Hong Kong Med J 2015 Feb;21(1):85–7 | Epub 5 Dec 2014
DOI: 10.12809/hkmj144455
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
COMMENTARY
Has dengue found its home in Hong Kong?
CM Poon, BSSC1; SS Lee, MD, FHKAM (Medicine)2
1 Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
2 Stanley Ho Centre for Emerging Infectious Diseases, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Prof SS Lee (sslee@cuhk.edu.hk)
In Hong Kong, the recent report of locally acquired
dengue cases has raised concern about the
introduction of dengue virus in our community.
Within just over a month, three people with no
travel history during the incubation period have
been confirmed to have dengue virus infection. As
there was no epidemiological link between the third
patient and the previous two, multiple sources of
infection and the possibility of ongoing transmission
within Hong Kong are suggested. Temporally, the
emergence of these three cases follows closely the
large outbreak in Guangdong, which affected more
than 40 000 people. Given the scale of the outbreak,
the Department of Health of Guangdong Province
has released updates on the dengue situation on a
daily basis from 22 September 2014 to the end of
October 2014.1 Dengue activity peaked between 29
September and 13 October, with almost 10 000 cases
reported each week. More than 80% of the cases
were notified in Guangzhou.
Epidemiological information for the
Guangdong outbreak can be accessed on the Internet,
which allows us to map the dengue distribution by
city and the changes over time with the Geographic
Information System. The scale of the outbreak has
evidently expanded geographically in Guangdong
since its onset, as shown by the biweekly standard
deviational ellipses, each of which would have
covered approximately 68% of the dengue cases in
the respective fortnight (Fig). Guangzhou remained
the spatial centre of the outbreak during these weeks.
A similar temporal distribution of dengue cases was
observed in other cities in the Pearl River Delta
(PRD), while places further away from the delta were
subsequently affected at a diminished level, like a
ripple effect. Researchers from Mainland China
had previously suggested the PRD and Chao Shan
Area as the two main hubs in Guangdong where
indigenous dengue cases have clustered since the
turn of the century.2 It appears, therefore, that this
latest outbreak is the result of extensive transmission
of dengue virus from the PRD hub.
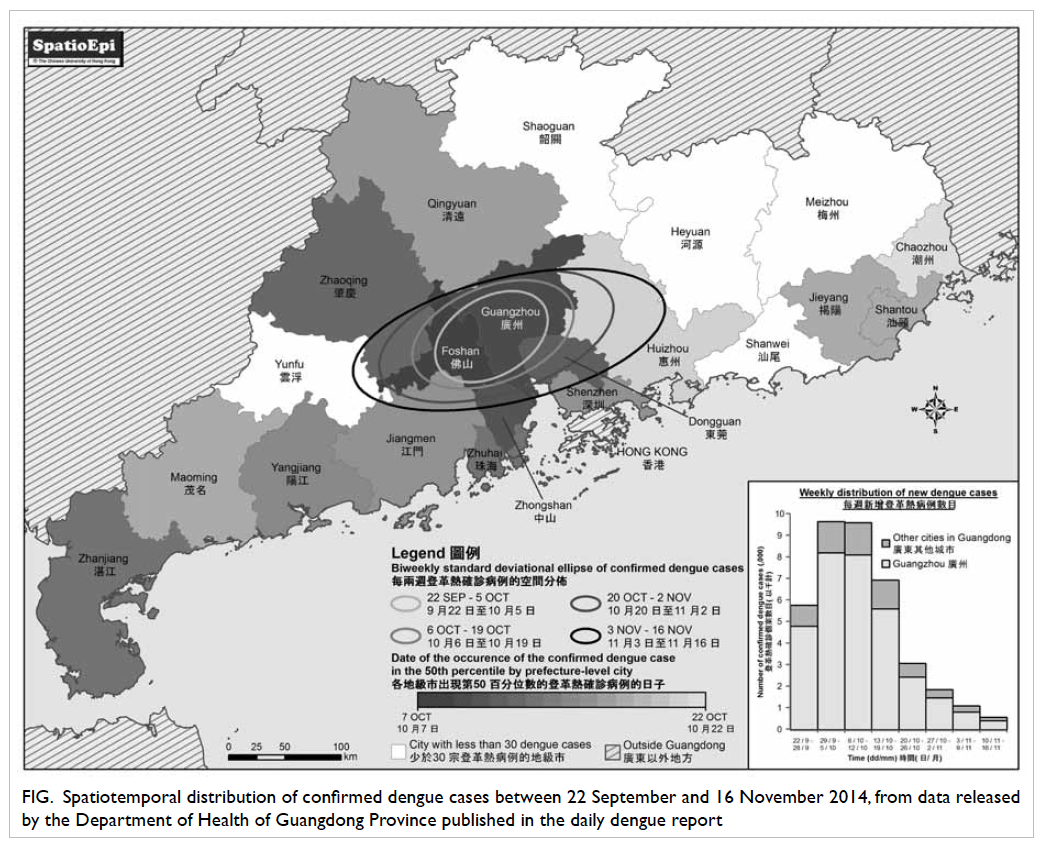
Figure. Spatiotemporal distribution of confirmed dengue cases between 22 September and 16 November 2014, from data released by the Department of Health of Guangdong Province published in the daily dengue report
Even before the change in sovereignty, the PRD
was gradually transformed into a Chinese region
where many inhabitants move within and between
cities every day. With the rising number of people
who live in the PRD, Hong Kong is no different to a
prefecture-level city of Guangdong. Currently, Hong
Kong’s population comprises an estimated 200 000
mobile residents, with the majority travelling
back and forth to Guangdong.3 Mainland China’s
transport system, with its increasing efficiency,
not only reduces the travel time between cities in
Guangdong, but also accelerates the propagation of
infections in the region. In the past decade, dengue
outbreaks have been reported in places as far apart
as Zhejiang and Henan,4 5 6 a scenario proving that
human mobility is crucial in predisposing to virus
spread. It is conceivable that the latest Guangdong
outbreak has led to concurrent maintenance of
multiple pools of actively infected people commuting
in the PRD who, in the presence of the Aedes
mosquitoes, have enabled the virus to find a new
home base. Beginning in August 2014, three local
dengue cases were reported in Macau,7 coinciding
with the reported onset of the Guangdong outbreak.
The Guangdong epidemic undoubtedly increases
the likelihood of exposure to dengue virus among
mobile residents, the magnitude of which varies
with the place, time, and duration of their stay, as
well as their commuting frequency. Geographically,
dengue virus transmission might be more likely to
occur in some districts in Hong Kong. This does
not necessarily imply a direct association with the
size of the vector population, but the heterogeneous
distribution of mobile residents in the territory.3
Theoretically, one useful way to guard Hong
Kong from importation of dengue virus is to track
the situation in neighbouring countries. However,
the uniqueness of the natural history of dengue virus
infection poses a challenge to accurately predicting
its epidemiological risk. Clinically, dengue fever is
typically a self-limiting condition with an incubation
period ranging between 3 and 14 days.8 Since most
people infected with dengue virus have no or mild
signs or symptoms, a significant proportion are never
diagnosed or reported.9 Consequently, estimation
from the size and distribution of actively infected
people can hardly reflect dengue epidemiology.
Serological testing of dengue antibody may infer
the proportional distribution of the population
with previous exposure to the virus, but is unable to
determine the actual size of the ‘infective’ population.
A study in 2007 to 2009 in Hong Kong gave a
prevalence of positive dengue immunoglobulin G
of 1.6%, a figure that cannot provide insight into
the current epidemiological status.10 Importantly,
clinical testing plays no direct role in public health
interventions. It is also almost impossible to
intercept asymptomatic travellers or those in latent
infection from entering Hong Kong. Introduction of
dengue virus into our neighbourhood is therefore
often silent and discovered after several waves of
transmissions.
Upon identification of a locally acquired
dengue case in Hong Kong, epidemiological
investigations and vector control measures have
been conducted immediately to prevent secondary
spread. Coincidentally, the three recently reported
local dengue patients either worked or lived in
the vicinity of a construction site, and breeding of
mosquito larvae was found during site inspection.11
This reminds us of the lesson from the outbreak
related to a construction site in Ma Wan in 2002,
affecting 16 workers and residents nearby.12 Whereas
the previous outbreak was limited to an island, the
residences and suspected infection sources of the
three recent local dengue patients were sporadically
distributed in several densely populated districts,
suggesting a higher risk for subsequent evolvement
of dengue endemicity. Prior to the Ma Wan outbreak,
a major dengue outbreak occurred in Macau in
the preceding year, with a total of 1418 reported
cases.13 Although the exact source of the Macau
outbreak could not be ascertained, it also occurred
at a time when infrastructure development was
gaining momentum. Dengue dissemination could
well be the outcome of a perfect match between
increasing human mobility and urban development.
Understandably vector surveillance is a key
component of current environmental measures.
Ovitraps have been strategically established in 44
locations in Hong Kong to monitor the extensiveness
of the distribution of Aedes mosquitoes.14 However,
the insufficient geographical coverage of ovitraps
limits their ability to detect the invasion of dengue
virus as Aedes albopictus has a short flight range of
no more than 200 metres.15 Thus, the eggs sampled
in the ovitraps can only represent the mosquitoes
in a confined geographic area. Nevertheless, the
importance of eliminating mosquito breeding sites
in our highly urbanised environment cannot be
overemphasised. This is, however, a real challenge,
even for dengue-endemic Singapore where the
hygiene standard is enviably high.16
Even without any new locally acquired
dengue cases in the coming months, the medical
profession should remain vigilant and advise on
effective prevention. The virus can hide in the eggs
of infected mosquitoes this winter and becomes
reactivated when the weather is favourable for
breeding again.17 The next round of local dengue
transmission could probably begin after the spring,
merging with the anticipated upsurge of indigenous
dengue infections in the PRD every late summer,2
which are our potential external sources of dengue
virus. In consideration of the incident local dengue
cases and our dengue endemic neighbours, there is
no doubt that dengue virus has found its home in
Hong Kong. It is just a matter of whether this is a
temporary home or a permanent base.
References
1. The Department of Health of Guangdong Province. Updates on
epidemics [in Chinese]. Available from: http://www.gdwst.gov.cn/a/yiqingxx/. Accessed 18 Nov 2014.
2. Li Z, Yin W, Clements A, et al. Spatiotemporal analysis of
indigenous and imported dengue fever cases in Guangdong
province, China. BMC Infect Dis 2012;12:132. CrossRef
3. Census and Statistics Department, Hong Kong. 2011
Population Census. Available from: http://www.census2011.gov.hk/en/index.html. Accessed 18 Nov 2014.
4. Xu G, Dong H, Shi N, et al. An outbreak of dengue virus
serotype 1 infection in Cixi, Ningbo, People’s Republic
of China, 2004, associated with a traveler from Thailand
and high density of Aedes albopictus. Am J Trop Med Hyg
2007;76:1182-8.
5. Wu JY, Lun ZR, James AA, Chen XG. Dengue fever in
mainland China. Am J Trop Med Hyg 2010;83:664-71. CrossRef
6. Huang XY, Ma HX, Wang HF, et al. Outbreak of dengue
fever in Central china, 2013. Biomed Environ Sci
2014;27:894-7.
7. Health Bureau, Macao. Infectious disease information.
Available from: http://www.ssm.gov.mo/portal/csr/ch/main.aspx. Accessed 27 Nov 2014.
8. Rudolph KE, Lessler J, Moloney RM, Kmush B, Cummings
DA. Incubation periods of mosquito-borne viral infections: a systematic review. Am J Trop Med Hyg 2014;90:882-91. CrossRef
9. Chastel C. Eventual role of asymptomatic cases of dengue
for the introduction and spread of dengue viruses in non-endemic
regions. Front Physiol 2012;3:70. CrossRef
10. Lo CL, Yip SP, Leung PH. Seroprevalence of dengue in the
general population of Hong Kong. Trop Med Int Health
2013;18:1097-102. CrossRef
11. Hong Kong SAR Government press release. Government
enhances anti-mosquito measures in prevention of dengue
fever. 21 November 2014. Available from: http://www.info.gov.hk/gia/general/201411/11/P201411110853.htm. Accessed 27 Nov 2014.
12. Ma SK, Wong WC, Leung CW, et al. Review of vector-borne
diseases in Hong Kong. Travel Med Infect Dis
2011;9:95-105. CrossRef
13. Almeida AP, Baptista SS, Sousa CA, et al. Bioecology and
vectorial capacity of Aedes albopictus (Diptera: Culicidae)
in Macao, China, in relation to dengue virus transmission.
J Med Entomol 2005;42:419-28. CrossRef
14. Audit Commission, Hong Kong. Director of Audit’s Report
No. 63. Available from: http://www.aud.gov.hk/pdf_e/e63ch05.pdf. Accessed 24 Nov 2014.
15. Marini F, Caputo B, Pombi M, Tarsitani G, della Torre A.
Study of Aedes albopictus dispersal in Rome, Italy, using
sticky traps in mark-release-recapture experiments. Med
Vet Entomol 2010;24:361-8. CrossRef
16. Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35
years of vector control in Singapore. Emerg Infect Dis
2006;12:887-93. CrossRef
17. Lee HL, Rohani A. Transovarial transmission of dengue
virus in Aedes aegypti and Aedes albopictus in relation to
dengue outbreak in an urban area in Malaysia. Dengue
Bulletin 2005;29:106-11.
Find HKMJ in MEDLINE:

