Hong Kong Med J 2014 Dec;20(6):495–503 | Epub 12 Sep 2014
DOI: 10.12809/hkmj144245
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Dermoscopy for common skin problems in Chinese children using a novel Hong Kong–made dermoscope
David CK Luk, MSc, FHKAM (Paediatrics); Sam YY Lam, MB, ChB, MRCPCH; Patrick CH Cheung, FRCPCH, FHKAM (Paediatrics); Bill HB Chan, FRCPCH, FHKAM (Paediatrics)
Department of Paediatrics and Adolescent Medicine, United Christian Hospital, Kwun Tong, Hong Kong
Corresponding author: Dr David CK Luk (davidluk98@hotmail.com)
Abstract
Objective: To evaluate the dermoscopic features of
common skin problems in Chinese children.
Design: A case series with retrospective qualitative
analysis of dermoscopic features of common skin
problems in Chinese children.
Setting: A regional hospital in Hong Kong.
Participants: Dermoscopic image database, from
1 May 2013 to 31 October 2013, of 185 Chinese
children (aged 0 to 18 years).
Results: Dermoscopic features of common
paediatric skin problems in Chinese children were
identified. These features corresponded with the
known dermoscopic features reported in the western
medical literature. New dermoscopic features were
identified in café-au-lait macules.
Conclusion: Dermoscopic features of common
skin problems in Chinese children were consistent
with those reported in western medical literature.
Dermoscopy has a role in managing children with
skin problems.
New knowledge added by this
study
- This is the first research on dermoscopy in Chinese children.
- Dermoscopic features reported in western medical literature could be identified in Chinese children.
- Routine use of dermoscopy has a role in managing paediatric skin problems.
Introduction
Skin complaints are common in both community-based
and hospital paediatric practices. The range
of skin problems is diverse, including categories
like inflammatory conditions (eg eczema, psoriasis),
birthmarks (eg haemangiomas, port-wine stains,
melanocytic naevi), infectious skin diseases, and hair
and nail problems.
In many situations, the clinical diagnosis
of paediatric skin problems is straightforward
but masquerading conditions also exist.1 2 Although histopathological examination can confirm the
clinical diagnosis, skin biopsy in young children
may require special arrangements such as sedation.
In recent years, the gap between clinical and
histopathological examination of skin lesions has
been filled by various skin imaging modalities.3 4 As a simple, quick,5 non-invasive clinical technique,
dermoscopy has gained popularity in the examination
of skin in western countries.6 7 Dermoscopy refers to the examination of skin with a handheld device to reveal surface and subsurface skin structures. This is
achieved by an optical system to magnify, illuminate,
and remove light flare and reflection from the skin
surface. It provides the link between eyeball clinical
inspection and histopathological examination. With more than 1500 articles published after its
introduction in 1980s, dermoscopy has been
established as a routine skin examination technique
in many western countries.8 As dermoscopy is
extremely useful in the diagnosis of malignant
melanoma9 and is able to reduce the need for skin
biopsy,10 11 it is most widely used in the management
of pigmented skin lesions. Recently, dermoscopic
features of a wide range of non-pigmented skin
problems have also been reported.12 13 With the
uncovering of more dermoscopic features,
dermoscopy has gained importance in the diagnosis of skin lesions, and its benefits in educating medical
students and use in family practice have also been
published recently.14 15 16
Medical researches on dermoscopy in Chinese populations, however, have rarely been published.17 18 19
With the understanding that ethnicity may affect
dermoscopic findings,20 the aim of this study was to
identify dermoscopic features in Chinese children
and assess if those are in line with internationally
published features.
The clinical use and research on dermoscopy
in children worldwide had been limited by both patient
factors and equipment factors. Camera-mounted
dermoscope required lengthy setup and was not user-friendly
in busy clinics. In addition, babies and young
children might not stay still during the examination.
To ensure an efficient dermoscopic examination and
a good-quality dermoscopy image capture, a novel
device developed by the biomedical engineering team
of the Hong Kong Productivity Council was applied.
This study evaluated the dermoscopic features of
common skin problems in Chinese children using
the dermoscopy image database established with the
dermoscope.
Methods
A retrospective analysis of the dermoscopic features
of skin lesions of Chinese children (aged 0-18 years)
was performed. The study was approved by the
local Research Ethics Committee and conducted
at the Department of Paediatrics and Adolescent
Medicine, United Christian Hospital, Hong Kong,
using the dermoscopic image database from 1 May
2013 to 31 October 2013, which contained only
cases with symptoms and signs classical for their
respective clinical diagnoses. These images were
examined by a paediatrician trained in dermoscopy.
A two-step algorithm was used to assess dermoscopy
images with the first step aiming at differentiating
melanocytic from non-melanocytic lesions and the
second step on specific pattern analysis. The results
of the analysis were categorised according to the
clinical diagnoses.
Literature search of the MEDLINE database
was performed to identify specific dermoscopic
features for each clinical diagnosis. The key words
used in the literature search were “dermoscopy,
dermatoscopy, dermoscope, dermatoscope”.
Key words specific to individual clinical diagnosis
were used to facilitate the search. It was then
assessed if the dermoscopic features of this study
corresponded with those from published medical
literature.
During the study period, all dermoscopy
images were captured by the novel Hong Kong–made dermoscope. It was an all-in-one pocket-size
autofocusing polarised digital dermoscope with
Wi-Fi and USB connectivity capable of capturing
dermoscopy image of all ages including young infants within 5 seconds
(Fig). An extensible optic barrel was hinged on the body
of the dermoscope so that both gross photos and 10-times magnified dermoscopy photos could be taken.
The images were then uploaded to a computerised
dermoscopy image database.
Results
Dermoscopic images of 185 Chinese children (86
boys and 99 girls) suffering from 22 skin conditions
were retrieved. The mean age of these children was
5.2 years (range, 2 days to 17 years). The top 12 diagnoses reported (in descending order of frequency)
were port-wine stain (n=42), melanocytic naevus
(n=41), haemangioma (n=30), café-au-lait macule
(CALM; n=15), sebaceous naevus (n=8), viral wart
(n=7), atopic dermatitis (n=6), alopecia areata (n=5),
cutis aplasia (n=5), psoriasis (n=4), scabies (n=3),
and molluscum contagiosum (n=3).
The dermoscopic features of these 12 diagnoses
were further analysed. They were grouped into four
main disease categories: birthmarks (pigmentary
and vascular), infections, hair problems, and
inflammatory dermatoses. Forty-two dermoscopic
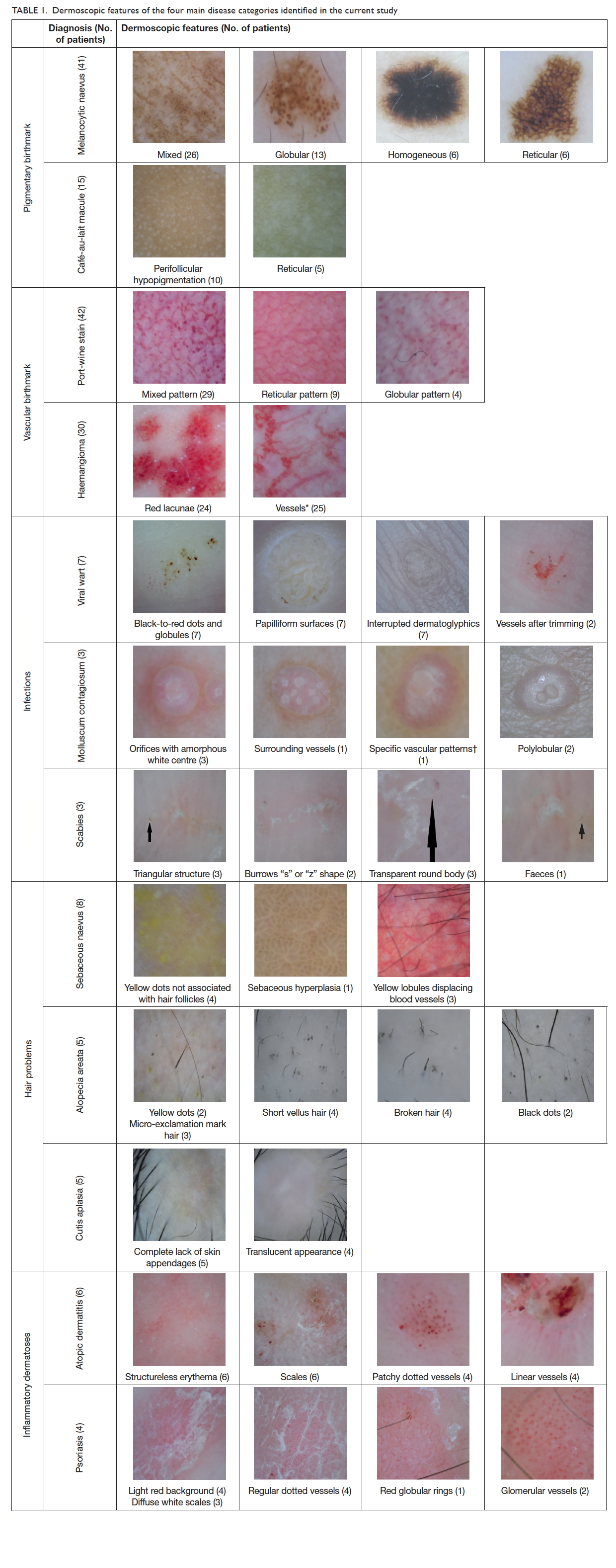
features were identified (Table 1).
In the pigmentary birthmark category, there were
41 children with 51 melanocytic naevi (mean age, 7.3
years). The most common dermoscopic pattern of
melanocytic naevus was mixed, followed by globular,
homogeneous, and reticular. In the mixed pattern
naevi, globular-homogeneous was the commonest
(n=13), followed by globular-reticular-homogeneous
(n=6), reticular-homogeneous (n=4), and globular-reticular
(n=3). There were 15 children with CALM
(mean age, 3.5 years). All 10 children with facial
CALM showed a homogeneous brown patch with
perifollicular hypopigmentation. The five children
with CALM on neck showed a reticular pattern.
In the vascular birthmark category, there were
42 children with port-wine stains (mean age, 6.5
years) and 30 infants with infantile haemangiomas (mean
age, 6 months). For those with port-wine stains,
both globular (n=4) and reticular (n=9) vascular
patterns were identified but the most common
dermoscopic pattern was mixed pattern (n=29) with
both globular and reticular components. Among the
30 infantile haemangiomas, 25 had vessels of various
morphologies, and red lacunae were noted in 24.
None of the haemangiomas had melanocytic pattern.
In the infectious diseases category, there were
seven children with viral warts on hands or feet (mean
age, 10.5 years). Thrombosed capillaries presented
as black-to-red dots, and papilliform surfaces and
interrupted skin lines were identified in all cases. All
three children with molluscum contagiosum (mean
age, 5 years) showed orifices but vessels and specific
vascular patterns could be found in only one case.
In the three cases of scabies (mean age, 12.5 years),
triangular head, transparent body, and burrows were
present.
Patients with patchy alopecia were identified in
the hair category. Eight patients had sebaceous naevus
(mean age, 8.4 years), with four having yellow dots
not associated with hair follicles, three having yellow
lobules displacing blood vessels, and one having
sebaceous hyperplasia. In the five patients with
alopecia areata (mean age, 12 years), dermoscopic
features including yellow dots (n=2), black dots
(n=2), short vellus hair (n=4), broken hair (n=4), and
micro-exclamation mark hair (n=3) were noted. Five
patients with cutis aplasia (mean age, 3 years) were
featured by a complete lack of skin appendages (n=5)
and translucent appearance (n=4).
There were six patients with atopic dermatitis
(mean age, 6.5 years) and four patients with psoriasis
(mean age, 11 years) in the inflammatory dermatoses
category. Atopic eczema was featured by structureless
erythema (n=6), scales (n=6), and patchy dotted
vessels (n=4) while the psoriasis patients had light
red background (n=4), diffuse white scales (n=3),
regular dotted vessels (n=4), and glomerular vessels
(n=2).
Discussion
Our study documented the dermoscopic findings
of common paediatric skin conditions in Chinese
children. In the analysis of the dermoscopy images using
a two-step algorithm, the first step was differentiation
between melanocytic and non-melanocytic lesions.
This study identified typical melanocytic patterns
(globular and reticular pattern21) in melanocytic
naevi, and absence of melanocytic patterns in all
haemangiomas. This two-step algorithm analysis
of skin lesions confirmed the findings on clinical
inspection and provided a standard approach to
dermoscopic examination even in difficult cases.
Birthmarks are very common in children.
Salmon patches occur in half of the neonates22 23 and infantile haemangiomas in one tenth of premature babies,
while the prevalence of capillary malformations
(port-wine stain) has been reported to be 0.3% to
2.1%.24 25 Within the vascular birthmark category,
both port-wine stains and haemangiomas could
present as neonatal erythematous patches. As
lacunae pattern was commonly identified in
haemangiomas but not in port-wine stains, it may
serve to differentiate haemangiomas from port-wine
stains. An early diagnosis of haemangiomas facilitates
timely management as some may rapidly proliferate
or develop complications in the first few months of
life. In our series, the majority of the port-wine stain
lesions showed a mixed pattern with both globular
and reticular components (n=29/42) while reticular
(n=9/42) and globular (n=4/42) patterns were less
common. The ectatic capillary plexus was situated
deeper in the dermis in those with a reticular pattern
than those with a globular pattern; this difference
may have treatment and prognostic implications on response to laser treatment.13 As such,
laser treatment strategy aiming at the deeper dermal
layer would be required to improve treatment
results.
In the pigmentary birthmark category, both
congenital and acquired melanocytic naevi were
included. The common dermoscopic patterns
of globular, reticular, homogeneous, and mixed
reported in our series were in line with those
reported in the western medical literature.26 27 28 29 As
dermoscopy improves the detection of melanomas,30
its use was suggested in the monitoring of congenital
melanocytic naevi (CMN), especially the smaller
CMN.31 32 33 Sequential digital dermoscopy imaging
can also reduce the unnecessary excision of
suspicious pigmented skin lesions.34 This has been
emphasised in children with epidermolysis bullosa
who are at risk of developing skin cancers, and in
whom overtreatment of the fragile skin should be avoided.35
This is the first report of dermoscopic features
of CALM in medical literature. It was noted that the
dermoscopic patterns of CALM might vary according
to the location on the body. All the 10 cases of facial
CALM showed homogeneous brown patches with
perifollicular hypopigmentation while the five cases
of CALM on the neck had a faint brown reticular
pattern. As it may be difficult to differentiate CALM
from CMN in infancy by inspection,36 dermoscopy
provides a quick and non-invasive diagnostic tool to
guide subsequent management.
In the infectious disease category, all viral
warts were on the hands or feet, and all of them
showed the classical features of thrombosed
capillaries present as black-to-red dots and
globules on papilliform surfaces with interrupted
skin lines. These findings were consistent with
features reported in the medical literature.37 The
confirmation of the diagnosis of viral wart before
initiating treatment is important because acral
melanoma, which is more common in Chinese, has
been reported to be misdiagnosed as viral wart with
disastrous consequences.38 39 40 Moreover, dermoscopy
could help guide treatment by identifying residual
warty structures or confirming complete resolution
of warts.37
In our series, there were three children with
scabies who had either the classical dermoscopic
sign of ‘triangular structure’41 or the round bodies42 43
of the scabies mite. The “z”- or “s”-shaped burrows
were also well depicted on dermoscopy in two
of them. Dermoscopy is a simple, accurate, and
rapid44 technique for diagnosing scabies even in
inexperienced hands.45 In a study involving 756
patients, dermoscopic examination for scabies was
found to be 91% sensitive and 86% specific.45 It
greatly enhances treatment decisions45 and allows
fast introduction of proper treatment.42 Diagnosing
scabies in children by dermoscopy is child-friendly
as it requires no skin scrappings, thus, causing no
fear or pain.42 In addition, demonstration of scabies
mite to patient may foster treatment adherence in
both patients and asymptomatic family members.44
Molluscum contagiosum is a common skin
infection in children46 and is highly contagious47
with outbreaks reported.48 In our three children with
molluscum contagiosum, the reported dermoscopic
features included orifices with amorphous white
centre and polylobular appearance surrounded
by vessels.49 50 When the typical clinical features
of molluscum contagiosum are not apparent,
dermoscopy can be helpful for diagnosis.51
Three common causes of patchy alopecia in
children were reported in this study. For neonates
or infants with congenital patchy alopecia, the
differentiation between sebaceous naevus and
cutis aplasia may be difficult.52 In our study, the
dermoscopic features of sebaceous naevi with yellow
dots unassociated with hair follicles, sebaceous
hyperplasia, and yellow lobules displacing blood
vessels53 were demonstrated. On the other hand, cutis
aplasia showed a complete lack of skin appendages
and skin translucency.52 While no specific treatment
is usually needed for cutis aplasia, surgical excision
of sebaceous naevi is often advised with its potential
for developing into basal cell carcinoma.54
The lifetime risk of alopecia areata in the
general population is approximately 1.7% and as
many as 60% of patients with alopecia areata have
disease onset before 20 years of age.55 The clinical
features of hair loss vary with clinical subtypes.56 In
our series of five children with alopecia areata, black
dots, yellow dots, short vellus hair, broken hair, and
micro-exclamation mark hairs were noted. These
dermoscopic features may be useful clinical indicators
in alopecia areata which have both diagnostic and
prognostic values.57 58
Concerning the inflammatory dermatoses category, clinical similarities exist between atopic
dermatitis and psoriasis as both are chronic pruritic
scaly erythematous skin conditions. It is known
that characteristic dermoscopic vascular patterns
facilitate differentiation of psoriasis from atopic
dermatitis.59 In our study, the patchy dotted vessels60
and linear vessels of atopic dermatitis could be
differentiated from the red globular rings61 and
glomerular vessels62 of psoriasis.
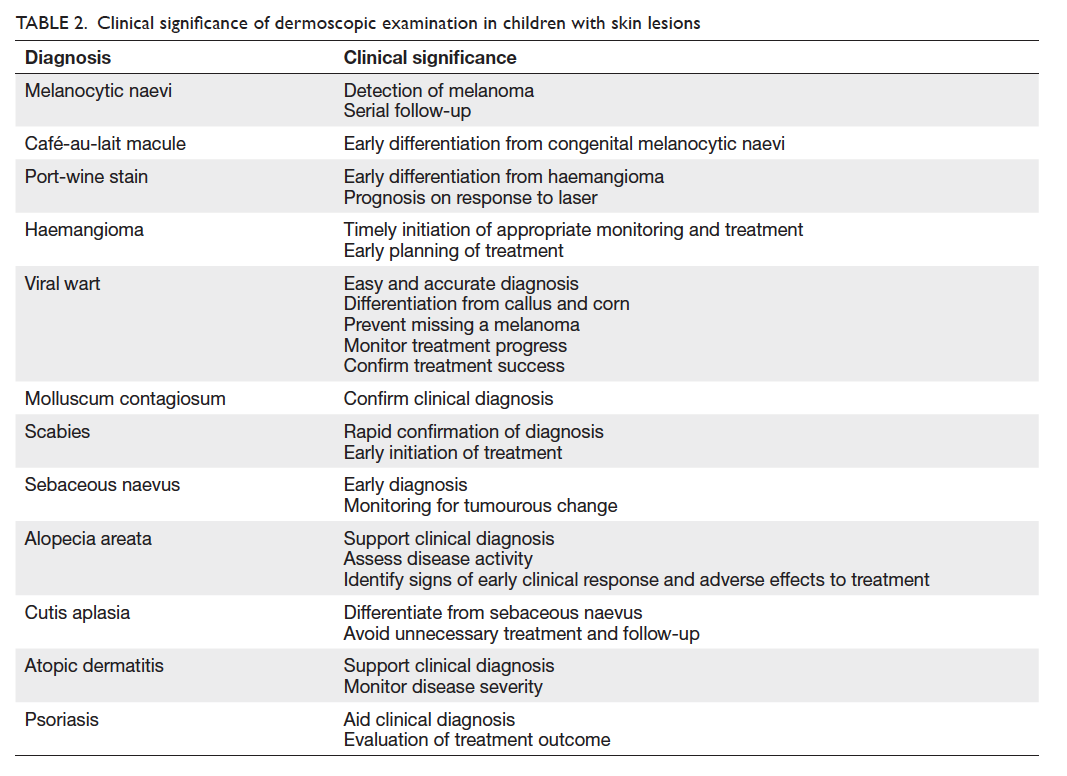
The clinical significance of dermoscopy in
children’s skin conditions is summarised in Table 2.
Although various dermoscopic features of
skin problems could be identified in this study,
it had several limitations. First, the dermoscopy
database only contained images captured during
routine clinical service when the clinical features were classical of their respective diagnosis. As such, the database was
not representative of all common skin problems in
children. In addition, with the small case numbers
for some of the diseases such as atopic dermatitis
and psoriasis, further research is required to confirm
our preliminary findings. Moreover, features of skin
diseases in children are age-dependent and phase-dependent
but these factors were not evaluated in
the present study.
Conclusion
Dermoscopy is a well-established skin examination
tool with known dermoscopic features for many diagnoses.
Our study confirmed that the dermoscopic features
reported in the medical literature could be identified
in Chinese children. While the value of dermoscopy
in diagnostic, prognostic, and disease monitoring
is being unveiled, further studies are required to
understand its role in various
paediatric skin diseases.
Acknowledgements
We would like to thank Ms Carol YB Liu and Mr Bryan MK So of Hong Kong
Productivity Council and Hong Kong Innovation
and Technology Fund for the support on the dermoscopy device for this study.
Declaration
David CK Luk acted as advisor to Hong Kong
Productivity Council on the development of
dermoscope prototype. No conflicts of interests
were declared by authors.
References
1. Ng BC, San CY, Lau EY, Yu SC, Burd A. Multidisciplinary
vascular malformations clinic in Hong Kong. Hong Kong
Med J 2013;19:116-23.
2. Hon KL, Leung TF, Cheung HM, Chan PK. Neonatal
herpes: what lessons to learn. Hong Kong Med J 2012;18:60-2.
3. Koehler MJ, Lange-Asschenfeldt S, Kaatz M. Non-invasive
imaging techniques in the diagnosis of skin diseases.
Expert Opin Med Diagn 2011;5:425-40. CrossRef
4. Sharif SA, Taydas E, Mazhar A, et al. Noninvasive clinical
assessment of port-wine stain birthmarks using current
and future optical imaging technology: a review. Br J
Dermatol 2012;167:1215-23. CrossRef
5. Jaimes N, Dusza SW, Quigley EA, et al. Influence of time
on dermoscopic diagnosis and management. Australas J
Dermatol 2013;54:96-104. CrossRef
6. Venugopal SS, Soyer HP, Menzies SW. Results of
a nationwide dermoscopy survey investigating the
prevalence, advantages and disadvantages of dermoscopy
use among Australian dermatologists. Australas J Dermatol
2011;52:14-8. CrossRef
7. Engasser HC, Warshaw EM. Dermatoscopy use by US
dermatologists: a cross-sectional survey. J Am Acad
Dermatol 2010;63:412-9, 419.e1-2.
8. Tasli L, Kaçar N, Argenziano G. A scientometric analysis
of dermoscopy literature over the past 25 years. J Eur Acad
Dermatol Venereol 2012;26:1142-8. CrossRef
9. Inoue Y, Menzies SW, Fukushima S, et al. Dots/globules on
dermoscopy in nail-apparatus melanoma. Int J Dermatol
2014;53:88-92. CrossRef
10. Argenziano G, Catricalà C, Ardigo M, et al. Dermoscopy
of patients with multiple nevi: improved management
recommendations using a comparative diagnostic
approach. Arch Dermatol 2011;147:46-9. CrossRef
11. van der Rhee JI, Bergman W, Kukutsch NA. The impact
of dermoscopy on the management of pigmented lesions
in everyday clinical practice of general dermatologists: a
prospective study. Br J Dermatol 2010;162:563-7. CrossRef
12. Haliasos EC, Kerner M, Jaimes-Lopez N, et al. Dermoscopy
for the pediatric dermatologist part I: dermoscopy of
pediatric infectious and inflammatory skin lesions and hair
disorders. Pediatr Dermatol 2013;30:163-71. CrossRef
13. Haliasos EC, Kerner M, Jaimes N, et al. Dermoscopy for
the pediatric dermatologist, part ii: dermoscopy of genetic
syndromes with cutaneous manifestations and pediatric
vascular lesions. Pediatr Dermatol 2013;30:172-81. CrossRef
14. Liebman TN, Goulart JM, Soriano R, et al. Effect of
dermoscopy education on the ability of medical students
to detect skin cancer. Arch Dermatol 2012;148:1016-22. CrossRef
15. Herschorn A. Dermoscopy for melanoma detection in
family practice [in English, French]. Can Fam Physician
2012;58:740-5, e372-8.
16. Chen LL, Liebman TN, Soriano RP, Dusza SW, Halpern
AC, Marghoob AA. One-year follow-up of dermoscopy
education on the ability of medical students to detect skin
cancer. Dermatology 2013;226:267-73. CrossRef
17. Ye Y, Zhao Y, Gong Y, et al. Non-scarring patchy alopecia
in patients with systemic lupus erythematosus differs from
that of alopecia areata. Lupus 2013;22:1439-45. CrossRef
18. Tan C, Min ZS, Xue Y, Zhu WY. Spectrum of dermoscopic
patterns in lichen planus: a case series from China. J Cutan
Med Surg 2014;18:28-32.
19. Chan G, Ho H. A study of dermoscopic features of
pigmented basal cell carcinoma in Hong Kong Chinese.
Hong Kong J Dermatol Venereol 2008;16:189-96.
20. de Moura LH, Duque-Estrada B, Abraham LS, Barcaui CB,
Sodre CT. Dermoscopy findings of alopecia areata in an
African-American patient. J Dermatol Case Rep 2008;2:52-4. CrossRef
21. Fortina AB, Zattra E, Bernardini B, Alaibac M, Peserico
A. Dermoscopic changes in melanocytic naevi in children
during digital follow-up. Acta Derm Venereol 2012;92:427-9. CrossRef
22. Boon LM, Enjolras O, Mulliken JB. Vascular malformations.
In: Irvine AD, Hoeger PH, Yan AC, editors. Harper’s
textbook of pediatric dermatology. 3rd ed. Oxford: Wiley-Blackwell; 2011: 112.1-112.24. CrossRef
23. Leung AK, Barankin B, Hon KL. Persistent salmon patch
on the forehead and glabellum in a Chinese adult. Case Rep
Med 2014;2014:139174.
24. Jacobs AH, Walton RG. The incidence of birthmarks in the
neonate. Pediatrics 1976;58:218-22.
25. Hidano A, Purwoko R, Jitsukawa K. Statistical survey of
skin changes in Japanese neonates. Pediatr Dermatol
1986;3:140-4. CrossRef
26. Aguilera P, Puig S, Guilabert A, et al. Prevalence study
of nevi in children from Barcelona. Dermoscopy,
constitutional and environmental factors. Dermatology
2009;218:203-14. CrossRef
27. Belloni Fortina A, Zattra E, Romano I, Bernardini B,
Alaibac M. Clinical and dermoscopic features of nevi in
preschool children in Padua. Dermatology 2010;220:53;
author reply 54. CrossRef
28. Scope A, Dusza SW, Marghoob AA, et al. Clinical and
dermoscopic stability and volatility of melanocytic nevi
in a population-based cohort of children in Framingham
school system. J Invest Dermatol 2011;131:1615-21. CrossRef
29. Zalaudek I, Schmid K, Marghoob AA, et al. Frequency of
dermoscopic nevus subtypes by age and body site: a cross-sectional
study. Arch Dermatol 2011;147:663-70. CrossRef
30. Haliasos HC, Zalaudek I, Malvehy J, et al. Dermoscopy
of benign and malignant neoplasms in the pediatric
population. Semin Cutan Med Surg 2010;29:218-31. CrossRef
31. Nehal KS, Oliveria SA, Marghoob AA, et al. Use of and
beliefs about dermoscopy in the management of patients
with pigmented lesions: a survey of dermatology residency
programmes in the United States. Melanoma Res
2002;12:601-5. CrossRef
32. Marghoob AA. Congenital melanocytic nevi. In: Marghoob
AA, Malvehy J, Braun RP, editors. Atlas of dermoscopy. London: Informa Healthcare; 2012: 147-58.
33. Rocha CR, Grazziotin TC, Rey MC, Luzzatto L, Bonamigo
RR. Congenital agminated melanocytic nevus—case
report. An Bras Dermatol 2013;88(6 Suppl 1):170-2. CrossRef
34. Gulia A, Massone C. Advances in dermoscopy for detecting
melanocytic lesions. F1000 Med Rep 2012;4:11.
35. de Queiroz Fuscaldi LA, Buçard AM, Alvarez CD, Barcaui
CB. Epidermolysis bullosa nevi: report of a case and review
of the literature. Case Rep Dermatol 2011;3:235-9. CrossRef
36. Bishop JA. Melanocytic naevi and melanoma. In: Irvine
AD, Hoeger PH, Yan AC, editors. Harper’s textbook of
pediatric dermatology. 3rd ed. Oxford: Wiley-Blackwell;
2011: 109.1-109.28. CrossRef
37. Bae JM, Kang H, Kim HO, Park YM. Differential diagnosis
of plantar wart from corn, callus and healed wart with the
aid of dermoscopy. Br J Dermatol 2009;160:220-2. CrossRef
38. Dalmau J, Abellaneda C, Puig S, Zaballos P, Malvehy J. Acral
melanoma simulating warts: dermoscopic clues to prevent
missing a melanoma. Dermatol Surg 2006;32:1072-8. CrossRef
39. Rosen T. Acral lentiginous melanoma misdiagnosed
as verruca plantaris: a case report. Dermatol Online J
2006;12:3.
40. Burd A, Bhat S. An update on the management of
cutaneous melanoma. Hong Kong J Dermatol Venereol
2008;16:143-8.
41. Prins C, Stucki L, French L, Saurat JH, Braun RP.
Dermoscopy for the in vivo detection of sarcoptes scabiei.
Dermatology 2004;208:241-3. CrossRef
42. Kamińska-Winciorek G. Entomodermoscopy in scabies—is it a safe and friendly screening test for scabies in children?
Acta Dermatovenerol Croat 2012;20:117-9.
43. Executive Committee of Guideline for the Diagnosis, Ishii
N. Guideline for the diagnosis and treatment of scabies in
Japan (second edition). J Dermatol 2008;35:378-93.
44. Fox G. Diagnosis of scabies by dermoscopy. BMJ Case Rep
2009;2009.
45. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard
dermoscopy for diagnosing scabies. J Am Acad Dermatol
2007;56:53-62. CrossRef
46. Netchiporouk E, Cohen BA. Recognizing and managing
eczematous id reactions to molluscum contagiosum virus
in children. Pediatrics 2012;129:e1072-5. CrossRef
47. Marsal JR, Cruz I, Teixido C, et al. Efficacy and tolerance
of the topical application of potassium hydroxide (10%
and 15%) in the treatment of molluscum contagiosum:
randomized clinical trial: research protocol. BMC Infect
Dis 2011;11:278. CrossRef
48. Oren B, Wende SO. An outbreak of molluscum
contagiosum in a kibbutz. Infection 1991;19:159-61. CrossRef
49. Zaballos P, Ara M, Puig S, Malvehy J. Dermoscopy of
molluscum contagiosum: a useful tool for clinical diagnosis
in adulthood. J Eur Acad Dermatol Venereol 2006;20:482-3. CrossRef
50. Morales A, Puig S, Malvehy J, Zaballos P. Dermoscopy of
molluscum contagiosum. Arch Dermatol 2005;141:1644. CrossRef
51. Mun JH, Ko HC, Kim BS, Kim MB. Dermoscopy of giant
molluscum contagiosum. J Am Acad Dermatol 2013;69:
e287-8. CrossRef
52. Neri I, Savoia F, Giacomini F, Raone B, Aprile S, Patrizi
A. Usefulness of dermatoscopy for the early diagnosis of
sebaceous naevus and differentiation from aplasia cutis
congenita. Clin Exp Dermatol 2009;34:e50-2. CrossRef
53. Kim NH, Zell DS, Kolm I, Oliviero M, Rabinovitz HS. The
dermoscopic differential diagnosis of yellow lobularlike
structures. Arch Dermatol 2008;144:962. CrossRef
54. Bomsztyk ED, Garzon MC, Ascherman JA. Postauricular
cerebriform sebaceous nevus: case report and literature
review. Ann Plast Surg 2008;61:637-9. CrossRef
55. Hon KL, Leung AK. Alopecia areata. Recent Pat Inflamm
Allergy Drug Discov 2011;5:98-107. CrossRef
56. Finner AM. Alopecia areata: clinical presentation,
diagnosis, and unusual cases. Dermatol Ther 2011;24:348-54. CrossRef
57. Rudnicka L, Rakowska A, Olszewska M. Trichoscopy: how
it may help the clinician. Dermatol Clin 2013;31:29-41.
58. Inui S, Nakajima T, Nakagawa K, Itami S. Clinical
significance of dermoscopy in alopecia areata: analysis of
300 cases. Int J Dermatol 2008;47:688-93. CrossRef
59. Lallas A, Apalla Z, Tzellos T, Lefaki I. Photoletter to the
editor: dermoscopy in clinically atypical psoriasis. J
Dermatol Case Rep 2012;6:61-2. CrossRef
60. Lallas A, Kyrgidis A, Tzellos TG. Accuracy of dermoscopic
criteria for the diagnosis of psoriasis, dermatitis, lichen
planus and pityriasis rosea. Br J Dermatol 2012;166:1198-205. CrossRef
61. Vázquez-López F, Zaballos P, Fueyo-Casado A, Sánchez-Martín J. A dermoscopy subpattern of plaque-type
psoriasis: red globular rings. Arch Dermatol 2007;143:1612. CrossRef
62. Micali G, Lacarrubba F, Musumeci ML, Massimino D,
Nasca MR. Cutaneous vascular patterns in psoriasis. Int J
Dermatol 2010;49:249-56. CrossRef