but the gap widened (with 10%-15%
difference) with higher disease severity. This may
be due to a lower physiological reserve in the ≥80s
that manifests when illness is severe. This study
could not demonstrate how physiological reserve
diminishes with age. As this was a retrospective
observational study, we cannot tell whether the
greater hazard for death in those ≥80 years is really
related to a ‘lower’ physiological reserve, or whether
ICU doctors/family are more likely to withhold/withdraw life support. The decision to limit therapy
involves assessment of a patient’s quality of life; such
data were not available in this study. These findings
also indicate the importance of early management of
clinical deterioration in those aged ≥80 years. When
disease severity progresses, mortality risk increases
much faster among those ≥80 years than in those
aged 60 to 79 years.
With regard to the level of treatment in the
ICU, previous studies have shown that very elderly
patients receive less aggressive treatment than
younger patients.15 16 17 18 In our cohort, the elderly
patients were less likely to receive renal replacement
therapy. Mechanical ventilation, however, was
commonly performed even in those aged ≥80 years
(55.5%), which is in contrast to previous studies.4 19 20
This may have been due to a difference in case-mix
and clinical practice. Lerolle et al21 showed that
the intensity of ICU treatment has increased and
survival has improved over a decade for those aged
≥80 years. Ihra et al4 also showed that the prognosis
of those aged >80 years improved by 3% per year.
Thus admission of such patients to ICU for a trial
period of therapy is warranted.
The impact of age on mortality has been
demonstrated in our study and previous studies.3 8 18 Similar to previous studies, however, the presence of
significant co-morbidities, disease severity, and use of
mechanical ventilation also independently predicted
mortality.3 4 22 These findings are not surprising and indicate that the decision to refuse ICU care for those
aged ≥80 years should be based not on age alone, but
also on multiple factors listed in Tables 2 and 3. Co-morbidities
may manifest as impaired pre-admission
functional status or increment of complication
rate during hospital stay. Functional status usually
includes physical, cognitive, and social functioning.
Impaired functioning in daily life is more prevalent
in the elderly patients and independently predicts
mortality.23 24 Previous studies have also shown that
elderly patients have a higher surgical complication
rate and risk of nosocomial infection.25 26 With regard
to mechanical ventilation, animal study has shown
that ageing increases susceptibility to injurious
mechanical ventilation–induced pulmonary injury.27
Although no human study has confirmed this finding,
survival rates in patients with acute respiratory
failure correlate with age and decrease with duration
of mechanical ventilation.28 29
Post-ICU discharge mortality is determined
by care in general wards and end-of-life decisions.
Calculating the ratio of hospital deaths versus ICU
deaths can provide some insight into this issue. A
higher ratio implies that more patients die in the
general ward than in the ICU. In our study, the ratio
was the same across the three groups of elderly
patients (1.67), indicating a similar level of care after
ICU discharge. Our finding was similar to the study
by Andersen and Kvåle,19 but our ratios were lower
than those in other overseas studies.3 4 30
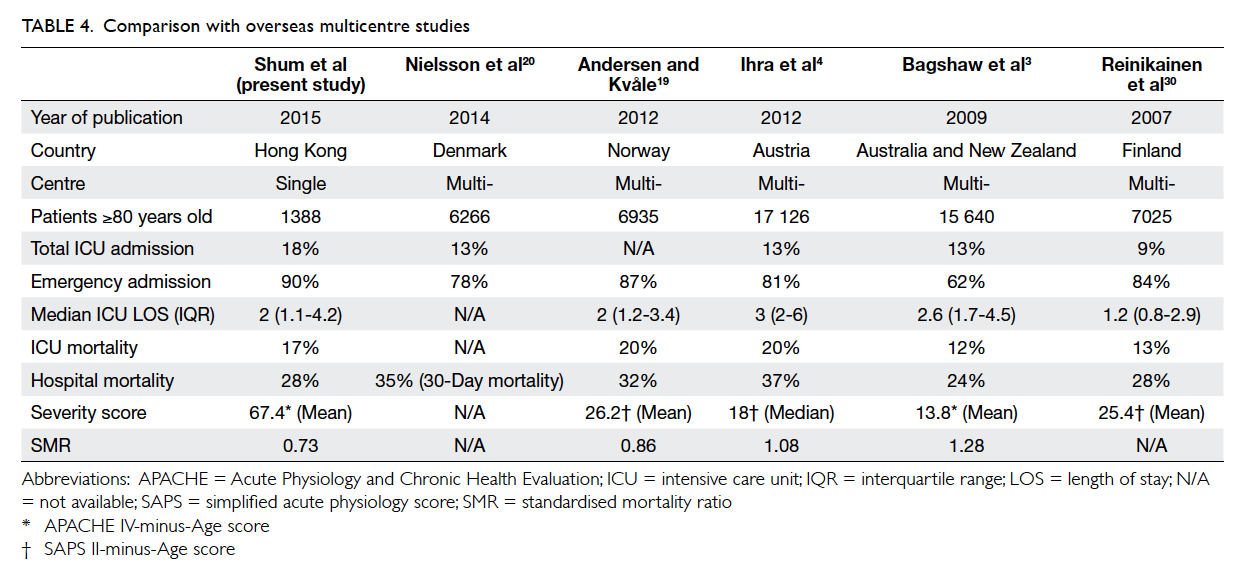
Compared with other multicentre
studies,3 4 19 20 30 we admitted more patients aged ≥80
years (18% vs 9-13%) [Table 4]. The median ICU
length of stay was comparable. Similar to them4 19 30
(except Bagshaw study3), most ICU admissions for
≥80 year olds were emergency in nature and carried
a higher hospital mortality. A study conducted by
Bagshaw et al3 had a relatively higher proportion of
elective cases (38%) and explains their apparently
lower hospital mortality compared with others. It is
difficult to compare disease severity across different
studies as severity scoring systems are inconsistent.
The performance of ICU for these groups of patients,
however, can be assessed by the SMR that represents
the ratio of observed versus expected mortality
based on the severity score. An SMR of <1 indicates
better-than-expected performance and >1 indicates
suboptimal performance. Our SMR was slightly
lower than other overseas studies and this might
indicate a better outcome for those ≥80 years old.
Another possible explanation for this phenomenon
is that the severity scores adopted by other studies,
namely SAPS II and APACHE II score, were
developed in the 1990s and may not be appropriate
to the modern ICU setting.31 32 33
The triage decision for ICU admission is
always a difficult task for the critical care physician.
The potential benefit of ICU care should be weighed
against the multiple risks, namely iatrogenic
complications from invasive monitoring and
treatments, higher exposure to nosocomial infection
and ICU delirium, in which the elderly patients
are more vulnerable.34 35 36 We do not have any data
from a randomised controlled trial that can advise
whether we should place an upper age limit on
ICU admission. Our study showed that more than
70% of critically ill patients aged ≥80 years could
be discharged home and their 180-day survival rate
was >60%. This is firm evidence to support ICU
admission for those ≥80 years old. Post-discharge
functional outcome is another valuable parameter
and warrants consideration during triage decision.
Such information, however, was not available in our
study. The decision to discharge patients from ICU
and hospital depends not only on clinical factors,
but also on operational factors (eg bed occupancy
and manpower issue). This may induce bias in
assessment of patient outcome when using ICU or
hospital mortality alone. Using 180-day mortality, as
in our study, will resolve this problem.
Is it cost-effective to treat elderly patients in
the ICU? It is difficult to conduct randomised study
of this issue because of ethical considerations. An
observational study by Edbrooke et al37 examined the
cost-effectiveness of ICU admission by comparing
patients who were accepted into ICU after ICU
triage with those who were not, while attempting
to adjust such comparison for confounding factors.
Their study showed that ICU admission not only
improved survival, but the cost per life saved
decreased as severity of illness increased. The cost
decreased substantially for patients with predicted
mortality higher than 40%. The elderly patients have
significant co-morbidities and higher disease severity
that contributed to elevated predicted hospital
mortality. Therefore, they may benefit more from
ICU care at a lower cost. Chelluri et al38 investigated
the relationship between age and hospital cost for
those patients who received prolonged mechanical
ventilation. Daily and total costs for hospitalisation
were less for older patients than younger patients.
One would think that the lower hospital cost was
due to higher mortality and consequent shorter
length of stay of elderly patients, but it is not the
case. The relationship between age and costs was
independent of hospital mortality, resuscitation
status, and discharge location. More studies are
required to clarify the potential health economic
impact associated with increased ICU admission for
these elderly patients.
Our study has several limitations. First, we
have no information about the decision to limit or
withdraw therapy. This may contribute to some of
the differences between the oldest-old and other
groups of patients. Second, the pre-ICU admission
functional statuses and post-discharge quality-of-life
assessment were not available. Functional status
before ICU admission correlates with long-term
outcome, and the absence of such information may
have induced bias in this study.7 39 Many elderly
patients deteriorate with critical illness that requires
ICU care and improve after hospital discharge,
although quality of life fails to return to the pre-admission
level even after a prolonged period.40 41
Therefore, quality of life should be assigned the
same weighting as mortality when determining a
patient’s outcome. Third, other confounders such as
smoking or nutritional status were not recorded and
might have affected prognosis. Fourth, the follow-up
duration was short and long-term outcome could not
be assessed. Finally, this was a single-centre study
and the findings may not be applicable to other
institutions.
Conclusions
The proportion of critically ill patients aged ≥80 years
increased over 5 years. Age, disease severity, and
presence of co-morbidities independently predicted
180-day mortality. Despite having more significant
co-morbidities, greater disease severity, and higher
ICU/hospital/180-day mortality rate than those
aged 60 to 79 years, 71.8% of those aged ≥80 years
could be discharged home and 62.2% survived >180
days from ICU admission. This provides evidence
to support ICU admission for those aged ≥80 years.
We recommend further studies to explore the long-term
functional outcome of these critically ill elderly
patients and the potential health economic impact
associated with increased ICU admission for those
aged ≥80 years.
References
1. Hong Kong Population Projections 2012-2041.
Available from: http://www.statistics.gov.hk/pub/B1120015052012XXXXB0100.pdf. Accessed Nov 2014.
2. Song X, MacKnight C, Latta R, Mitnitski AB, Rockwood K.
Frailty and survival of rural and urban seniors: results from
the Canadian Study of Health and Aging. Aging Clin Exp
Res 2007;19:145-53. Crossref
3. Bagshaw SM, Webb SA, Delaney A, et al. Very old patients
admitted to intensive care in Australia and New Zealand: a
multi-centre cohort analysis. Crit Care 2009;13:R45. Crossref
4. Ihra GC, Lehberger J, Hochrieser H, et al. Development
of demographics and outcome of very old critically ill
patients admitted to intensive care units. Intensive Care
Med 2012;38:620-6. Crossref
5. Garrouste-Orgeas M, Montuclard L, Timsit JF, Misset
B, Christias M, Carlet J. Triaging patients to the ICU: a
pilot study of factors influencing admission decisions and
patient outcomes. Intensive Care Med 2003;29:774-81.
6. Dragsted L, Qvist J. Epidemiology of intensive care. Int J
Technol Assess Health Care 1992;8:395-407. Crossref
7. de Rooij SE, Abu-Hanna A, Levi M, de Jonge E. Factors that
predict outcome of intensive care treatment in very elderly
patients: a review. Crit Care 2005;9:R307-14. Crossref
8. Fuchs L, Chronaki CE, Park S, et al. ICU admission
characteristics and mortality rates among elderly and very
elderly patients. Intensive Care Med 2012;38:1654-61. Crossref
9. Chelluri L, Pinsky MR, Donahoe MP, Grenvik A. Long-term
outcome of critically ill elderly patients requiring
intensive care. JAMA 1993;269:3119-23. Crossref
10. Kaarlola A, Tallgren M, Pettilä V. Long-term survival,
quality of life, and quality-adjusted life-years among
critically ill elderly patients. Crit Care Med 2006;34:2120-6. Crossref
11. Ip SP, Leung YF, Ip CY, Mak WP. Outcomes of critically
ill elderly patients: is high-dependency care for geriatric
patients worthwhile? Crit Care Med 1999;27:2351-7. Crossref
12. Definition of an older or elderly person. Available from:
http://www.who.int/healthinfo/survey/ageingdefnolder/en/. Accessed 1 Oct 2014.
13. Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute
Physiology and Chronic Health Evaluation (APACHE)
IV: hospital mortality assessment for today’s critically ill
patients. Crit Care Med 2006;34:1297-310. Crossref
14. Hong Kong Monthly Digest of Statistics. Census and
Statistics Department, Hong Kong SAR; 2014: 5.
15. Hamel MB, Davis RB, Teno JM, et al. Older age,
aggressiveness of care, and survival for seriously ill,
hospitalized adults. SUPPORT Investigators. Study to
Understand Prognoses and Preferences for Outcomes and
Risks of Treatments. Ann Intern Med 1999;131:721-8. Crossref
16. Somme D, Maillet JM, Gisselbrecht M, Novara A, Ract C,
Fagon JY. Critically ill old and the oldest-old patients in
intensive care: short- and long-term outcomes. Intensive
Care Med 2003;29:2137-43. Crossref
17. Boumendil A, Aegerter P, Guidet B; CUB-Rea Network.
Treatment intensity and outcome of patients aged 80 and
older in intensive care units: a multicenter matched-cohort
study. J Am Geriatr Soc 2005;53:88-93. Crossref
18. Brandberg C, Blomqvist H, Jirwe M. What is the importance
of age on treatment of the elderly in the intensive care unit?
Acta Anaesthesiol Scand 2013;57:698-703. Crossref
19. Andersen FH, Kvåle R. Do elderly intensive care unit
patients receive less intensive care treatment and have
higher mortality? Acta Anaesthesiol Scand 2012;56:1298-305. Crossref
20. Nielsson MS, Christiansen CF, Johansen MB, Rasmussen
BS, Tønnesen E, Nørgaard M. Mortality in elderly
ICU patients: a cohort study. Acta Anaesthesiol Scand
2014;58:19-26. Crossref
21. Lerolle N, Trinquart L, Bornstain C, et al. Increased
intensity of treatment and decreased mortality in elderly
patients in an intensive care unit over a decade. Crit Care
Med 2010;38:59-64. Crossref
22. Fuchs L, Novack V, McLennan S, et al. Trends in severity of
illness on ICU admission and mortality among the elderly.
PLoS One 2014;9:e93234. Crossref
23. Covinsky KE, Justice AC, Rosenthal GE, Palmer RM,
Landefeld CS. Measuring prognosis and case mix in
hospitalized elders. The importance of functional status. J
Gen Intern Med 1997;12:203-8. Crossref
24. Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz
RI, Concato J. Importance of functional measures in
predicting mortality among older hospitalized patients.
JAMA 1998;279:1187-93. Crossref
25. Blot S, Cankurtaran M, Petrovic M, et al. Epidemiology and
outcome of nosocomial bloodstream infection in elderly
critically ill patients: a comparison between middle-aged,
old, and very old patients. Crit Care Med 2009;37:1634-41. Crossref
26. Deiner S, Westlake B, Dutton RP. Patterns of surgical care
and complications in elderly adults. J Am Geriatr Soc
2014;62:829-35. Crossref
27. Nin N, Lorente JA, De Paula M, et al. Aging increases the
susceptibility to injurious mechanical ventilation. Intensive
Care Med 2008;34:923-31. Crossref
28. Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M,
Steinberg KP, Bernard GR. Recovery rate and prognosis
in older persons who develop acute lung injury and the
acute respiratory distress syndrome. Ann Intern Med
2002;136:25-36.
29. Feng Y, Amoateng-Adjepong Y, Kaufman D, Gheorghe C,
Manthous CA. Age, duration of mechanical ventilation,
and outcomes of patients who are critically ill. Chest
2009;136:759-64. Crossref
30. Reinikainen M, Uusaro A, Niskanen M, Ruokonen E.
Intensive care of the elderly in Finland. Acta Anaesthesiol
Scand 2007;51:522-9. Crossref
31. Breslow MJ, Badawi O. Severity scoring in the critically ill:
part 2: maximizing value from outcome prediction scoring
systems. Chest 2012;141518-27. Crossref
32. Breslow MJ, Badawi O. Severity scoring in the critically ill:
part 1—interpretation and accuracy of outcome prediction
scoring systems. Chest 2012;141:245-52. Crossref
33. Juneja D, Singh O, Nasa P, Dang R. Comparison of newer
scoring systems with the conventional scoring systems
in general intensive care population. Minerva Anestesiol
2012;78:194-200.
34. Nguyen YL, Angus DC, Boumendil A, Guidet B. The
challenge of admitting the very elderly to intensive care.
Ann Intensive Care 2011;1:29. Crossref
35. Mercier E, Giraudeau B, Ginies G, Perrotin D, Dequin
PF. Iatrogenic events contributing to ICU admission: a
prospective study. Intensive Care Med 2010;36:1033-7. Crossref
36. Zhang Z, Pan L, Ni H. Impact of delirium on clinical
outcome in critically ill patients: a meta-analysis. Gen
Hosp Psychiatry 2013;35:105-11. Crossref
37. Edbrooke DL, Minelli C, Mills GH, et al. Implications
of ICU triage decisions on patient mortality: a cost-effectiveness
analysis. Crit Care 2011;15:R56. Crossref
38. Chelluri L, Mendelsohn AB, Belle SH, et al. Hospital costs
in patients receiving prolonged mechanical ventilation:
does age have an impact? Crit Care Med 2003;31:1746-51. Crossref
39. Boumendil A, Somme D, Garrouste-Orgeas M, Guidet B.
Should elderly patients be admitted to the intensive care
unit? Intensive Care Med 2007;33:1252-62. Crossref
40. Hennessy D, Juzwishin K, Yergens D, Noseworthy T, Doig
C. Outcomes of elderly survivors of intensive care: a review
of the literature. Chest 2005;127:1764-74. Crossref
41. Roch A, Wiramus S, Pauly V, et al. Long-term outcome in
medical patients aged 80 or over following admission to an
intensive care unit. Crit Care 2011;15:R36. Crossref