Hong Kong Med J 2014;20:74–7 | Number 1, February 2014
DOI: 10.12809/hkmj133780
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Middle cerebral artery infarction in a cancer patient: a fatal case of Trousseau’s syndrome
Peter YM Woo, MB, BS, MRCS (Edin)1;
Danny TM Chan, MB, ChB, FRCS (Edin)1;
Tom CY Cheung, MB, ChB, FRCR2;
XL Zhu, BMed (Jinan) FRCS (Edin)1;
WS Poon, MB, ChB (Glasg), FRCS (Edin)1
1 Division of Neurosurgery, Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin,
New Territories, Hong Kong
2 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin,
New Territories, Hong Kong
Corresponding author: Prof WS Poon (wpoon@cuhk.edu.hk)
Abstract
Trousseau’s syndrome is defined as any unexplained
thrombotic event that precedes the diagnosis of an
occult visceral malignancy or appears concomitantly
with a tumour. This report describes a young,
previously healthy man diagnosed to have an acute
middle cerebral arterial ischaemic stroke and lower-limb
deep vein thrombosis, who subsequently
succumbed to pulmonary arterial embolism. During
the course of his illness, he was diagnosed to have
a malignant pleural effusion secondary to an occult
adenocarcinoma. This report highlights the need
for a high degree of suspicion for occult malignancy
and non-bacterial thrombotic endocarditis in
young (<60 years old) ischaemic stroke patients
with no identifiable conventional cardiovascular
risks. In selected patients, transoesophageal
echocardiography is the diagnostic investigation
of choice, since transthoracic imaging is not sensitive. Screening tests for serum tumour markers
and prompt heparinisation of these patients are
suggested whenever ischaemic stroke secondary to
malignancy-induced systemic hypercoagulability is
suspected.
Case report
A 37-year-old Korean man, who was a non-smoker
and previously healthy, experienced a sudden-onset
right hemiparesis in April 2011. The patient was
travelling in Guangzhou, China and was hospitalised
within 4 hours of symptom onset. He was diagnosed
to have a left middle cerebral artery (MCA)
territory massive infarct based on plain computed
tomography (CT). Contrast-enhanced magnetic
resonance arteriography indicated a left proximal
MCA occlusion (Fig a). The patient was treated
conservatively and neither thrombolytic therapy nor
operative management was given. Three days later,
he was transferred to Hong Kong.
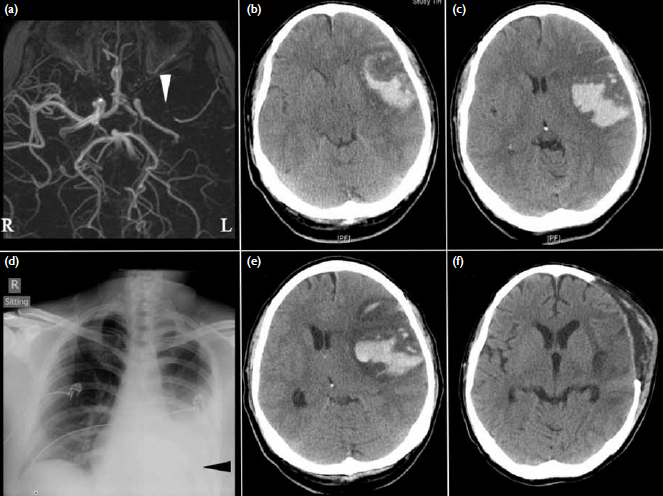
Figure. (a) Contrast-enhanced magnetic resonance angiograms on the day of symptom onset showing a left proximal middle cerebral artery (MCA) occlusion (white arrowhead). Brain computed tomography (CT) conducted 4 days later revealing haemorrhagic conversion of a left MCA region infarction with obliteration of (b) the basal cisterns and (c) midline shift. (d) Evidence of a left pleural effusion (black arrowhead) is present on the admission chest X-ray. (e) Deterioration in consciousness 8 days after symptom onset correlated with increased midline shift secondary to increased peri-haematomal oedema. (f) The 2-week postoperative CT showing satisfactory decompression and resolution of the intracerebral haematoma
On examination the patient had expressive
aphasia and right hemiplegia. He was able to obey
commands, had a Glasgow Coma Scale score of 11/15
(E4V1M6) and was afebrile. His blood pressure was
not high and his pulse was regular, nor were carotid
bruits or heart murmurs detected.
Repeat CT of the brain showed a massive
left MCA territory infarction with haemorrhagic
conversion and a chest X-ray revealed a left pleural
effusion (Figs 1b-d). An electrocardiogram (ECG)
showed no arrhythmias and initial blood test results
showed a raised white cell count of 15.4 x 109 /L
with neutrophilia. Other than that, the complete
blood count, fasting serum glucose, lipid profile,
and autoimmune markers were all within normal
limits. The patient was initially managed medically with close neurological observations. In view of the
neutrophilia, the patient was provisionally diagnosed
to have bacterial endocarditis with complicating
chest infection and parapneumonic pleural effusion.
An echocardiogram was arranged and empirical
intravenous antibiotics started.
Four days later, the patient became increasingly
drowsy and an emergency decompressive
craniectomy and duraplasty was performed (Figs 1e-f). The procedure was uneventful and the patient
was stabilised.
As for delineating the cause for the stroke, all
specimen culture results showed no evidence of an
underlying infection. Percutaneous aspiration of the
pleural effusion and pleural biopsy were performed.
The biopsy showed no evidence of malignancy, but
fluid cytology yielded adenocarcinoma cells. Serum
tumour markers, including carcinoembryonic
antigen and alpha-fetoprotein, were normal. The
patient developed sinus tachycardia with an ECG
showing a right heart strain pattern, before further
cardiological tests (positron emission tomography
and echocardiogram) could be performed. Blood for
troponin-T and D-dimer levels were also elevated.
The patient was subsequently diagnosed to have
bilateral lower-limb deep vein thrombosis (DVT)
extending from the external iliac vein to the popliteal
vein. Thoracic CT was consistent with pulmonary
embolism to the right lower lobe pulmonary arteries.
Subcutaneous low-molecular-weight heparin was started and an inferior vena cava (IVC) filter
was placed. The patient's condition continued
to deteriorate and he developed disseminated
intravascular coagulopathy and respiratory failure.
Progression of the pulmonary embolism was
suspected, but the patient was deemed unsuitable
for systemic thrombolysis due to his recent
neurosurgery. The patient succumbed 2 weeks
after the operation. A post-mortem examination
was arranged, but waived by the coroner upon the
family's request. The cause of death was pulmonary
embolism contributed by an occult malignancy.
Discussion
Ischaemic stroke is the second most frequent
neurological finding after brain metastases in post-mortem
studies of cancer patients.1 From an autopsy
study, cerebral infarction was observed in 15% of
3426 patients diagnosed with cancer, of whom half
were previously symptomatic.1 In a recent single-centre
retrospective review of 5106 patients admitted
for ischaemic stroke, 24 (0.47%) were diagnosed to
have an underlying malignancy. In this subgroup of patients, the mean age was relatively young (52
years) and there was a lower incidence of the typical
vascular risk factor profiles as noted in larger stroke
cohort studies.2
Trousseau first described the association between vascular thrombosis and cancer in his
monograph of a peculiar migratory thrombophlebitis
in 1865 and diagnosed the syndrome on himself
2 years later when he succumbed to gastric
adenocarcinoma.3 4 There are several definitions for
Trousseau's syndrome including "the occurrence
of thrombophlebitis migrans with visceral cancer"
and "spontaneous recurrent venous thromboses
and/or arterial emboli caused by non-bacterial
thrombotic endocarditis (NBTE) in a patient with
malignancy".4 But a prima facie definition should be
any "unexplained thrombotic event that precede[s]
the diagnosis of an occult visceral malignancy or
appear[s] concomitantly with the tumor".4
It is estimated that 15% of cancer patients will
suffer from a thromboembolic event during the
course of their illness and up to 50% have evidence
of venous thromboembolism on post-mortem
examination.5 The underlying pathophysiological
mechanisms can be broadly classified as either
due to the malignancy itself or as an iatrogenic
complication of oncological treatment such as
radiotherapy-induced arteriopathy. In turn, tumour-related
ischaemic cerebrovascular events can be
due to systemic hypercoagulability as part of a
paraneoplastic syndrome, tumour emboli secondary
to vessel infiltration, or contiguous compression of
an artery. For this patient the thoracic CT revealed no
evidence of infiltration of mediastinal great vessels or
a cardiac lesion, so the possibility of arterial tumour
emboli was unlikely. Brain magnetic resonance
imaging also did not show brain metastasis. The
fact that the patient developed extensive vascular
thrombosis with bilateral lower-limb DVT and
pulmonary embolism in spite of an IVC filter
suggests that the cause of the stroke may have been
due to malignancy-associated hypercoagulability.
The biological basis for systemic coagulopathy
in Trousseau's syndrome has yet to be defined.
It is thought to result from a complex interplay of
tumour cell secretion of procoagulants and the
host's blood vessels. When macrophages interact
with malignant cells, there is a release of cytokines
such as interleukin-1, interleukin-6, and tumour
necrosis factor that can lead to endothelial damage
and thrombogenesis on viable surfaces.6 Tumour
cells may also secrete tissue factor and cysteine
proteases with thromboplastin-like properties
that activate coagulation factors VII and X. Finally
mucin-producing adenocarcinomas can lead to
direct non-enzymatic activation of factor X.7 In
this state of hypercoagulability, the two commonest
underlying mechanisms for ischaemic stroke are
related to cardiogenic embolism from NBTE or
cerebral intravascular coagulation.8
Non-bacterial thrombotic endocarditis
Up to half of patients with NBTE develop systemic emboli, most often to the cerebral vasculature and
result in stroke, but some emboli also end up in the
pulmonary, cardiac, and renal circulations.6 This
may have been the underlying cause for the patient's
massive MCA infarction and pulmonary embolism.
Formally known as marantic endocarditis, the
condition is characterised by sterile platelet and fibrin
rich thrombi that form on previously undamaged
heart valves.9 The incidence is largely unknown
but according to Graus et al's autopsy series,1 it
was noted in 1.6% of adult cancer patients. Often,
it is encountered in patients with advanced-stage
malignancies, in particular adenocarcinomas of the
lung or gastro-intestinal tract; as in this patient,
rarely NBTE can be a harbinger of occult cancer.8 10
Its pathogenesis is incompletely understood, but
is possibly due to cytokine-mediated inflammatory
valvular tissue injury that predisposes to thrombus
formation.6 For several reasons, NBTE is difficult
to diagnose. Thus, though aortic and mitral valves
are the most commonly affected, patients seldom
have detectable cardiac murmurs.6 11 Concomitantly,
immunocompromised patients can suffer from
infective endocarditis that confounds the diagnosis.
Finally, transthoracic echocardiography is not
sufficiently sensitive in detecting the smaller friable
valvular vegetations of NBTE.12 Careful selection
of patients for transoesophageal echocardiography,
the preferred diagnostic test, is claimed to be
preferable.6 Patients without end-stage malignancy,
acceptable performance status of 3 or less on the
Eastern Cooperative Oncology Group scale and with
non-debilitating stroke are suitable candidates. For
this patient, the diagnosis of NBTE was inferred in
view of the negative culture and serology results and
failed response to systemic antibiotics.
Treatment is directed at the underlying
malignancy coupled with systemic anticoagulation.
Since patients often present with
metastatic disease, curative options are limited and
anticoagulation should be administered indefinitely,
since thromboembolic events tend to recur after
discontinuation.6 Unfractionated heparin, given
either intravenously or subcutaneously, has been
proven to be most effective.6 Alternatively, low-molecular-weight heparin could be considered, but
evidence about its utility is anecdotal. In contrast,
vitamin K antagonists such as warfarin are not
recommended, as recurrent thromboembolic events
are common and expose the patient to unnecessary
bleeding risks.6 13 The exact reason for warfarin
resistance is unknown, but it has been suggested
that thrombosis secondary to cytokine-induced
inflammation is preferentially mediated by non-vitamin-K–dependent coagulation factors.13
Cerebral intravascular coagulation
Cerebral intravascular coagulation is a post-mortem pathological diagnosis made when cardiac
valvulopathy is excluded. Originally described in
autopsy studies among patients with leukaemia
and breast cancer, this condition involves diffuse
fibrinous occlusions of small cerebral blood vessels
leading to micro-infarcts. Patients often present
with progressive encephalopathy and seldom
develop focal neurological deficits, although partial
seizures have been reported.14 No reliable laboratory
or radiological investigations are available to detect
this disorder. Patients are usually in their preterminal
state and management is often supportive.8
Conclusion
This patient suffered from a fatal manifestation of
Trousseau's syndrome. One should be cognisant
of the diagnosis of NBTE as a cause of ischaemic
stroke in any patient with a known or suspected
underlying malignancy. This is particularly true
if new heart murmurs are detected in a cancer
patient. Conversely, for a young patient (<60 years
old), ischaemic stroke with no overt vascular risk
factors should be considered for cancer screening. In
selected cases, transoesophageal echocardiography
appears to be the investigation of choice for NBTE.
Anticoagulation, preferably with unfractionated
heparin, should be administered as soon as possible
in ischaemic stroke patients whenever such an event
is a suspected consequence of an occult or overt
malignancy causing systemic hypercoagulability.
References
1. Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985;64:16-35. Crossref
2. Taccone FS, Jeangette SM, Blecic SA. First-ever stroke
as initial presentation of systemic cancer. J Stroke
Cerebrovasc Dis 2008;17:169-74. Crossref
3. Trousseau A. Lectures on clinical medicine, delivered at
the Hotel-Dieu, Paris. 2nd ed. Philadelphia: Lindsay &
Blakiston; 1865: 281-332.
4. Varki A. Trousseau's syndrome: multiple definitions and
multiple mechanisms. Blood 2007;110:1723-9. Crossref
5. Deitcher SR. Cancer and thrombosis: mechanisms and
treatment. J Thromb Thrombolysis 2003;16:21-31. Crossref
6. el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic
endocarditis in cancer patients: pathogenesis, diagnosis,
and treatment. Oncologist 2007;12:518-23. Crossref
7. Bick RL. Cancer-associated thrombosis. N Engl J Med
2003;349:109-11. Crossref
8. Rogers LR. Cerebrovascular complications in cancer patients. Neurol Clin 2003;21:167-92. Crossref
9. Gross L, Friedberg CK. Nonbacterial thrombotic
endocarditis. Classification and general description. Arch
Intern Med (Chic) 1936;58:620-40. Crossref
10. Edoute Y, Haim N, Rinkevich D, Brenner B, Reisner
SA. Cardiac valvular vegetations in cancer patients: a
prospective echocardiographic study of 200 patients. Am
J Med 1997;102:252-8. Crossref
11. Rosen P, Armstrong D. Nonbacterial thrombotic
endocarditis in patients with malignant neoplastic
diseases. Am J Med 1973;54:23-9. Crossref
12. Lee RJ, Bartzokis T, Yeoh TK, Grogin HR, Choi D,
Schnittger I. Enhanced detection of intracardiac sources
of cerebral emboli by transesophageal echocardiography.
Stroke 1991;22:734-9. Crossref
13. Bell WR, Starksen NF, Tong S, Porterfield JK. Trousseau's
syndrome. Devastating coagulopathy in the absence of
heparin. Am J Med 1985;79:423-30. Crossref
14. Collins RC, Al-Mondhiry H, Chernik NL, Posner JB.
Neurologic manifestations of intravascular coagulation
in patients with cancer. A clinicopathologic analysis of 12
cases. Neurology 1975;25:795-806. Crossref

