Hong Kong Med J 2014;20:205–12 | Number 3, June 2014 | Epub 30 Jan 2014
DOI: 10.12809/hkmj134080
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Ibuprofen versus indomethacin treatment of
patent ductus arteriosus: comparative effectiveness and
complications
NM Chan, MRCPCH, FHKAM (Paediatrics); CW
Law, MB, BS, FHKAM (Paediatrics); KF Kwan, FHKAM (Paediatrics)
Department of Paediatrics, Queen Elizabeth
Hospital, 30 Gascoigne Road, Kowloon, Hong Kong
Corresponding author: Dr NM Chan (cnm312@ha.org.hk)
Abstract
Objectives: To compare
the effectiveness and complications of intravenous ibuprofen
versus indomethacin treatment of patent ductus arteriosus in
preterm infants.
Design: Retrospective
case series.
Setting: A tertiary
referral centre in Hong Kong.
Patients: A total of 95
infants who had received at least one course of indomethacin or
ibuprofen for closure of patent ductus arteriosus from January
2008 to December 2011 were studied.
Main outcome measures: Following
the total switch from indomethacin to ibuprofen in clinical use
in April 2010, outcomes of infants receiving indomethacin and
ibuprofen were compared. The primary outcomes including rates of
failed medical closure and recourse to surgical ligation were
compared. The secondary outcomes including rates of all-cause
mortality, bronchopulmonary dysplasia, intestinal complications
(necrotising enterocolitis, spontaneous intestinal perforation),
change in urine output and serum creatinine, and progression of
any intraventricular haemorrhage were also evaluated.
Results: The failure
rate of medical treatment was similar in the indomethacin and
ibuprofen groups, with 16 (31%) such infants in the indomethacin
group and 14 (33%) in the ibuprofen group; for ibuprofen this
yielded a relative risk of 1.06 (95% confidence interval, 0.66-1.67; P=0.852). The proportion of infants having surgical
ligation was also similar. A higher rate of intestinal
complications (necrotising enterocolitis or spontaneous
intestinal perforation) was encountered in our ibuprofen group
(P=0.043). No significant difference was observed in other
secondary outcomes determined.
Conclusion: In our
clinical practice, ibuprofen and indomethacin were shown to be
equally effective for medical closure of patent ductus
arteriosus in premature infants. With the higher rates of
intestinal complications and similar effects on renal function
in the ibuprofen group, we conclude that ibuprofen may not have
fewer adverse effects than indomethacin.
New knowledge added by this
study
- Ibuprofen was shown to be as effective as indomethacin for the medical closure of patent ductus arteriosus in premature infants in clinical practice in Hong Kong.
- Ibuprofen may not have fewer adverse effects than indomethacin, as it was associated with higher rates of intestinal complications and similar effects on renal function.
- Close monitoring for adverse effects is recommended in infants with patent ductus arteriosus treated with either indomethacin or ibuprofen.
Introduction
Patent ductus arteriosus (PDA) is a common
problem in preterm infants. Its occurrence is associated with
prematurity and respiratory distress syndrome (RDS).1 2 A
persistent left to right shunt in preterm neonates may be
associated with neonatal morbidities, including bronchopulmonary
dysplasia (BPD), intraventricular haemorrhage (IVH), and
necrotising enterocolitis (NEC).3
Pharmacological closure of PDAs with
indomethacin was first described in 1970s.4 Reported complications associated with the use
of indomethacin included renal impairment,5 NEC, spontaneous intestinal perforation,6 and impaired cerebral blood flow.7 Ibuprofen, another cyclo-oxygenase inhibitor,
has been investigated as an alternative to indomethacin for the
same purpose. Published randomised controlled trials reported that
ibuprofen was as efficacious as indomethacin for PDA closure, and
some studies claimed that it had fewer adverse effects and gave
rise to less renal impairment than indomethacin.8 9 10 11
The use of ibuprofen for closure of PDA has
been increasing in clinical practice worldwide. In Hong Kong,
indomethacin has been replaced by ibuprofen since 2010 due to
interruption of the supply of indomethacin from the pharmaceutical
company. Local data on its effectiveness and safety in clinical
practice are very limited. A study comparing the use of ibuprofen
versus indomethacin for this purpose could provide valuable data
for clinicians regarding their use in clinical practice. At our
unit, intravenous indomethacin had been used for treatment of PDA
in preterm infants until April 2010. After that date, ibuprofen
was used due to cessation of the supply of indomethacin from the
pharmaceutical company supplying our hospital. We therefore set
out to compare the two infant cohorts for treatment effectiveness
and complications when used in our local setting.
Methods
Patients and study design
This retrospective study was conducted in
the neonatal intensive care unit (NICU) of Queen Elizabeth
Hospital, a tertiary referral centre in Hong Kong with a level III
neonatal intensive care service. The subjects in this study were
all preterm infants admitted to the unit with their date of birth
from 1 January 2008 to 31 December 2011 inclusive, and who had
received at least one course of medical treatment for closure of a
PDA with either indomethacin or ibuprofen. Due to the total switch
from indomethacin to ibuprofen in clinical practice for this
purpose in April 2010, we had information on two groups of
infants—the indomethacin cohort (date of birth from 1 January 2008
to April 2010) and the ibuprofen cohort (date of birth from l
April 2010 to 31 December 2011).
Preterm infants were defined as those who
were born with less than 37 weeks of gestation. In our unit,
preterm infants with clinical features suggestive of PDA, namely
heart murmur, hypotension, hyperactive precordium, and increased
ventilator settings were assessed by paediatric cardiologists. The
diagnosis was then confirmed by echocardiography. Infants with a
haemodynamically significant PDA were evaluated for medical
closure with indomethacin/ibuprofen. Corresponding infants with
features of heart failure, hypotension or who were
ventilator-dependent were considered to have a haemodynamically
significant PDA. Baseline assessments of these patients included
platelet count, serum creatinine and electrolytes levels, urine output,
and cranial ultrasound. Common contra-indications for the receipt
of indomethacin/ibuprofen included thrombocytopenia, bleeding
tendency, progressing IVH, NEC, and impaired renal function.
Indomethacin was given at 0.1 mg/kg intravenously at 24-hour
intervals for six doses or 0.2 mg/kg intravenously every 24 hours
for three doses. Ibuprofen was given at 10 mg/kg, 5 mg/kg, and 5
mg/kg intravenously every 24 hours for a total of three doses.
During the treatment courses, the infants were monitored for
potential drug side-effects. Enteral feeding was withheld during
the treatment course. Ductal closure was defined as persistent
disappearance of the heart murmur; some of whom also had
echocardiographic confirmation. Infants who failed the first
course of medical treatment were re-evaluated and received a
second course. Infants who failed two courses of medical treatment
were considered for surgical ligation of the PDA in another
tertiary referral centre in Hong Kong. Apart from the switch from
indomethacin to ibuprofen in April 2010, the clinical practice for
PDA management remained unchanged.
Data collection
Eligible infants were identified by the
Clinical Data Analysis and Reporting System, and their medical
records were retrieved for data extraction. The neonatal
demographic variables and baseline characteristics of both groups
were collected and compared. The effectiveness of the drugs was
primarily measured by (1) the failure rate of PDA closure after
medical treatment, and (2) rate of recourse to surgical ligation.
Secondary outcomes included all-cause mortality before discharge,
BPD, adverse effects on renal function, gastro-intestinal
complications (NEC and spontaneous intestinal perforation), and
IVH. Occurrence of BPD was defined as (1) the use of supplement
oxygen at 28 days of life or (2) the use of supplement oxygen at
36 weeks’ postmenstrual age. Adverse effects on renal function
were inferred by the magnitude of any serum creatinine and/or
urine output change. Necrotising enterocolitis was diagnosed and
classified according to modified Bell’s staging.12 Intraventricular haemorrhage was classified
according to the standard grading system.13
Statistical analyses
The two groups of infants receiving
indomethacin or ibuprofen were compared using independent sample t
tests for continuous normally distributed data, while the Wilcoxon
rank-sum test was used for continuous non-normal data. Chi squared
and Fisher’s exact tests were used as appropriate for categorical
variables. The relative risks (RRs) of the outcome measures
between the two groups were determined. The extent of change in
urine output and serum creatinine level during the treatment
course (within-subject effect) and the difference in change
between the two groups (between-subject effect) were analysed by
repeated measures analysis of variance. Potential confounding
factors for medical closure of the PDA,8
including gender, gestational age, RDS grading, PDA ductal
diameter, and day of starting treatment were evaluated by logistic
regression. Significant factors were then entered into a
multivariate logistic regression model to determine adjusted odds
ratios. In all the analyses, a P value of less than 0.05 was
considered significant. The statistical analysis was performed
using the Statistical Package for the Social Sciences (Windows
version 16.0; SPSS Inc, Chicago [IL], US). The sample size
estimation was based on the primary outcome measure: the
difference in proportion of infants with medical closure between
the two groups. The sample size calculation for a moderate effect
size of 0.3, power of 80%, and an alpha of 0.05 indicated that
around 40 subjects were needed in each group.
This study was approved by the Research
Ethics Committee, Kowloon Central Cluster, Hospital Authority.
Results
Baseline characteristics
In all, 96 infants had medical treatment
for closure of a PDA during the study period; 52 (55%) received
indomethacin only and 43 (45%) received ibuprofen only. One
infant, who received both indomethacin and ibuprofen during the
transitional period, was excluded. There were no significant
differences in the demographic variables and baseline
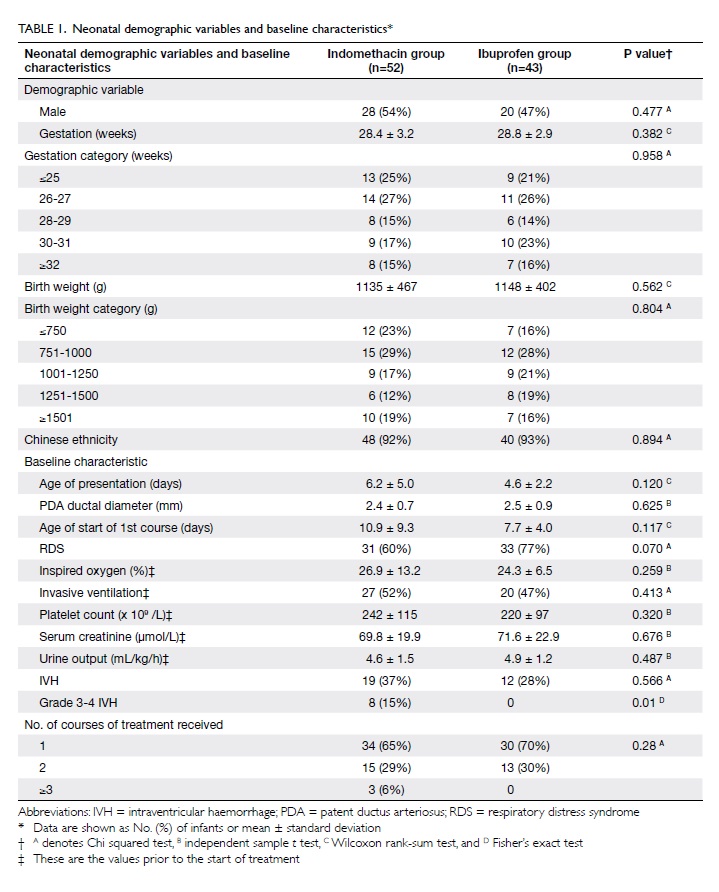
characteristics of the two groups (Table 1), except for a higher proportion
with severe IVH (grades 3 and 4) in the indomethacin group
(P=0.01). There was no significant difference between these groups
with respect to the number of infants receiving one or two courses
of treatment (Table 1).
Primary outcomes
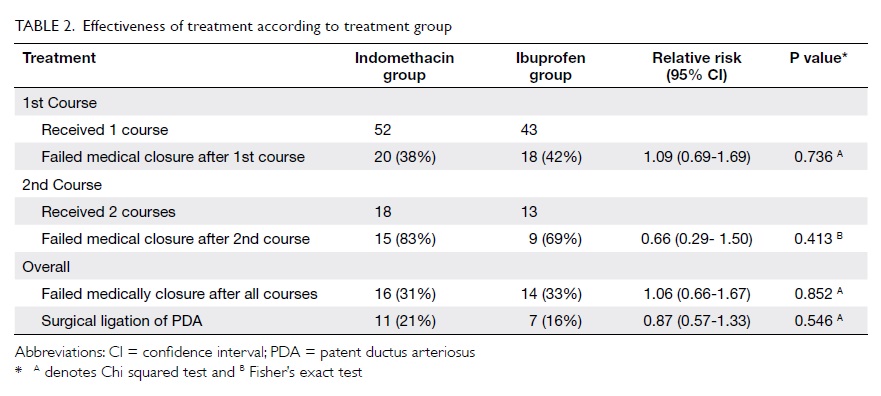
Regarding the effectiveness of treatment,
20 (38%) of the infants in the indomethacin group and 18 (42%) in
the ibuprofen group failed medical treatment after the first
course; the RR of failure for the latter compared to indomethacin
was 1.09, the 95% confidence interval (CI) being 0.69 to 1.69 (Table 2). Considering all courses of
treatment with indomethacin or ibuprofen, 16 (31%) in the former
group and 14 (33%) in the latter group failed medical treatment.
Eleven (21%) infants in the indomethacin group and seven (16%) in the
ibuprofen group underwent surgical ligation of the PDA. For the
primary outcome measure, both groups were very comparable.
Factors with a potential to affect medical
closure of PDA were evaluated. Among them, gestational age, RDS,
and age at the start of medical treatment were shown to be
significantly related to the rate of surgical ligation of PDA,
with borderline significance for age at start of treatment in the
univariate analysis (Table 3). When the above-mentioned
significant factors were used in the multivariate analysis model,
there was no significant difference between the two groups in
terms of the rate of surgical ligation (adjusted odds ratio=0.94;
95% CI, 0.27-3.26; P=0.923).
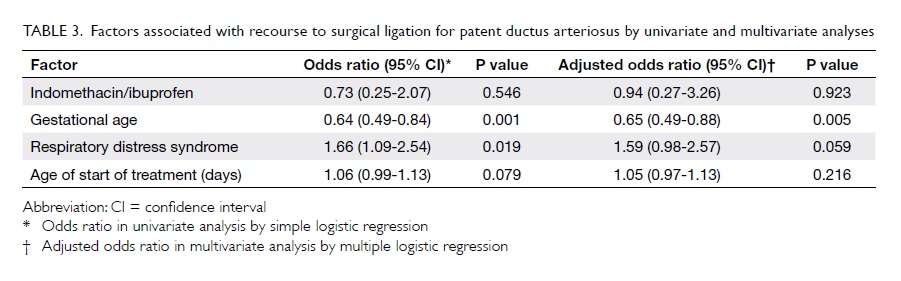
Table 3. Factors associated with recourse to surgical ligation for patent ductus arteriosus by univariate and multivariate analyses
Secondary outcomes
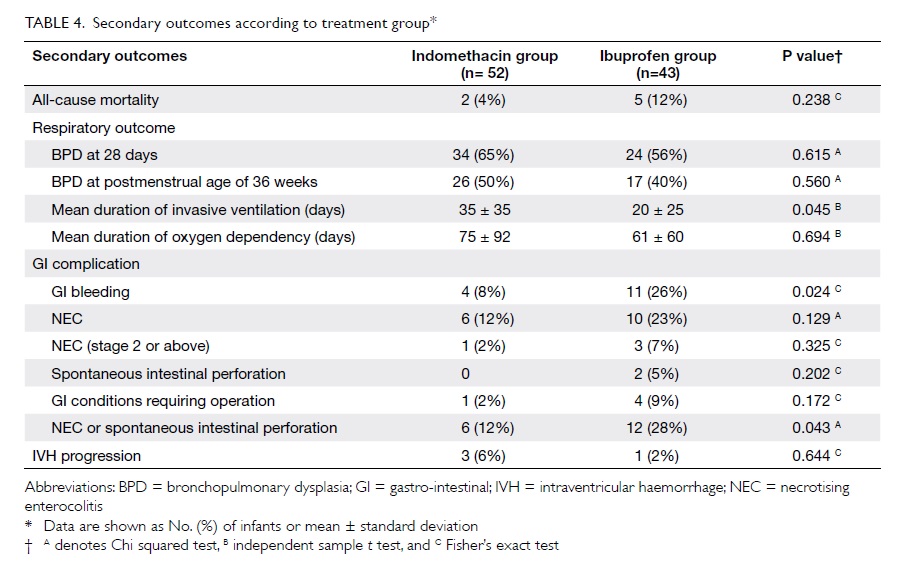
Mortality
Within the study cohort, two (4%) infants
in the indomethacin group and five (12%) in the ibuprofen group
died before being discharged, but this yielded no statistically
significant difference in all-cause mortality.
Respiratory outcomes
The rates of BPD were also similar in both
groups (P=0.615 for use of supplement oxygen at 28 days and
P=0.560 for use of supplement oxygen at 36 weeks’ postmenstrual
age). The mean duration of invasive ventilation for the
indomethacin group, however, was significantly longer than that
for ibuprofen group (mean ± standard deviation, 35 ± 35 vs 20 ± 25
days; P=0.045), while the mean duration of oxygen dependency was
similar (P=0.694; Table 4).
Gastro-intestinal effects
Although not statistically significant,
there was a higher rate of spontaneous intestinal perforation in
the ibuprofen group (5% vs 0%, P=0.202), a higher rate of NEC (23%
vs 12%, P=0.129), and NEC stage 2 or above (7% vs 2%, P=0.325) in
the ibuprofen group (Table 4). On the other hand, on considering
infants with NEC or spontaneous intestinal perforation together,
there was a significantly higher rate in the ibuprofen than
indomethacin group (P=0.043), and the same was true for
gastro-intestinal bleeding (P=0.024).
Renal effects
Mean baseline serum creatinine
concentrations and urine outputs were similar in the two groups (Table 1). Renal function related to the
first course of treatment with indomethacin or ibuprofen was also
studied. For within-subject effects, there were significant
decreases in urine output (P<0.001) and increases in serum
creatinine level (P=0.004) over time during treatment. For
between-subjects effect, there was no significant difference in
the changes of serum creatinine (P=0.829) and urine output
(P=0.498) in the two groups, indicating that both drugs had a
significant and comparable effect on renal function as measured by
serum creatinine level and urine output (Fig).
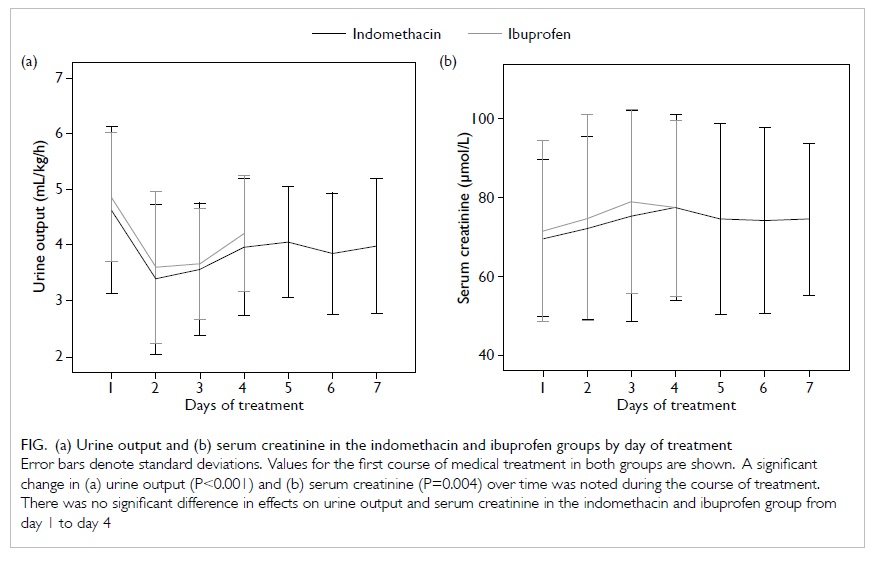
Figure. (a) Urine output and (b) serum creatinine in the indomethacin and ibuprofen groups by day of treatment
Intraventricular haemorrhage
A larger proportion of infants in the
indomethacin group had severe IVH at baseline. However, in both
groups the rates of progression of IVH after treatment were
similar (P=0.644).
Discussion
Our study compared the effectiveness and
side-effects of intravenous indomethacin versus ibuprofen in
treating PDA in preterm infants in two cohorts of Hong Kong
patients. Our results demonstrate no significant difference in
baseline characteristics between the two groups, thus justifying
comparison of the cohorts. The two drugs appear to have similar
effectiveness as measured by the rate of medical closure and
surgical ligation rate of PDAs; such finding was also consistent
with previous randomised controlled trials8 9 10 11and
cohort
studies.14 15 Even after potential confounding factors
(discussed in the previous literature8)
were controlled, the effectiveness of the two drugs did not differ
significantly.
A higher all-cause mortality rate was
observed in the ibuprofen group, although this did not reach
statistical significance. The mortality case analysis was limited
by the small number of deaths in each group; the power calculated
was only 25%. Similar findings were reported by Katakam et al.15 When considering the individual cases, we
observed that two infants in the ibuprofen group might have died
of drug-related complications, namely spontaneous intestinal
perforation and acute renal failure. Although one should not be
biased by individual cases, these deaths illustrate the potential
for fatal complications related to this drug.
We found no significant difference in the
risk of BPD in the two groups; such result was consistent with
that of a recent Cochrane review.16
By contrast another review by Jones et al17
concluded that intravenous ibuprofen may be associated with an
increased risk of BPD when compared with intravenous indomethacin.
These inconsistencies may be related to the definitions of BPD
that were used. Our study considered BPD using the two most
commonly used definitions (supplement oxygen use at 28 days and at
36 weeks' postmenstrual age, separately). Notably, similar rates
of BPD were observed in the two groups for both definitions. By
contrast, the duration of invasive ventilation was significantly
longer in the indomethacin group. In this respect, a possible
explanation and limitation of our study was that there may have
been a gradual change in ventilation strategy over time, with a
trend towards non-invasive ventilation.18
Regarding evaluation of possible
gastrointestinal complications, two conditions (NEC and
spontaneous intestinal perforation) have been described. Both are
believed to be associated with impaired mesenteric blood flow due
to a PDA as well as the use of cyclo-oxygenase inhibitors, though
some recent studies have reported on the difference in clinical
presentations and histological findings between these two
entities.19 20 We observed a statistically higher rate of intestinal
complications (NEC or spontaneous intestinal perforation) in the ibuprofen group
(P=0.043). In contrast, the latest Cochrane
review16 reported less NEC
in the ibuprofen group (RR=0.68; 95% CI, 0.47-0.99). The
management practice of preterm infants in our unit, including the
feeding regimen, remained unchanged during the study period. Thus,
this particular inconsistency could not be attributed to any known
factors. Kushnir and Pinheiro14
studied 350 infants and also reported a higher rate of NEC in
ibuprofen than indomethacin users (8% vs 4%; P=0.08). Rao et al19 studied 102 infants with
PDA treated with ibuprofen, and reported a 9% rate of spontaneous
intestinal perforation and 6% rate of NEC; such figures were
comparable to those in our ibuprofen cohort. These findings
suggest that compared with preterm infants treated with
indomethacin, intestinal complications appear to be more common in
those receiving ibuprofen.
We found that indomethacin and ibuprofen
had a similar effect on renal function, though previous literature
8 9 15 16 17indicated that ibuprofen had less effect on
renal blood flow and renal function. This inconsistency could be
related to differences in how measurement of renal function was
carried out. We evaluated the change in serum creatinine and urine
output during the course of treatment. The change in these
parameters, rather than the absolute values, might be better
parameters to assess due to variations in serum creatinine with
gestational age and the age of the infants.21 Another problem was the timing of
measurements. Akima et al22
evaluated the renal effects of indomethacin and reported a
significant increase in serum creatinine level on day 2 and day 7 of
treatment when compared with the controls. Due to differences in
the duration of treatment courses with the two drugs, the best
time to carry out comparisons remains unclear. Moreover, as
observed in one of our infants given ibuprofen who also developed
acute renal failure 2 days after the completion of second course,
there could be delayed and cumulative effects on renal function
with repeat treatment courses. This was also shown by Kushnir and
Pinheiro,14 whereby
indomethacin had a more prominent effect on renal function during
the first course while both drugs led to equal adversity at the
second and third courses. However, the retrospective design of our
study was a limitation as some data (especially on day 4 and
later) in the ibuprofen group were influenced by the course
lasting only 3 days, whilst data on the repeat courses of
treatment were less complete. Hence, our study evaluated the first
4 days of the first course of treatment, and evaluation of repeat
courses was excluded. With regard to the significant renal effects
of ibuprofen and indomethacin noted in our study, we recommend
close monitoring of renal function when either drug is used.
Special cautions may be necessary for repeat courses of treatment.
Till now, published studies on the efficacy
and safety of ibuprofen versus indomethacin were mainly randomised
trials. The subjects in randomised trials were selected using
inclusion and exclusion criteria that may be less representative
of the whole spectrum of infants in clinical practice. For
instance, randomised trials by Van Overmeire et al8 and Lago et al9
only studied infants with PDA treatment given in the first 2 to 4
days of life and RDS was an inclusion criterion. Our study
included all infants that were treated within the study period,
maximising the representativeness of the sample. Being a
retrospective study to investigate the effectiveness and
complications related to drug therapy in a clinical setting, the
allocation of treatment was not randomised or blinded. However,
selection bias was minimised as the drug treatment each infant
received was only determined by the month and year they were
admitted to the neonatal unit. On the other hand, being a study
from two contiguous time periods, there may have been minor
modifications of clinical practice despite that both infant
cohorts being managed by the same group of clinicians and there
being no change in departmental guidelines for management of PDAs.
Our study shared the limitations of most
previous studies. Our sample size estimation was based on the
primary outcome (the rate of successful medical closure). As the
sample size was limited by the number of eligible infants within
the study period, the effect size adopted in the sample size
estimation was 0.3, which was moderate compared to other similar
studies. Moreover, with respect to adverse outcome evaluation,
infant numbers with positive findings were small, which affected
the precision of our analyses. Another limitation was that two
regimens of the indomethacin were used in our hospital: 0.1
mg/kg/dose every 24 hours for six doses (prolonged course) and 0.2
mg/kg/dose every 24 hours for three doses (short course).
Fortunately, this heterogeneity within the group was small, as the
majority of infants received the prolonged course (46 out of 52).
Moreover, previous studies comparing these two regimens showed
that their efficacy did not differ significantly.23 24
As for the generalisability of our study, variations in management
of symptomatic PDA do exist between centres,25 26
and there is no consensus approach. Our practice, for trial of a
second course of indomethacin or ibuprofen before considering
surgical ligation, entailed intense monitoring for adverse
effects, which was consistent with common practice.23 Thus, our study could provide useful
information for other NICUs to consider for the management of PDA
in preterm infants.
Conclusion
In clinical practice, intravenous ibuprofen
is as effective as indomethacin for the medical closure of PDAs in
premature infants. However, owing to the higher rates of
intestinal complications after ibuprofen therapy, we conclude that
it may not have fewer adverse effects than indomethacin.
Neonatologists are therefore advised to cautiously monitor for
possible side-effects in preterm infants receiving either
indomethacin or ibuprofen for the treatment of PDAs.
Declaration
No conflicts of interests were declared by authors.
References
1. Kitterman JA, Edmunds LH Jr,
Gregory GA, Heymann MA, Tooley WH, Rudolph AM. Patent ductus
arteriosus in premature infants — incidence, relation to pulmonary
disease and management. N Engl J Med 1972;287:473-7. CrossRef
2. Thibeault DW, Emmanouilides GC,
Nelson RJ, Lachman RS, Oh W. Patent ductus arteriosus complicating
the respiratory distress syndrome in preterm infants. J Pediatr
1975;86:120-6. CrossRef
3. Clyman RI, Chorne N. Patent
ductus arteriosus: evidence for and against treatment. J Pediatr
2007;150:216-9. CrossRef
4. Friedman WF, Hirschklau MJ,
Printz MP, Pitlick PT, Kirkpartrick SE. Pharmacologic closure of
patent ductus arteriosus in the premature infant. N Engl J Med
1976;295:526-9. CrossRef
5. Seyberth HW, Rascher W,
Hackenthal R, Wille L. Effect of prolonged indomethacin therapy on
renal function and selected vasoactive hormones in very low birth
weight infants with symptomatic patent ductus arteriosus. J
Pediatr 1983;103:979-84. CrossRef
6. Fujii AM, Brown E, Mirochnick M,
O'Brien S, Kaufman G. Neonatal necrotizing enterocolitis with
intestinal perforation in extremely premature infants receiving
early indomethacin treatment for patent ductus arteriosus. J
Perinatol 2002;22:535-40. CrossRef
7. Edwards AD, Wyatt JS, Richardson
C, et al. Effects of indomethacin on cerebral haemodynamics in
very preterm infants. Lancet 1990;335:1491-5. CrossRef
8. Van Overmeire B, Smets K,
Lecoutere D, et al. A comparison of ibuprofen and indomethacin for
closure of patent ductus arteriosus. N Engl J Med 2000;343:674-81. CrossRef
9. Lago P, Bettiol T, Salvadori S,
et al. Safety and efficacy of ibuprofen versus indomethacin in
preterm infants treated for patent ductus arteriosus: a randomised
controlled trial. Eur J Pediatr 2002;161:202-7. CrossRef
10. Mosca F, Bray M, Lattanzio M,
Fumagalli M, Tosetto C. Comparative evaluation of the effects of
indomethacin and ibuprofen on cerebral perfusion and oxygenation
in preterm infants with patent ductus arteriosus. J Pediatr
1997;131:549-54. CrossRef
11. Su BH, Lin HC, Chiu HY, Hsieh
HY, Chen HH, Tsai YC. Comparison of ibuprofen and indomethacin for
early-targeted treatment of patent ductus arteriosus in extremely
premature infants: a randomized controlled trial. Arch Dis Child
Fetal Neonatal Ed 2008;93:F94-9. CrossRef
12. Kliegman RM, Walsh MC.
Neonatal necrotizing enterocolitis: pathogenesis, classification,
and spectrum of illness. Curr Probl Pediatr 1987;17:213-88. CrossRef
13. Papile LA, Burstein J,
Burstein R, Koffler H. Incidence and evolution of subependymal and
intraventricular hemorrhage: a study of infants with birth weights
less than 1,500 gm. J Pediatr 1978;92:529-34. CrossRef
14. Kushnir A, Pinheiro JM.
Comparison of renal effects of ibuprofen versus indomethacin
during treatment of patent ductus arteriosus in contiguous
historical cohorts. BMC Clin Pharmacol 2011;11:8. CrossRef
15. Katakam LI, Cotton CM,
Goldberg RN, Dang CN, Smith PB. Safety and effectiveness of
indomethacin versus ibuprofen for treatment of the patent ductus
arteriosus. Am J Perinatol 2010;27:425-9. CrossRef
16. Ohlsson A, Walia R, Shah SS.
Ibuprofen for the treatment of patent ductus arteriosus in preterm
and/or low birth weight infants. Cochrane Database Syst Rev
2010;(4):CD003481.
17. Jones LJ, Craven PD, Attia J,
Thakkinstian A, Wright I. Network meta-analysis of indomethacin
versus ibuprofen versus placebo for PDA in preterm infants. Arch
Dis Child Fetal Neonatal Ed 2011;96:F45-52. CrossRef
18. Ramanathan R, Sardesai S. Lung
protective ventilatory strategies in very low birth weight
infants. J Perinatol 2008;28 Suppl 1:S41-6. CrossRef
19. Rao R, Bryowsky K, Mao J,
Bunton D, McPherson C, Mathur A. Gastrointestinal complications
associated with ibuprofen therapy for patent ductus arteriosus. J
Perinatol 2011;31:465-70. CrossRef
20. Attridge JT, Clark R, Walker
MW, Gordon PV. New insights into spontaneous intestinal
perforation using a national data set: (2) two populations of
patients with perforations. J Perinatol 2006;26:185-8. CrossRef
21. Bueva A, Guignard JP. Renal
function in preterm neonates. Pediatr Res 1994;36:572-7. CrossRef
22. Akima S, Kent A, Reynolds GJ,
Gallagher M, Falk MC. Indomethacin and renal impairment in
neonates. Pediatr Nephrol 2004;19:490-3. CrossRef
23. Tammela O, Ojala R, Iivainen
T, et al. Short versus prolonged indomethacin therapy for patent
ductus arteriosus in preterm infants. J Pediatr 1999;134:552-7. CrossRef
24. Siu KL, Wan KM, Law CW, Lee
WH. Indomethacin regimes for patent ductus arteriosus in premature
newborn. HK J Paediatr (New Series) 1997;2:180-1.
25. Amin SB, Handley C,
Carter-Pokras O. Indomethacin use for the management of patent
ductus arteriosus in preterms: a web-based survey of practice
attitudes among neonatal fellowship program directors in the
United States. Pediatr Cardiol 2007;28:193-200. CrossRef
26. Brissaud O, Guichoux J. Patent
ductus arteriosus in the preterm infant: a survey of clinical
practices in French neonatal intensive care units. Pediatr Cardiol
2011;32:607-14. CrossRef