Hong Kong Med J 2014;20:152–5 | Number 2, April 2014
DOI: 10.12809/hkmj133942
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Pulmonary artery sarcoma diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration
Johnny WM Chan, FRCP, FHKAM (Medicine)1;
Stephanie YY Chu, MRCP, FHKAM (Medicine)1;
Connie HK Lam, MRCP, FHKAM (Medicine)1;
WH O, MRCP, FHKAM (Medicine)1;
OY Cheung, FRCPath, FHKAM (Pathology)2;
TL Kwan, FRCR, FHKAM (Radiology)3;
Alex KC Leung, FRCR, FHKAM (Radiology)4;
WL Law, MRCP, FHKAM (Medicine)1
1 Department of Medicine, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Department of Pathology, Queen Elizabeth Hospital, Jordan, Hong Kong
3 Department of Radiology and Imaging, Queen Elizabeth Hospital, Jordan, Hong Kong
4 Department of Clinical Oncology, Queen Elizabeth Hospital, Jordan, Hong Kong
Corresponding author: Dr JWM Chan (chanwmj@ha.org.hk)
Abstract
Pulmonary artery sarcoma is a rare disease with poor
prognosis that has not been reported in Hong Kong.
Its clinical and radiological presentation frequently
mimics pulmonary embolism. Diagnosis is usually
delayed until surgery, which is the treatment option
that provides the best survival. Endobronchial
ultrasound-guided transbronchial needle aspiration
is an effective non-surgical technique for lymph node
staging of lung cancer and diagnosis of mediastinal
lesions via bronchoscopy. Here we discuss a case
of pulmonary artery sarcoma diagnosed by this
method, the second one in the literature, which
serves to illustrate its potential use for early and
minimally invasive diagnosis of the condition.
Although such aspiration is a safe procedure, tissue
sampling of extravascular extensions is advisable
wherever possible.
Case report
A 66-year-old non-smoker Chinese female was
hospitalised after her first episode of haemoptysis
(approximate volume, 100 mL) in February 2012. She
reported being in good health, except for an episode
of right lower lobe pneumonia about 6 months before
presentation, which was treated with antibiotics.
Despite radiological recovery, she complained of
occasional dry cough, malaise, and weight loss of
approximately 10 pounds in the subsequent months.
At admission, she was afebrile, without
dyspnoea, and with unremarkable physical findings.
Apart from mild anaemia (haemoglobin, 107 g/L)
and slightly elevated erythrocyte sedimentation
rate of 47 mm/h, other laboratory tests including
white cell and platelet counts, C-reactive protein,
coagulation profile, and liver and renal function
tests were within normal limits. Culture, acid-fast
staining, and cytological examination of the
sputum did not reveal any abnormality. Radiological
examination of the chest revealed a peripheral
shadow measuring 1.5 cm in diameter in the right
middle zone. Thoracic computed tomography (CT)
revealed a huge contrast-enhancing mass with low,
non-homogeneous attenuation filling the right
pulmonary artery (PA), extending up to the right upper and lower segmental pulmonary arteries, as
well as into the mediastinum. Patchy foci were also
noted in the pulmonary trunk (Fig 1). Peripheral
soft tissue densities were noted in the right middle
lobe, which may have been either infarcts or
tumour deposits. Bronchoscopy and endobronchial
ultrasound-guided transbronchial needle aspiration
(EBUS-TBNA) were performed to examine the
airways and to obtain histological samples from
the mediastinal extension of the mass. No bleeding
source or significant endobronchial lesions were
identified during bronchoscopy. A heterogeneous
mass with distinct margins extending from the lower
end of trachea to the right hilar regions along the right
main bronchus was revealed by EBUS. Endobronchial
ultrasound-guided transbronchial needle aspiration
of the mass was performed at the lower end of
trachea (Fig 2a). Histological examination revealed
tumour fragments with spindle cells lying in myxoid
stroma with no definite differentiation (Fig 2b).
Immunohistochemistry revealed strongly positive
smooth muscle actin expression, while CD-31,
EMA, CK, calretinin, and S-100 were all negative.
These findings were consistent with a diagnosis of
pulmonary artery sarcoma (PAS). Echocardiogram
revealed heterogeneous masses inside the main pulmonary trunk, and between the aorta and the
anterior aspect of left atrium, with no evidence of
valvular dysfunction or pulmonary hypertension. Due to the presence of extensive disease and central
location of the tumour, aggressive surgery was not
considered appropriate by cardiothoracic surgeons.
The patient declined palliative chemotherapy or
radiotherapy and defaulted from follow-up.
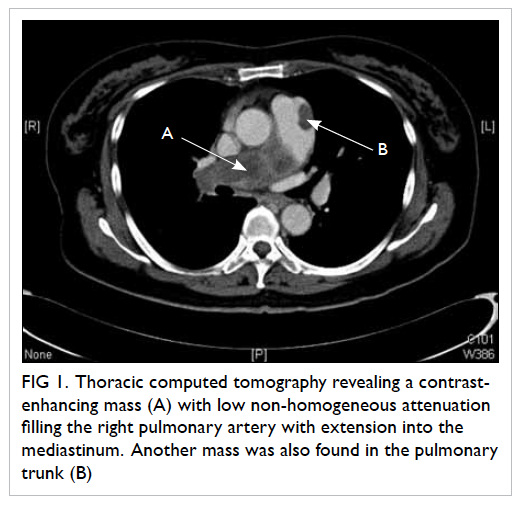
Figure 1. Thoracic computed tomography revealing a contrastenhancing mass (A) with low non-homogeneous attenuation filling the right pulmonary artery with extension into the mediastinum. Another mass was also found in the pulmonary trunk (B)
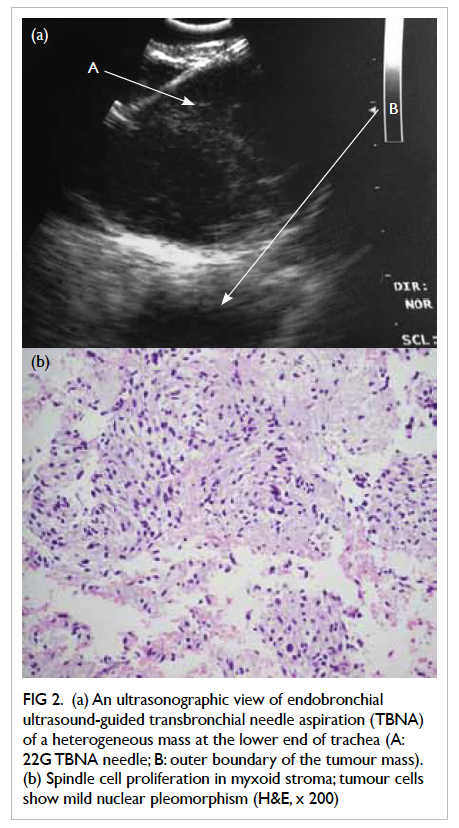
Figure 2. (a) An ultrasonographic view of endobronchial ultrasound-guided transbronchial needle aspiration (TBNA) of a heterogeneous mass at the lower end of trachea (A: 22G TBNA needle; B: outer boundary of the tumour mass). (b) Spindle cell proliferation in myxoid stroma; tumour cells show mild nuclear pleomorphism (H&E, x 200)
Discussion
Pulmonary artery sarcoma is a rare condition that
was first reported in 1923 from an autopsy.1 Apart
from a few case series and reviews,2 3 4 5 6 most cases were
only isolated case reports. Although it is sometimes
divided into intimal and mural types, and further
histological subtypes, the adherent nature of the
tumour and the frequent lack of tissue differentiation
sometimes do not allow such classifications, such as
in our patient. As both the clinical and radiological
features can resemble closely that of pulmonary
embolism (PE), some reported cases were initially
treated with anticoagulation therapy but without
response.3 4 A definite diagnosis is usually delayed
and made either at autopsy or intra-operatively
with frozen sections.5 While dyspnoea, cough,
and chest pain are the commonest presenting
symptoms,2 3 4 5 haemoptysis has also been reported.3 7
Lung involvement is commonly found,3 4 although
the lung lesion in our patient could represent either
tumour deposit or infarct, and both could have led
to haemoptysis. On CT scan, the characteristics of
PAS often mimic PE. Other features that support a
diagnosis of PAS include a low-attenuation defect
filling the whole lumen of main or proximal PA and
extravascular extension,6 both of which were present
in our patient. Considering the CT scan findings,
along with the absence of clinical features and risk
factors of vascular thrombosis, malignancy was
suspected in our patient. Apart from CT scan, both
magnetic resonance imaging (MRI; with gadolinium contrast enhancement)4 and fluorodeoxyglucose–positron emission tomography (FDG-PET)7 have
been described as useful for the diagnosis of PAS.
Imaging like CT and MRI may have enabled us to
determine the exact nature of peripheral lung lesion
in our patient.
The prognosis of PAS is poor, with a median
survival of 1.5 months without any treatment.2 If
possible, radical resection of the tumour should
be considered since median survival has been
demonstrated to be significantly better with
this option (36.5 ± 20.2 months) than that with
incomplete resection such as tumour debulking
and thromboendarterectomy (median survival,
11 ± 3 months).4 Radical resections are usually
major operations that might involve extensive
resections and subsequent reconstructions and/
or prosthetic replacement of PA, pneumonectomy
or thromboendarterectomy, and often requiring
cardiopulmonary bypass.4 5 Multimodality treatment
including neoadjuvant or adjuvant chemotherapy
and radiation, together with surgery, might further
improve survival.3 4 Owing to the rarity of PAS,
currently there are no prospective data to guide
the best management practices for PAS. Despite
the apparent prognostic superiority of curative
resection, an adequate cardiopulmonary reserve
for the major surgery and the absence of extensive
disease that precludes radical resection would be
necessary. In view of the extensive disease, radical
tumour resection was not considered in our patient.
A definite diagnosis at an early stage would improve
the chance of survival of PAS patients. However,
in the past, such an opportunity was usually not
available unless a patient underwent surgery.
Endobronchial ultrasound-guided transbronchial
needle aspiration is a minimally invasive
diagnostic procedure. The efficacy and safety of
EBUS-TBNA, via the bronchoscopic route, in
mediastinal and hilar lymph node staging of lung
cancer have been well documented, with high
sensitivity, specificity, and diagnostic accuracy of
92.3%, 100% and 98%, respectively.8 This treatment
modality can offer a more cost-effective and less
invasive diagnostic option than mediastinoscopy.9
It is also useful for diagnosing central pulmonary
lesions adjacent to the bronchus10 and non-malignant
conditions.11 Performed in an endoscopic suite,
the procedure involves using a special endoscopic
instrument incorporating a 7.5-MHz curvilinear
ultrasonic transducer at the tip of a flexible
bronchoscope, which allows needle aspiration of
target lesions under real-time ultrasound guidance
via a 22-gauge needle. The procedure is very safe
and can be performed under local anaesthesia and
conscious sedation.8 9 10 11 Our case is the second report
in medical literature which illustrates the potential
applicability of EBUS-TBNA in a histological diagnosis for PAS through a non-surgical route. In
contrast to our case, in the first report describing
two patients in literature, the TBNA involved
puncturing of the pulmonary arterial wall in one of
the patients undergoing the procedure.12 Although
PA puncture in EBUS-TBNA has been reported to
be safe,13 self-limiting intramural haematoma and
haemopneumomediastinum have been described
after an accidental EBUS-TBNA puncture,14 and so,
the safety of EBUS-TBNA in the routine differential
diagnosis of PAS and pulmonary thromboembolism
have been doubted.15 In contrast, our patient had
suspicious radiological features of PAS and absence
of clinical features and risks for PE. We believe
using EBUS-TBNA to approach extravascular
extension of the lesion in such patients offers a
relatively non-invasive means for early diagnosis of
PAS. However, the safety of PA punctures in EBUS-TBNA
needs to be clarified in large studies. It must
be remembered that performing EBUS-TBNA in
patients with acute PE and pulmonary hypertension
may be associated with a high risk of complications
such as respiratory distress and bleeding.15 Thus, the
routine use of EBUS-TBNA as a tool to distinguish
PE from the rarer PAS is not encouraged. While MRI
or FDG-PET may be considered before performing
invasive diagnostic investigations,4 the present
report suggests that EBUS-TBNA can potentially
provide clinicians a diagnostic alternative to surgical
exploration for patients suspected of having PAS.
Conclusion
Pulmonary artery sarcoma is a rare disease with
poor prognosis, which is often diagnosed late and
frequently mimics PE. Endobronchial ultrasound-guided
transbronchial needle aspiration may be a
safe and easy option for the early diagnosis of the
condition. Extravascular extension of the lesion on
thoracic CT provides a diagnostic clue for PAS, and
also offers a safe approach for performing EBUS-TBNA.
Declaration
No conflicts of interest were declared by authors.
References
1. Mandelstamm M. Uber primare neubildungen des herzens [in German]. Virchows Arch 1923;245:43-54. CrossRef
2. Krüger I, Borowski A, Horst M, de Vivie ER, Theissen P, Gorss-Fengels W. Symptoms, diagnosis and therapy of primary sarcomas of pulmonary artery. Thorac Cardiovasc Surg 1990;38:91-5. CrossRef
3. Huo L, Moran CA, Fuller GN, Gladish G, Suster S. Pulmonary artery sarcoma: a clinicopathologic and immunohistochemical study of 12 cases. Am J Clin Pathol 2006;125:419-24. CrossRef
4. Blackmon SH, Rice DC, Correa AM, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg 2009;87:977-84. CrossRef
5. Mayer E, Kriegsmann J, Gaumann A, et al. Surgical treatment of pulmonary artery sarcoma. J Thorac Cardiovasc Surg 2001;121:77-82. CrossRef
6. Yi CA, Lee KS, Choe YH, Han D, Kwon OJ, Kim S. Computed tomography in pulmonary artery sarcoma: distinguishing features from pulmonary embolic disease. J Comput Assist Tomogr 2004;28:34-9. CrossRef
7. Tueller C, Fischer Biner R, Minder S, et al. FDG-PET in diagnostic work-up of pulmonary artery sarcomas. Eur Respir J 2010;35:444-6. CrossRef
8. Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. CrossRef
9. Navani N, Lawrence DR, Kolvekar S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med 2012;186:255-60. CrossRef
10. Tournoy KG, Rintoul RC, van Meerbeeck JP, et al. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer 2009;63:45-9. CrossRef
11. Wong M, Yasufuku K, Nakajima T, et al. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur Respir J 2007;29:1182-6. CrossRef
12. Park JS, Chung JH, Jheon S, et al. EBUS-TBNA in the differential diagnosis of pulmonary artery sarcoma and thromboembolism. Eur Respir J 2011;38:1480-2. CrossRef
13. Vincent B, Huggins JT, Doelken P, Silvestri G. Successful real-time endobronchial ultrasound-guided transbronchial needle aspiration of a hilar lung mass obtained by traversing the pulmonary artery. J Thorac Oncol 2006;1:362-4. CrossRef
14. Botana-Rial M, Nú-ez-Delgado M, Pallarés-Sanmartín A, et al. Intramural hematoma of the pulmonary artery and hemopneumomediastinum after endobronchial ultrasound-guided transbronchial needle aspiration. Respiration 2012;83:353-6. CrossRef
15. Montani D, Jaïs X, Sitbon O, Dartevelle P, Simonneau G, Humbert M. EBUS-TBNA in the differential diagnosis of pulmonary artery sarcoma and thromboembolism. Eur Respir J 2012;39:1549-50. CrossRef

