DOI: 10.12809/hkmj134091
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Generalised involuntary limb twitching after ingestion of Mesobuthus martensii Karsch (Quanxie) powder
PK Lam, FHKAM (Emergency Medicine), Dip Clin Tox1; TW Wong, FRCSEd, FHKAM (Emergency Medicine)1; YC Chan, FRCSEd, FHKAM (Emergency Medicine)2; Tony WL Mak, FRCPath, FHKAM (Pathology)3
1 Department of Accident and Emergency, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 Hong Kong Poison Information Centre, United Christian Hospital, Kwun Tong, Hong Kong
3 Hospital Authority Toxicology Reference Laboratory, Princess Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr PK Lam (lampkrex@hotmail.com)
Abstract
Mesobuthus martensii Karsch, commonly known as the Chinese scorpion or Manchurian scorpion,
has been used in traditional Chinese medicine as Quanxie to treat chronic pain, tetanus, tremors,
convulsion, and paralysis for more than a thousand years. We report a case of poisoning after ingestion
of a teaspoon of Quanxie powder. The patient presented with chest pain, dizziness, diaphoresis,
generalised involuntary limb twitching, and hypertonia around 15 minutes post-ingestion. The
patient recovered uneventfully after supportive management. Intravenous diazepam appeared to
be effective in alleviating limb twitching. Failure to accurately measure the dose and to boil before
consumption may have contributed to his clinical toxicities.
Case report
A 63-year-old man complained of chest pain,
dizziness, and generalised tremors 15 minutes after
ingestion of a teaspoon of herbal powder with water
in September 2012. He had started taking herbal
decoctions prescribed by a registered Chinese
medicine doctor 1 month ago because of suboptimal
pain control of his trigeminal neuralgia with western
medicine. He presented around 2 to 3 hours post-ingestion
to our emergency department because of
persistent symptoms. He was fully conscious but the
limb tremor was so severe that he could barely walk.
There was no numbness, headache, or any gastro-intestinal
(GI) symptom. Apart from trigeminal
neuralgia, he also had a history of ischaemic heart
disease and hypercholesterolaemia. The medication
on-hand included aspirin, famotidine, simvastatin,
metoprolol, isosorbide mononitrate, diclofenac,
dihydrocodeine, tramadol, and carbamazepine but
he denied overdosing of any of those drugs.
He had not taken any other herbs, over-the-counter
medicines, or other suspicious foods such as coral
reef fish and shellfish.
On arrival he was fully conscious with a
Glasgow Coma Scale score of 15/15. His vital signs
were as follows: blood pressure 126/95 mm Hg, pulse
rate 66 beats/min, respiratory rate 18 breaths/min,
oxygen saturation by pulse oximetry (SaO2) 99% on
supplemental oxygen 2 L/min via a nasal cannula, and
tympanic temperature 36.6°C. He appeared nervous
with diaphoresis. Both his pupils were 2 mm in size
and reactive to light. Cranial nerve examination
was unremarkable but generalised involuntary limb
twitching with hypertonia was evident. The muscle
power in his four limbs was 5/5. Hyperreflexia and
bilateral upgoing plantar reflexes were noted; there
was no ankle clonus. Cardiovascular examination
was unremarkable and his chest was clear. No
distended urinary bladder or abnormal bowel sounds
were noted. Repeated electrocardiograms showed
sinus rhythm with normal axis. First-degree heart
block was noted but there were no definite ischaemic
changes. The QRS duration and the corrected
QT interval were 89 ms and 431 ms, respectively.
Chest X-ray revealed marginal cardiomegaly with
clear lung field. The spot haemostix level was 6.2
mmol/L. Other blood tests including a complete
blood picture, urea and electrolytes, serum calcium
level, liver function tests, troponin-I, and arterial
blood gas were essentially unremarkable, except a
slightly raised creatine kinase (CK) level of 408 IU/L
(reference range, 24-180 IU/L), which was likely due
to generalised muscle twitching.
His chest pain decreased after administering
3 mg of intravenous (IV) morphine. Subsequently,
his blood pressure dropped to 66/48 mm Hg but
responded to fluid challenge with 500 mL of IV 0.9%
normal saline. Less limb twitching was noted after
administering 5 mg of IV diazepam. The provisional
diagnosis was suspected Chinese herbal medicine
(CHM)–related neurotoxicity but at the time of
presentation, the formula of the herbal decoction
was not available for identifying the culprit. The
patient was admitted to the intensive care unit
(ICU) for close monitoring in view of the unstable
haemodynamic status upon presentation, which may
have been the result of CHM toxicity or hypotensive
effect of morphine.
Seven sheets of CHM prescription formula
were subsequently traced back by the patient’s son
3 hours after admission. The Hong Kong Poison
Information Centre was consulted for opinion. Multiple ingredients, including Quanxie (全蝎, Mesobuthus martensii Karsch), were prescribed
according to the formula. However, the patient had
remained free of side-effects in the previous month
when he took the herbal decoctions as instructed.
Further questioning revealed that the patient had
found his pain control unsatisfactory even after
taking the herbal decoctions. After receiving verbal
advice from his Chinese medicine doctor, he took a
few pieces of scorpion from the herb package and
put them into a food blender. He took a teaspoon
of the powder directly with water; this was the first
time he took the herb in this form and fashion. He
developed symptoms soon after ingestion.
The patient’s twitching decreased gradually
after ICU admission but he remained hypertonic,
which warranted administering another dose of IV
diazepam 2 mg 10 hours after admission. Otherwise,
he was fully conscious with a stable haemodynamic
status. Computed tomography of the brain was
unremarkable. His symptoms gradually resolved
and he was discharged from the ICU and transferred
to the Emergency Medicine Ward 16 hours after
admission. His CK level peaked to 413 IU/L and
normalised on day 2. He was discharged 36 hours
after admission, and was totally asymptomatic on
follow-up 5 days later.
The patient’s serum and urine samples, together
with the unused herbs and herbal powder, were sent
to the Hospital Authority Toxicology Reference
Laboratory for further analysis. Poisoning due to
other toxic herbs, such as aconitine, strychnine, and
matrine was ruled out by liquid chromatography-mass
spectrometry of the leftover herbal powder.
However, detection of the toxic peptides of M
martensii was not possible as the laboratory was
not equipped to test for these toxins. The patient’s
urine sample revealed the presence of diclofenac,
dihydrocodeine, carbamazepine metabolite,
salicylic acid and famotidine but these were the
usual medications taken by the patient. The serum
salicylate level was far below the toxic level. Judging
from the history and absence of other toxic alkaloids
to explain the symptoms, the diagnosis of this
case was compatible with neurotoxicity associated
with the consumption of M martensii powder,
even though it could not be directly confirmed by
chemical analysis.
Discussion
Mesobuthus martensii Karsch (synonym: Buthus
martensii Karsch), commonly known as Chinese
scorpion or Manchurian scorpion (東亞鉗蝎 or 馬氏鉗蝎),
belongs to the Buthidae family, and is
widely distributed in China. The whole body of
the scorpion has been used in traditional Chinese
medicine as Quanxie for more than a thousand
years (Fig). It functions through the liver meridian, extinguishing wind, and stopping tremors and
convulsion. According to the literature in traditional
Chinese medicine, common indications include
chronic pain, tetanus, tremors, acute/chronic
childhood convulsions, paralysis, cerebral vascular
accident, and fire toxic nodules.1 Its use is contra-indicated
during pregnancy and should be avoided in cases of internal wind with blood deficiency. The
recommended dose is 3 to 6 g for herbal decoction2
and 0.5 to 1 g for herbal powder.3
The venom of M martensii is complex. At
least 51 long-chain peptides related to the sodium (Na+) channel toxin family and 18 peptides related
to the potassium (K+) channel toxin family have been described4 and more have yet to be discovered.
Neurotoxins affecting the Na+ channel consist of α (highly active in mammalian brain) and β
(highly active in insects) toxins. Many β toxins, which are not noxious to mammals, were found to have
analgesic properties in animal models without the risk of dependence.5 Recently, a novel peptide,
BmK-YA, was found to contain an enkephalin-like sequence and can activate the mammalian δ
opioid receptor.6 Novel peptides with antiepileptic (eg BmK
AEP),7 antitumour (BmK AGAP),8 and antibacterial
(BmKn2-7)9 effects in animal models have also been identified in the venom. These findings provide
molecular evidence to support its traditional use in Chinese medicine, but relevant clinical studies are
still lacking.
Although known to be toxic in traditional
Chinese medicine, so far, literature on Quanxie
poisoning has been rather limited. Many of the
reported cases were due to therapeutic overdose,10
which might be related to the way the scorpions are
processed. Traditionally, the captured scorpions are
put into water for a few hours to allow them to spit out
soil from gut, pass retained faeces, and clean the dirt
over their bodies. Thereafter they are either boiled
with plain water or salt water, resulting in ‘plain’
Quanxie (淡全蝎) and ‘salted’ Quanxie (鹽全蝎),
respectively. Boiling kills the scorpion and decreases
its toxicity. The addition of salt serves to preserve
the processed scorpions for prolonged periods. The
majority of the processing is undertaken in scorpion
farms and there is a lack of standardised protocol.11
The details of processing vary from place to place,
resulting in variable therapeutic effects, even with
the same dose. Moreover, many merchants try to
add more salt when processing salted Quanxie to
increase its weight, for more profit in sale. The salt
content in salted Quanxie could be up to 44.82% in
the market,12 resulting in inadequate therapeutic
effects with the recommended dose. Therefore,
many traditional Chinese medicine doctors may opt
to use a higher-than-recommended dose to achieve
the targeted effect, thus increasing the risk of clinical
toxicities.10
In our case, it is difficult to estimate the amount
of Quanxie actually consumed as the powder had
not been accurately weighed before consumption.
With a standard teaspoon, the patient could have
taken 5 to 6 g of the powder, which is higher than the
recommended dose. Failure to boil before ingestion
might also have contributed to his clinical toxicities.
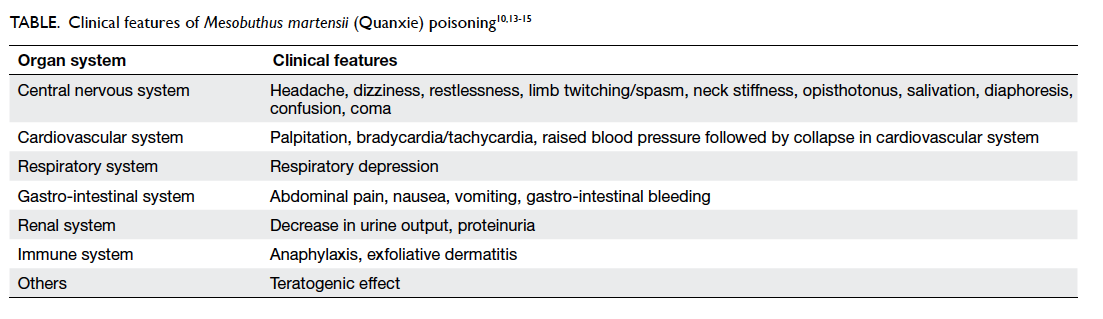
The clinical features of M martensii (Quanxie)
poisoning are summarised in the Table.10 13 14 15 There
is no specific antidote, such as anti-venom, for this
condition. The mainstay of treatment is supportive.
The toxicokinetics of Quanxie have not yet been
thoroughly studied. Judging from the rapid onset
of clinical symptoms after oral ingestion, we believe
that GI absorption is rapid. Gastro-intestinal
decontamination with gastric lavage or activated
charcoal can be considered if the patient presents
within 1 hour of ingestion and has a protected airway.
Such a time frame for GI decontamination has also
been recommended for other toxic herbs with rapid
GI absorption, such as aconitine. Clinicians may
choose to consider GI decontamination in patients
presenting beyond 1 hour after ingestion but the
benefit and risk should be carefully weighed on
a case-to-case basis. The benefit would certainly
decline with time, which may not justify the risk
of aspiration, especially when neurotoxic features
such as generalised twitching have already set in,
making the administration of activated charcoal
further difficult. As Quanxie has not been shown
to have enterohepatic circulation or prolonged GI
absorption, multi-dose activated charcoal is not
recommended. Benzodiazepines appeared effective
in alleviating limb twitching muscle spasm in our case but its role in the management of M martensii
poisoning remains to be elucidated.
Declaration
No conflicts of interests were declared by authors.
References
1. Li N, Yu M, Hu LN, Lin J. Collation of animal drugs —
Quanxie [in Chinese]. Jilin J Tradit Chin
Med 2009;29:805-6.
2. Committee of National Pharmacopoeia. Chinese
pharmacopoeia [in Chinese]. Vol 1. Beijing: Chemical
Industry Press; 2010: 133-4.
3. The editorial committee of “Chinese Herbals”, State
Administration of Traditional Chinese Medicine. Chinese
Materia Medica [in Chinese]. Vol 9. Shanghai: Shanghai
Science & Technology Press; 1999: 129-35.
4. Goudet C, Chi CW, Tytgat J. An overview of toxins and
genes from the venom of the Asian scorpion Buthus
martensii Karsch. Toxicon 2002;40:1239-58. CrossRef
5. Shao JH, Zhang R, Ge X, Yang B, Zhang JH. Analgesic
peptides in Buthus martensii Karsch: a traditional
Chinese animal medicine. Asian J Tradit Med 2007;2:45-50.
6. Zhang Y, Xu J, Wang Z, Zhang X, Liang X, Civelli O.
Bmk-YA, an encephalin-like peptide in scorpion venom.
PLoS One 2012;7:e40417. CrossRef
7. Zhou XH, Yang D, Zhang JH, Liu CM, Lei KJ. Purification
and N-terminal partial sequence of anti-epilepsy peptide
from venom of the scorpion Buthus martensii Karsch.
Biochem J 1989;257:509-17.
8. Liu YF, Zhang ZG, Mao YZ, et al. Production and antitumor
efficacy of recombinant Buthus martensii Karsch AGAP.
Asian J Tradit Med 2009;4:228-33.
9. Cao L, Dai C, Li Z, et al. Antibacterial activity and
mechanism of a scorpion venom peptide derivative in vitro
and in vivo. PLoS One 2012;7:e40135. CrossRef
10. Chang JM. Adverse effects of Quanxie [in Chinese]. J China Pharm 2003;14:484-5.
11. Gao ZJ. Brief review of the processing methods of
Quanxie [in Chinese]. J Community Med 2006;4:59-60.
12. Shao XH, Kong XS, Fang LH. Processing of Quanxie and
its clinical application [in Chinese]. Lishizhen Med Materia Medica Res 2006;17:232-3.
13. Chen XM. Analysis of adverse effects of Quanxie [in
Chinese]. Lishizhen Med Materia Medica Res 2003;14:635.
14. Xiao YC. A case report of neurotoxicity induced by
centipede and Quanxie [in Chinese]. China J Chin Materia Medica 1996;21:634.
15. Dai Y. A case report of free decoction for treatment of
Quanxie [in Chinese]. J Jilin Med Coll 2009;3:342.