Hong Kong Med J 2014;20:107–12 | Number 2, April 2014 | Epub 22 Jul 2013
DOI: 10.12809/hkmj133972
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Ultrasound-guided plugged percutaneous biopsy of solid organs in patients with bleeding tendencies
WK Tsang, MB, ChB, FRCR1; WH Luk, FRCR, FHKAM (Radiology)2; Adrian XN Lo, FRCR, FHKAM (Radiology)2
1 Department of Radiology and Nuclear Medicine, Tuen Mun Hospital,
Tuen Mun, Hong Kong
2 Department of Radiology and Organ Imaging, United Christian Hospital,
Kwun Tong, Hong Kong
Corresponding author: Dr WK Tsang (tsang_k@yahoo.com.hk)
Abstract
Objective: To establish and verify the utility of
plugging biopsy tracts, using a combination of
Gelfoam slurry and torpedo in the prevention of
post-biopsy bleeding in patients at high risk of
post-procedure haemorrhage following ultrasound-guided
percutaneous biopsy of solid organs.
Design: Case series.
Setting: Radiology Department of a regional hospital
in Hong Kong.
Patients: In our unit, all patients considered to
be at high risk of post-biopsy haemorrhage of a
solid organ underwent ultrasound-guided plugged
percutaneous biopsy from year 2005 to 2012.
Interventions: All the included patients had
undergone real-time ultrasound-guided biopsy of
solid organs (liver in 10 and spleen in one patient).
In all cases, a combination of a coaxial introducer
needle and Temno needle were used. After adequate
specimens were obtained, Gelfoam slurry (for distal
embolisation) followed by Gelfoam torpedo (for
proximal embolisation) were used to plug the biopsy
tract.
Main outcome measures: Technical success, any post-biopsy haemorrhage treated by transfusion
or other intervention, and plugging-related complications were reviewed for each patient.
Results: Technical success was achieved in all patients
and none experienced post-biopsy haemorrhage
treated by blood transfusion or any other intervention.
Conclusion: Plugging of the biopsy tract with
Gelfoam slurry followed by Gelfoam torpedo is a
direct and simple procedure that can safely and
effectively prevent haemorrhage in patients at high
risk of post-biopsy haemorrhage.
New knowledge added by this
study
- Plugging of the biopsy tract using a combination of Gelfoam slurry followed by Gelfoam torpedo is a new technique that has not been previously described.
- Plugging of the biopsy tract using a combination of Gelfoam slurry and torpedo is safe and easy to undertake and should be used in patients at high risk of post-biopsy haemorrhage.
Introduction
Ultrasound-guided percutaneous biopsy is a well-established
means for diagnosis of focal or diffuse
disease in solid organs. It is generally safe and
confers minimal risk of complications. However,
it is contra-indicated in patients with bleeding
tendencies, which means that histological diagnosis
may be lacking and sometimes life-saving treatment
cannot be commenced. Plugging of the biopsy tract
is a promising technique to decrease the risk of
post-biopsy haemorrhage, for which Gelfoam is the
most commonly used agent. In this article, we share
our experience in performing this procedure using
Gelfoam slurry followed by Gelfoam torpedo in patients at high risk of post-procedure haemorrhage
in our institution.
Methods
The Department of Radiology and Organ Imaging,
United Christian Hospital, is the main radiology
training centre of the Kowloon East Cluster, Hong
Kong. Apart from diagnostic imaging, we provide
both emergency and elective interventional radiology
services. In the form of a retrospective study
approved by our local ethics committee, since 2005,
it has been our standard practice to plug the biopsy
tract in all patients considered at risk of haemorrhage
after having ultrasound-guided percutaneous biopsy of a solid organ. Our departmental registry recorded
all the cases receiving plugged percutaneous biopsy
(PPB) of solid organs performed from 1 January 2005
to 30 September 2012. There was no reported refusal
of the procedure by any patient. Demographic data, indication for the biopsy and for plugging of
the biopsy tract, details of the biopsy technique,
biopsy results, and any episodes of post-biopsy
haemorrhage treated by transfusion or any other
type of intervention were reviewed for each patient.
Relevant details are listed in Table 1.
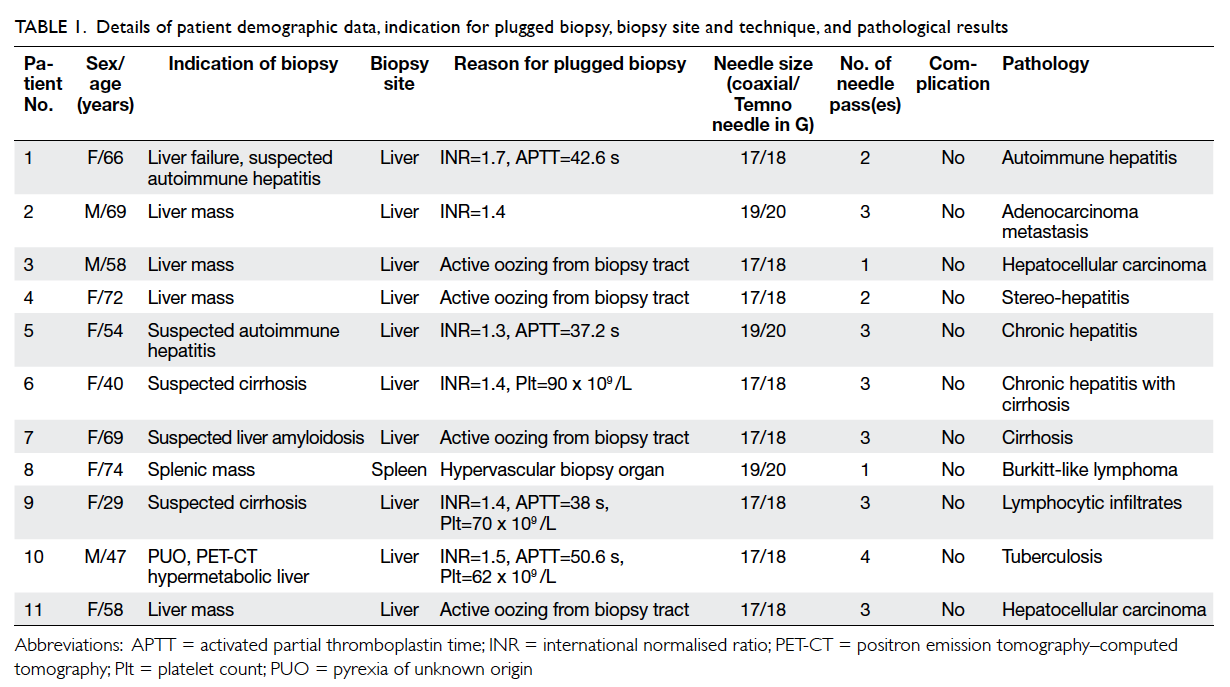
Table 1. Details of patient demographic data, indication for plugged biopsy, biopsy site and technique, and pathological results
Technique
All PPBs were performed under strict aseptic
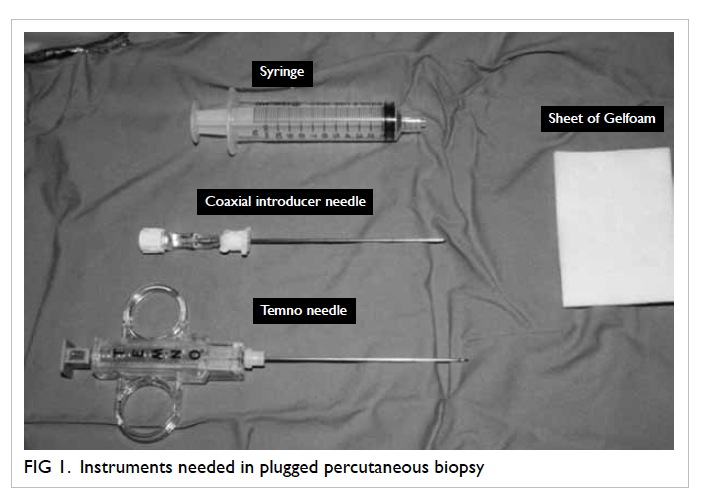
conditions with instruments as shown in Figure 1.
A biopsy path avoiding critical structures and major
vessels was selected under ultrasound guidance. The
length of the biopsy path starting from the organ
capsule to the target region was measured (Fig 2a). A
strip of Gelfoam of the same length and with a width of
approximately 2 mm was cut from a sheet of Gelfoam.
Before being cut, the sheet of Gelfoam was compressed
manually to expel all air bubbles. A Gelfoam torpedo
was formed by rolling the strip of Gelfoam into a
rod-like structure (Fig 2b). The remaining Gelfoam
sheet was then cut into tiny pledgets of around 2
mm x 2 mm in size. A syringe filled with Gelfoam
pledgets and another syringe filled with saline were
both connected to a 3-way stopcock. Macerating
the suspension with two syringes and a 3-way
stopcock allowed further decreases in size of the
pledgets into a slurry (Fig 2c). After the Gelfoam
torpedo and slurry were ready, the puncture site
was injected with local anaesthetic (5-10 mL of 1-2%
lignocaine) and a small skin incision was created.
Patients were then instructed to hold their breath
while a coaxial introducer needle (17G or 19G, CareFusion; Waukegan [IL], US) was advanced to
the target region. The stylet of the coaxial introducer
needle was removed, with the outer sheath held
firmly in place. A Temno biopsy needle (18G or 20G,
CareFusion) was then inserted through the sheath
under ultrasound guidance. Biopsy specimens were
obtained in a standard manner. After removal of
the Temno needle between passes, the stylet of the
coaxial introducer needle was reinserted into the
sheath to decrease the chance of haemorrhage. After
adequate specimens were obtained by inspection, 1 to
2 mL of Gelfoam slurry was injected into the sheath
of the coaxial introducer needle (Fig 3). The Gelfoam
torpedo was then placed at the hub of the sheath of
the coaxial introducer needle (Fig 4a) and pushed
by the stylet until the echogenic tip of the stylet was
advanced to the organ capsule (Fig 4b). The outer
sheath was then withdrawn while keeping the stylet
still (Fig 4c), so that the Gelfoam torpedo could be
deployed along it and therefore sealing the biopsy
tract. Finally, the entire coaxial introducer needle was
removed.
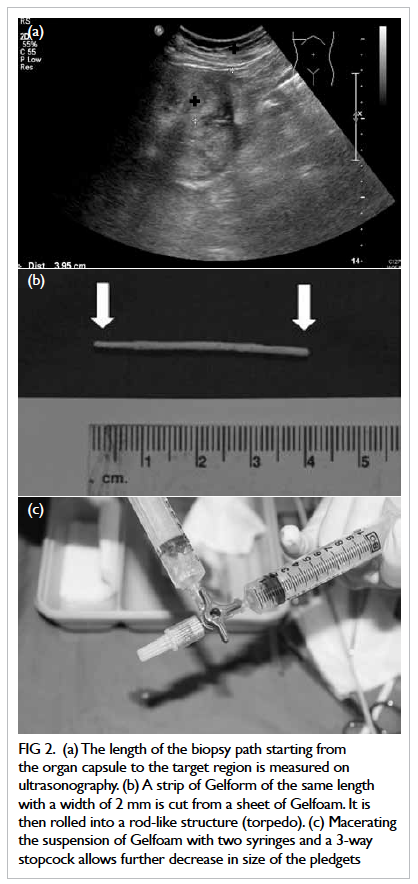
Figure 2. (a) The length of the biopsy path starting from the organ capsule to the target region is measured on ultrasonography. (b) A strip of Gelform of the same length with a width of 2 mm is cut from a sheet of Gelfoam. It is then rolled into a rod-like structure (torpedo). (c) Macerating the suspension of Gelfoam with two syringes and a 3-way stopcock allows further decrease in size of the pledgets
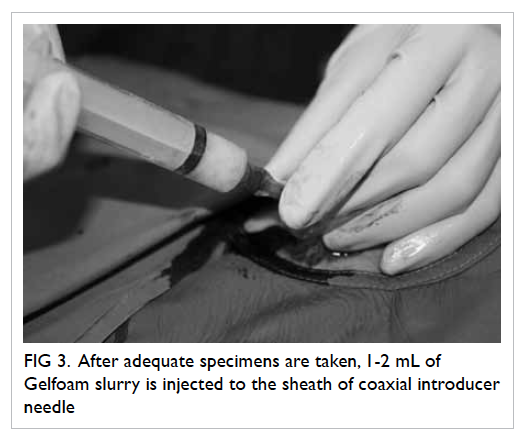
Figure 3. After adequate specimens are taken, 1-2 mL of Gelfoam slurry is injected to the sheath of coaxial introducer needle
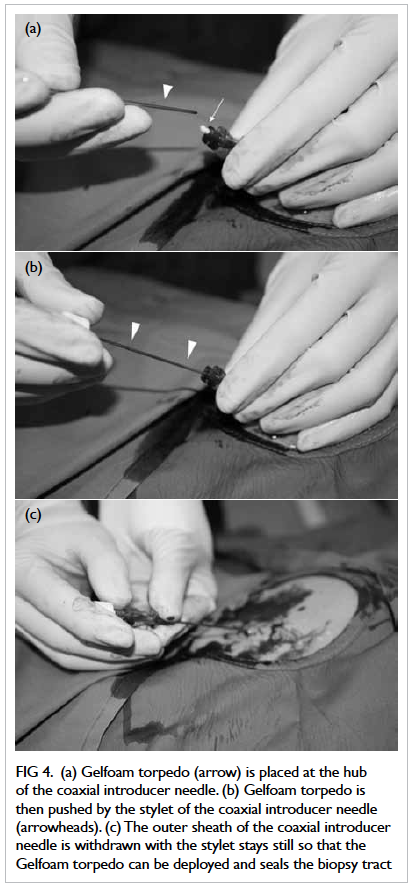
Figure 4. (a) Gelfoam torpedo (arrow) is placed at the hub of the coaxial introducer needle. (b) Gelfoam torpedo is then pushed by the stylet of the coaxial introducer needle (arrowheads). (c) The outer sheath of the coaxial introducer needle is withdrawn with the stylet stays still so that the Gelfoam torpedo can be deployed and seals the biopsy tract
Results
During a 7-year period, we performed 11 cases
of plugged percutaneous solid organ biopsy in 11
patients, all of whom were considered at high risk
of post-biopsy bleeding due to the reasons listed
in Table 1. The mean patient age was 58 (standard
deviation [SD], 14) years. Three patients were male
and eight were female. The target organ was the liver
in 10 cases and the spleen in one. The indications for
biopsy were to achieve a diagnosis of a focal mass
in five cases, and characterisation of diffuse hepatic
diseases in six (Table 1). The number of needle passes
ranged from one to four, with a mean of 2.5 (SD, 0.9).
In all cases, the combination of a coaxial introducer
needle and Temno needle (both by CareFusion) were
used. The combination of a 17G coaxial introducer
needle and 18G Temno needle was used in eight
biopsies, while the combination of a 19G coaxial
introducer needle and 20G Temno needle was used
thrice. All the biopsies were technically successful
in obtaining adequate specimens for a histological
diagnosis. None of the patients experienced post-biopsy
haemorrhage treated by transfusion or any
other form of intervention.
Discussion
Ultrasound-guided percutaneous solid organ biopsy
is a well-established means of diagnosing focal or
diffuse disease in solid organs. In general, it is safe
and confers minimal risk of complications. Major and
minor complication (mainly bleeding) rates of 0.8%
and 2-3.8%, respectively, have been reported.1 2 Many
factors increase the risk of post-biopsy haemorrhage,
which can be divided into lesional, technical,
and patient-related. Lesional factors consist of peripheral subcapsular location, close proximity to
major vessels, hypervascularity, and hypervascular
biopsy sites (such as the spleen). Technical factors
include increased numbers of needle passes, large
needle sizes, use of cutting needles, blind biopsies,
and less-experienced operators.3 Patient factors
include coagulopathy, platelet dysfunction or
thrombocytopenia, medications (eg antiplatelet
agents and anticoagulants), chronic liver disease,
haematological malignancy, presence of moderate-to-
severe ascites, and uncooperative patients.2 4 5
Some studies showed that peripheral blood
coagulation indices have a poor correlation with liver
bleeding time following laparoscopic biopsy, which
might be caused by low regional platelet counts,
clotting factor deficiencies in the liver parenchyma,
and the lack of mechanical compression of the biopsy
tract by inelastic tissue (eg cirrhotic liver).6 Therefore
operators should always be prepared for the
possibility of significant post-biopsy haemorrhage,
even in patients with normal clotting profiles and
platelet counts.
Obviously, the main contra-indication to
image-guided percutaneous solid organ biopsy is a
bleeding diathesis.2 However, histological diagnosis
is critical and even lifesaving, by means of achieving
correct treatment. In the past, transjugular liver
biopsy had been advocated in patients with bleeding
diathesis, massive ascites, and poor respiratory
control.7 8 However, this has multiple disadvantages.
In particular, it is not feasible for liver lesions
far from the major hepatic veins. Moreover, it is
technically demanding and associated with a high
rate of insufficient specimen retrieval for satisfactory
histological examination (11.2-29%).7 9 10 11 12 It can
also give rise to complications at the puncture site
(jugular vein) and induce arrhythmias during right
atrial passage. Haemoperitoneum is possible if the
liver capsule is perforated, which can sometimes be
fatal.
Plugged percutaneous biopsy is an alternative
to transjugular liver biopsy in patients at high risk
of bleeding.2 8 13 It was first described by Riley et al
in 1984.13 In plugged biopsy, the tract is embolised
(plugged) after the percutaneous biopsy, thus
decreasing the risk of haemorrhage. Multiple
studies on PPB have demonstrated at least a 95%
success rate in obtaining adequate specimens for
histological diagnoses. It is also a safe procedure with
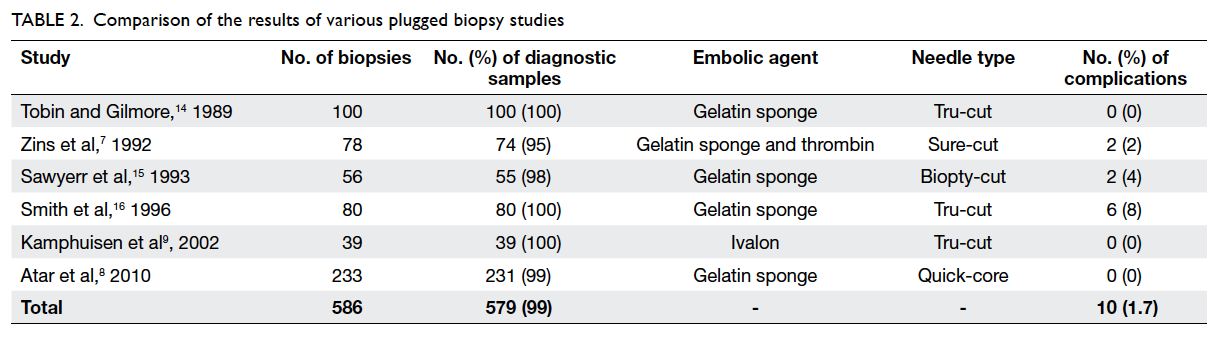
a complication rate of less than 2% (Table 27 8 9 14 15 16). It
has the obvious advantages of being direct and can be
used to biopsy focal hepatic lesions away from major
hepatic veins and in other organs. Also, a larger biopsy
needle can be used, which increases the chance of
obtaining adequate specimens. Finally, it does not
involve the vascular system or passage through the
right atrium and thus the relevant complications can
be avoided.
The most commonly used embolic agent is
Gelfoam, which is an absorbable compressed gelatin
sponge prepared from purified porcine skin.3 7 It is
capable of absorbing up to 45 times its weight of
whole blood, and induces haemostasis by speeding
up thrombus formation and providing structural
support for the clot. Gelfoam is a temporary embolic
agent, which is usually completely absorbed within
a few days or weeks, depending on the amount
used, the degree of saturation with blood, and the
application site. It is widely used in tract plugging as
it is relatively inexpensive and readily available. It is
easy to use and can be prepared in different forms,
depending on the site of application. In our centre,
Gelfoam was prepared in the form of torpedo and
slurry. The Gelfoam torpedo was made from tight
rolling of a small strip and used at the site of active
bleeding. Due to their larger size, Gelfoam torpedoes
can remain at the site of deployment instead of being
flushed away by blood. The drawback of the torpedo
is that distal embolisation cannot be achieved.
In contrast, Gelfoam slurry is suitable for distal
embolisation. It can be prepared by mixing tiny
Gelfoam pledgets with contrast or saline. Further
decrease in size of the pledgets can be created by
macerating the suspension with two syringes and
a 3-way stopcock. The syringe should be held nose
up as Gelfoam floats in fluid. The disadvantage
of slurry is that it is difficult to deploy at sites of
active bleeding, as the suspension can be flushed
away by blood. In our centre, we injected Gelfoam
slurry first for distal embolisation and then filled up
the rest of the biopsy tract with a torpedo. To the
best of our knowledge, plugging of the biopsy tract
using a combination of Gelfoam slurry followed by
Gelfoam torpedo is a new technique that has not
been previously described. Gelfoam is safe to use
most of the time, although there is a minute risk of
non-targeted embolisation of the biliary or vascular
systems and of becoming a nidus for microbial
growth.3
Apart from plugging of the biopsy tract,
there are other measures to decrease the risk of
bleeding in patients undergoing solid organ biopsy. First, as appropriate, we should try to correct any
coagulopathy by administration of fresh frozen
plasma, platelets, coagulation factors, and vitamin K,
whilst also withholding antiplatelet or anticoagulant
medications if at all feasible. Although not related to
the bleeding risk, red cell or whole blood transfusion
should be given before the biopsy to significantly
anaemic patients. Next, careful planning of the
method of biopsy is important. A safe biopsy path
not traversing vessels or critical structures should
be sought. Leaving adequate distance of normal
parenchyma from the organ capsule and the biopsy
site can also help mechanical compression of the
biopsy tract by virtue of tissue elasticity, after the
needle is removed. We have to strike a balance
between the tissue yield and the use of smaller
needles. The use of a coaxial system allows multiple
needle passes with just a single puncture. Reducing
ascites, if present with diuretics or paracentesis, can
also decrease the risk of haemorrhage.
One limitation of our study was the small
sample size. Second, it was a retrospective
observational study without a control group. A large-scale
prospective randomised controlled study may
be ideal to validate the efficacy and safety of PPB.
We share our experience in this small-scale study to
raise the awareness of this procedure (especially for
those not specialised in interventional radiology), as
it shows that PPB is a simple and safe method with
a high technical success rate that can help prevent
post-biopsy haemorrhage.
Conclusion
Plugging of the biopsy tract with Gelfoam slurry
followed by a Gelfoam torpedo is a direct, simple,
safe, and effective means of preventing haemorrhage
in patients at high risk of post-biopsy haemorrhage.
References
1. Hatfield MK, Beres RA, Sane SS, Zaleski GX. Percutaneous imaging-guided solid organ core needle biopsy: coaxial versus non coaxial method. AJR Am J Roentgenol 2008;190:413-7. CrossRef
2. Albeniz Arbizu E, Lopez San Roman A, Garcia Gonzalez M, et al. Fibrin-glue sealed liver biopsy in patients with a liver transplantation or in liver transplantation waiting list: preliminary results. Transplant Proc 2003;35:1911-2. CrossRef
3. Azar N, Delman T, Nakamoto D. Transcutaneous management of bleeding after solid organ biopsy what the radiologist needs to know and use. US Radiology 2011;3:53-6.
4. Chuah SY. Liver biopsy-past, present and future. Singapore Med J 1996;37:86-90.
5. Sherlock S, Dick R, Van Leeuwen DJ. Liver biopsy today. The Royal Free Hospital Experience. J Hepatol 1984;1:75-85. CrossRef
6. Ewe K. Bleeding after liver biopsy does not correlate with indices of peripheral coagulation. Dig Dis Sci 1981;26:388-93. CrossRef
7. Zins M, Vilgrain V, Gayno S, et al. US-guided percutaneous liver biopsy with plugging of the needle track: a prospective study in 72 high-risk patients. Radiology 1992;184:841-3.
8. Atar E, Ben Ari Z, Bachar GN, et al. A comparison of transjugular and plugged-percutaneous liver biopsy in patients with contraindications to ordinary percutaneous liver biopsy and an "in-house" protocol for selecting the procedure of choice. Cardiovasc Intervent Radiol 2010;33:560-4. CrossRef
9. Kamphuisen PW, Wiersma TG, Mulder CJ, de Vries RA. Plugged-percutaneous liver biopsy in patients with impaired coagulation and ascites. Pathophysiol Haemost Thromb 2002;32:190-3. CrossRef
10. Lebrec D, Goldfarb G, Degott C, Rueff B, Benhamou JP. Transvenous liver biopsy: an experience based on 1000 hepatic tissue samplings with this procedure. Gastroenterology 1982;83:338-40.
11. Velt PM, Choy OG, Shimkin PM, Link RJ. Transjugular liver biopsy in high-risk patients with hepatic disease. Radiology 1984;153:91-3.
12. Wolska-Krawczyk M, Krawczyk M, Katoh M, et al. Liver fibrosis: how many samples in transjugular liver biopsy are sufficient? Histological vs. clinical value. Abdom Imaging 2013;38:461-4. CrossRef
13. Riley SA, Ellis WR, Irving HC, Lintott DJ, Axon AT, Losowsky MS. Percutaneous liver biopsy with plugging of needle track: a safe method for use in patients with impaired coagulation. Lancet 1984;2:436. CrossRef
14. Tobin MV, Gilmore IT. Plugged liver biopsy in patients with impaired coagulation. Dig Dis Sci 1989;34:13-5. CrossRef
15. Sawyerr AM, McCormick PA, Tennyson GS, et al. A comparison of transjugular and plugged-percutaneous liver biopsy in patients with impaired coagulation. J Hepatol 1993;17:81-5. CrossRef
16. Smith TP, McDermott VG, Ayoub DM, Suhocki PV, Stackhouse DJ. Percutaneous transhepatic liver biopsy with tract embolization. Radiology 1996;198:769-74.