DOI: 10.12809/hkmj154629
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
LETTERS TO THE EDITOR
Heat treatment of biochemical samples to inactivate Ebola virus: does it work in practice?
Timothy B Nguyen, MD;
Vanessa Clifford, MB, BS;
Azni Abdul Wahab, MB, BS;
Vincent Sinickas, FRCPA, FRACP
Department of Pathology, Royal Melbourne Hospital, Parkville, Victoria, Australia
Corresponding author: Dr Vanessa Clifford (vanessa.clifford@mh.org.au)
To the Editor—We read with interest the article
by Chong et al1 on the effects of plasma heating
procedures on common biochemical tests. Heat
treatment at 60°C for 60 minutes has also been
suggested by our Australian guidelines as a means
to inactivate Ebola virus prior to routine laboratory
processing.2
We recently performed a similar study to
Chong et al1 in our laboratory at the Royal Melbourne
Hospital (Parkville, Australia). De-identified plasma
(n=29) and serum (n=38) venous samples were
collected in plastic BD vacutainer tubes (Becton,
Dickinson and Company, Franklin Lakes [NJ], US)
for electrolyte, liver function, and troponin testing on
the Architect c16000 analyser (Abbott Laboratories,
North Chicago [IL], US). After centrifugation, two
0.5-mL aliquots were obtained, one placed in a
heat block at 60°C for 60 minutes, and the other
paired sample left at room temperature. After heat
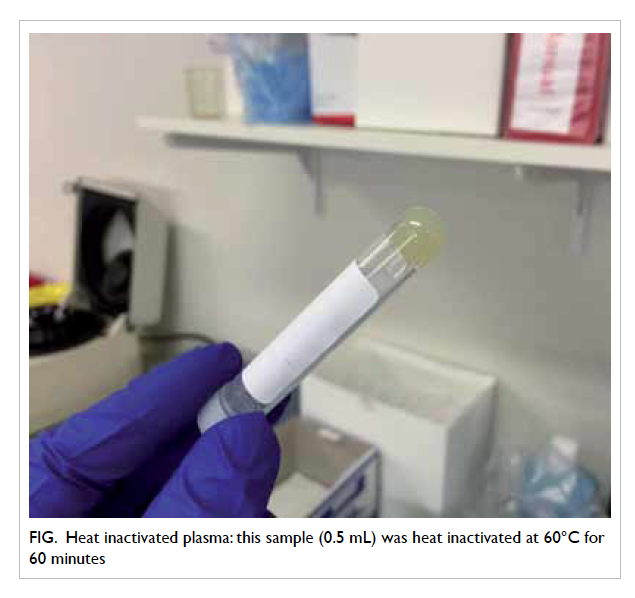
inactivation, 24/27 (89%) plasma samples and 25/36
(69%) serum samples changed to a viscous jelly-like
substance (Fig) and caused aspiration error on the
Architect analyser. Centrifugation, manual stirring,
and vortexing did not resolve the problem.
In serum samples that were not denatured
by heating, electrolyte measurements had a strong
correlation with results obtained by standard
testing. Nonetheless, enzyme tests (liver function
and troponin) showed a poor correlation (Table).
Interestingly, these problems have not been
previously reported.2 3 The use of a heat block instead of a water bath2 may have exposed our specimens to
unequal heating, hotspots, or spikes in temperature,
causing condensation or irreversible denaturing of
proteins.4
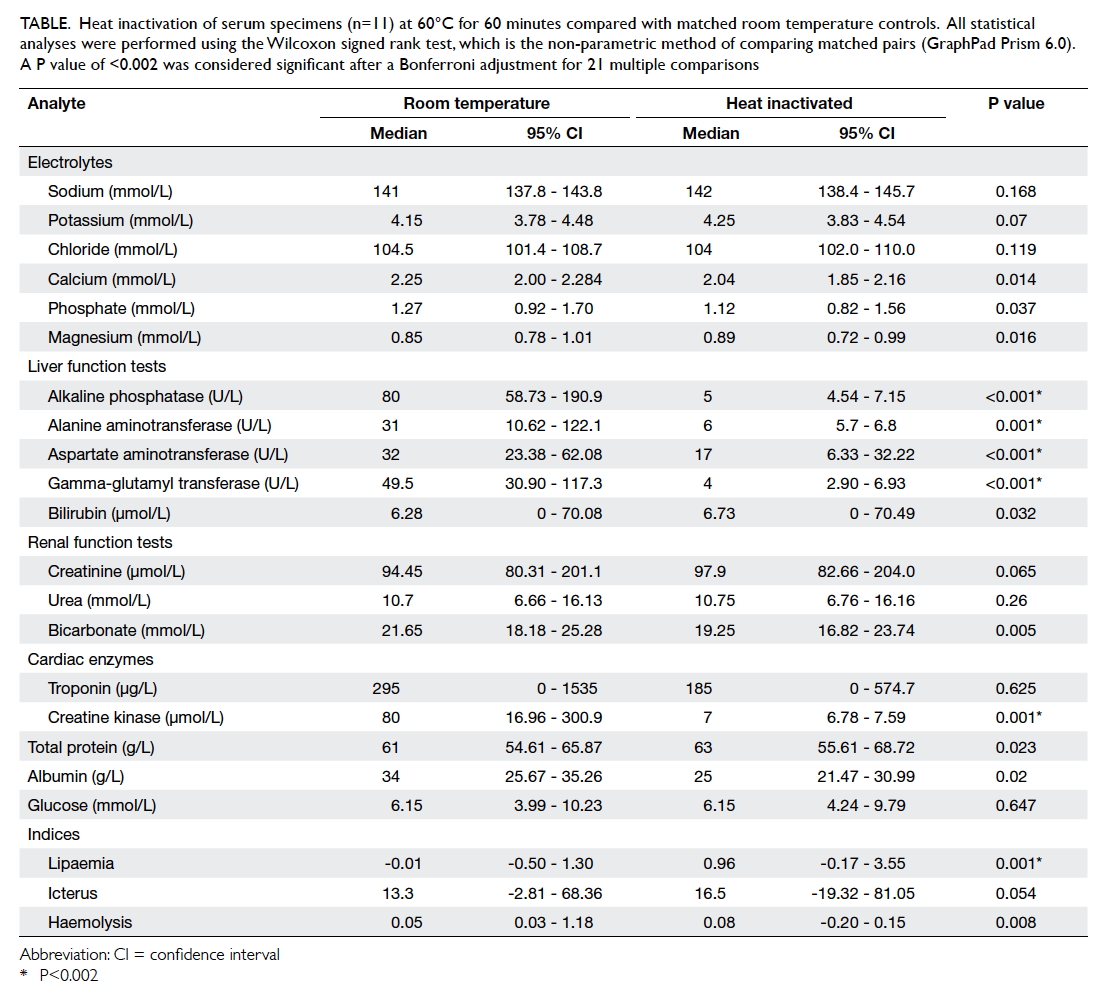
Table. Heat inactivation of serum specimens (n=11) at 60°C for 60 minutes compared with matched room temperature controls. All statistical analyses were performed using the Wilcoxon signed rank test, which is the non-parametric method of comparing matched pairs (GraphPad Prism 6.0). A P value of <0.002 was considered significant after a Bonferroni adjustment for 21 multiple comparisons
We found that, in our hands, it was not
possible to provide biochemical testing after heat
treatment, as recommended by current national and
international guidelines.
References
1. Chong YK, Ng WY, Chen SP, Mak CM. Effects of a plasma
heating procedure for inactivating Ebola virus on common
chemical pathology tests. Hong Kong Med J 2015;21:201-7. Crossref
2. Public Health Laboratory Network. Laboratory procedures
and precautions for samples collected from patients
with suspected viral haemorrhagic fevers: Australian
Government Department of Health; 2014. Available from:
http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-pubs-other-vhf.htm. Accessed 10 May 2015.
3. Bhagat CI, Lewer M, Prins A, Beilby JP. Effects of heating
plasma at 56 degrees C for 30 min and at 60 degrees C
for 60 min on routine biochemistry analytes. Ann Clin
Biochem 2000;37:802-4. Crossref
4. Wetzel R, Becker M, Behlke J, et al. Temperature behaviour
of human serum albumin. Eur J Biochem 1980;104:469-78. Crossref
Author’s reply
YK Chong, MB, BS;
WY Ng, MB, ChB, PhD;
Sammy PL Chen, FRCPA, FHKAM (Pathology);
CM Mak, FRCPA, FHKAM (Pathology)
Chemical Pathology Laboratory, Department of Pathology, Princess
Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr CM Mak (makm@ha.org.hk)
To the Editor—We would like to thank Nguyen et al
for their comments.
We concur with Nguyen et al that the use of
heat blocks rather than a water bath may result in
unequal heating, hotspots, or spikes in temperature.
In our previous experience with heating procedures
performed to determine alkaline phosphatase
isoenzymes,1 exposure of plasma to a temperature
of 65°C caused gelling of most plasma specimens
(unpublished observations). We suspect that the
gelling temperature of normal human plasma is 60°C
to 65°C.
In our experiments, we use a W14 water bath
with 14 L capacity (Sheldon Manufacturing Inc,
Cornelius [OR], US). The water bath has a much
higher heat capacity than heat blocks, due to the large
volume of water, as well as the considerably higher
specific heat capacity of water (4.1813 J g-1 K-1) when
compared with aluminium (0.897 J g-1 K-1),2 a metal
often used to manufacture heat blocks.
References
1. Panteghini M, Bais R. Serum enzymes. In: Burtis CA,
Ashwood ER, Bruns DE. Tietz textbook of clinical
chemistry and molecular diagnostics. St Louis, US:
Elsevier; 2012: 579. Crossref
2. Chung DD. Composite materials. London: Springer; 2010: 283. Crossref