Hong
Kong Med J 2017 Dec;23(6):594–8 | Epub 11 Aug 2017
DOI: 10.12809/hkmj165002
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Feasibility and safety of extended adjuvant
temozolomide beyond six cycles for patients with glioblastoma
Sonia YP Hsieh, MB, BS, MSc; Danny TM Chan, FRCS,
FHKAM (Surgery); Michael KM Kam, FRCR, FHKAM (Radiology); Herbert HF
Loong, MB, BS, MRCP (UK); WK Tsang, FRCR, FHKAM (Radiology); Darren MC
Poon, FRCR, FHKAM (Radiology); Stephanie CP Ng, PhD; WS Poon, FRCS, FHKAM
(Surgery)
CUHK Otto Wong Brain Tumour Centre, The Sir
Yue-kong Pao Centre for Cancer, Prince of Wales Hospital, Shatin, Hong
Kong
Corresponding author: Dr Danny TM Chan (tmdanny@surgery.cuhk.edu.hk)
Abstract
Introduction: Temozolomide is
the first chemotherapeutic agent proven effective for patients with
newly diagnosed glioblastoma. The drug is well tolerated for its low
toxicity. The current standard practice is concomitant chemoradiotherapy
for 6 weeks followed by 6 cycles of adjuvant temozolomide. Some
Caucasian studies have suggested that patients might benefit from
extended adjuvant cycles of temozolomide (>6 cycles) to lengthen both
progression-free survival and overall survival. In the present study, we
compared differences in survival and toxicity profile between patients
who received conventional 6-cycle temozolomide and those who received
more than 6 cycles of temozolomide.
Methods: Patients with newly
diagnosed glioblastoma without progressive disease and completed
concomitant chemoradiotherapy during a 4-year period were studied.
Progression-free survival was compared using Kaplan-Meier survival
curves. t Test, U test, and correlation were chosen
accordingly to examine the impact of age, extent of resection, MGMT
promoter methylation status and adjuvant cycles on progression-free
survival. For factors with a P value of <0.05 in univariate analyses,
Cox regression hazard model was adopted to determine the strongest
factors related to progression-free survival.
Results: The median
progression-free survival was 17.0 months for patients who received 6
cycles of temozolomide (n=7) and 43.4 months for those who received more
than 6 cycles (n=7) [P=0.007, log-rank test]. Two patients in the former
group and one in the latter group encountered grade 1 toxicity and
recovered following dose adjustment. Cycles of adjuvant temozolomide
were correlated with progression-free survival (P=0.016, hazard
ratio=0.68).
Conclusion: Extended cycles of
temozolomide are safe and feasible for Chinese patients with disease
responsive to temozolomide.
New knowledge added by this study
- Extended adjuvant temozolomide beyond 6 cycles is safe and feasible. The approach has improved progression-free survival.
- For glioblastoma patients with disease that is responsive to temozolomide, extended adjuvant cycles can be suggested.
Introduction
Glioblastoma multiforme (GBM) has been a conundrum
for all clinicians. The standard approach includes maximal safe resection,
irradiation with concurrent temozolomide (TMZ), and 6 cycles of adjuvant
TMZ.1 Addition of chemotherapy to
radiotherapy can prolong survival among GBM patients, with a median
increase in survival of 2.5 months.1
Since then, no further breakthrough treatment has emerged.
Of note, there is still insufficient evidence to
support 6 cycles as the optimal adjuvant amount of TMZ for GBM. Only few
studies have suggested that extended use of TMZ is safe and beneficial for
prolonged survival.2 The main
concern of extended use of TMZ is haematological toxicity. It is
attributed to the depletion of O6-methylguanine-DNA
methyltransferase (MGMT) protein activity in both GBM cells and
haematopoietic stem cells.3
Nonetheless, compared with other alkylating agents, the low toxicity
profile of TMZ has motivated clinicians to try its extended use after
balancing the benefits and side-effects for each patient.4
Our institution offers the option for GBM patients
with at least stable disease to step up to adjuvant cycles of TMZ. In this
study, we report the experience of extended use of TMZ and its impact on
newly diagnosed GBM patients.
Methods
Study design
We retrospectively reviewed the brain tumour
registry of the Chinese University of Hong Kong Otto Wong Brain Tumour
Centre, and identified patients with primary GBM during January 2010 to
December 2013. Those patients who received surgical intervention and
standard concomitant chemoradiotherapy after surgery (60-Gy irradiation
with concomitant TMZ for 6 weeks, then followed by at least 6 cycles of
adjuvant TMZ) were chosen as candidates for the study.
Surgical intervention
An experienced neuroradiologist was responsible for
determining the extent of resection (EOR) by reading the postoperative
day-1 magnetic resonance imaging (MRI) scans. A total resection indicated
that the entire preoperative contrast-enhanced lesion seen on T1-weighted
images was excised. If there was residual enhancement on T1-weighted
images as well as T1-subtraction sequence, the case would be labelled as
subtotal resection.
Irradiation and temozolomide protocol
As a standard practice, a postoperative irradiation
of 60 Gy was given to all patients within 4 weeks of surgery. Temozolomide
was prescribed concurrently during radiotherapy at 75 mg/d/m2
for 6 weeks, followed by 6 or more cycles of adjuvant TMZ at a dosage of
150-200 mg/d/m2 for 5 consecutive days every 28 days. After
completion of standard 6-cycle TMZ, all patients with at least stable
disease were offered the chance of extended TMZ, regardless of individual
prognostic factors. Whether or not they proceeded to extended TMZ was a
decision made principally by patients and their relatives and also with
neurosurgeons and clinical oncologists, on the basis of a detailed
assessment of the patient’s clinical performance (neurological status and
toxicity profile) and tumour response to TMZ. Anti-emetics were given
during the 5 days. To achieve early detection of TMZ toxicity,
haematological profile including complete blood picture with differential
count, and liver and renal functions were assessed monthly on about day 21
to day 25. Toxicity was graded according to the National Cancer Institute
Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.5
Follow-up schedule
All patients were followed up regularly with both
clinical and radiological assessments of disease status. They were seen by
a neuro-oncologist every 2 weeks after surgery, daily during irradiation,
and monthly when being given adjuvant TMZ. For radiological assessment,
patients were subjected to a scanning protocol with contrast-enhanced MRI
of the brain at postoperative day 1, 2 weeks after completion of
radiation, and every 3 months thereafter. These standardised protocols
ensured that disease progression of all patients could be monitored in a
timely manner. Disease progression was determined according to Macdonald’s
criteria. In short, deteriorating neurological status, increasing tumour
size, and appearance of new enhancement were suggestive of disease
progression.6
Statistical analysis
Progression-free survival (PFS) was calculated from
the date of surgical intervention to the date of progression. As the aim
of this study was to compare the therapeutic effect of standard 6-cycle
TMZ with that of more than 6 cycles of TMZ, only patients with neither
neurological deterioration nor radiological signs suggesting progression
for more than 28 days upon completion of the sixth cycle of adjuvant TMZ
were eligible. The MGMT promoter status and EOR were regarded as
categorical factors while age and cycles of adjuvant TMZ were assigned as
the continuous variables for which correlation was chosen as the tool for
analysis. t Test, U test, and correlation were chosen
accordingly to examine the impact on PFS of each factor. For those factors
with a P value of <0.05 in univariate analyses, a Cox regression hazard
model was adopted to determine those strongly related to PFS. All
statistical analyses were done using the SPSS (Windows version 22.0; IBM
Corp, Armonk [NY], US).
This audit review was conducted in accordance with
the principles outlined in the Declaration of Helsinki.
Results
Basic demographics
From January 2010 to December 2013, there were 14
patients who fulfilled the inclusion criteria. Their mean age at
presentation was 52 (range, 25-71) years with a male preponderance: 10
male versus 4 female patients. Total resection was achieved in seven
patients. For the remaining seven, six underwent subtotal resection and
one could only have tumour biopsy. The MGMT promoter status was available
in all cases and was methylated in 12 cases and unmethylated in the
remaining two. After completion of standard concomitant chemoradiotherapy
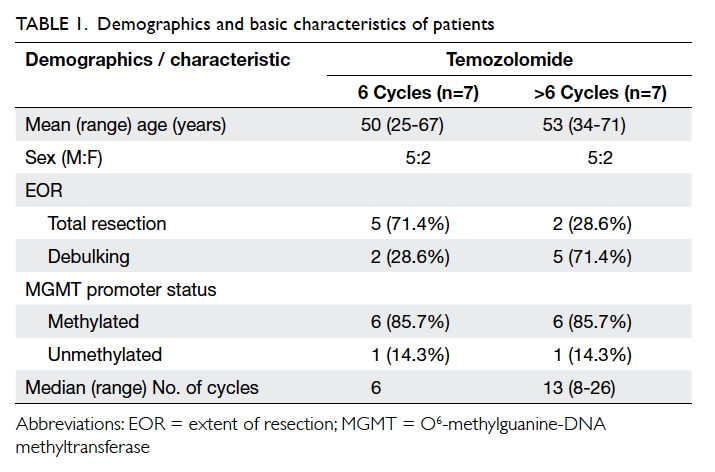
in all cases, extended adjuvant TMZ was initiated in seven cases (Table
1).
In total, 134 cycles of adjuvant TMZ were given,
with 92 cycles given to the seven patients who proceeded to extended
maintenance TMZ treatment. The median number of cycles given was 13
(range, 8-26) in the latter group. With regard to TMZ-related toxicity,
two patients in the 6-cycle group had grade 1 haematological toxicity
(thrombocytopaenia and neutropaenia) and one patient in the >6-cycle
group developed mildly deranged alanine aminotransferase (ALT; grade 1,
defined as “more than upper limit of normal and less than three times the
upper limit of normal by CTCAE”5)
during the fifth cycle of adjuvant TMZ that subsequently subsided.
Survival and associated prognostic factor
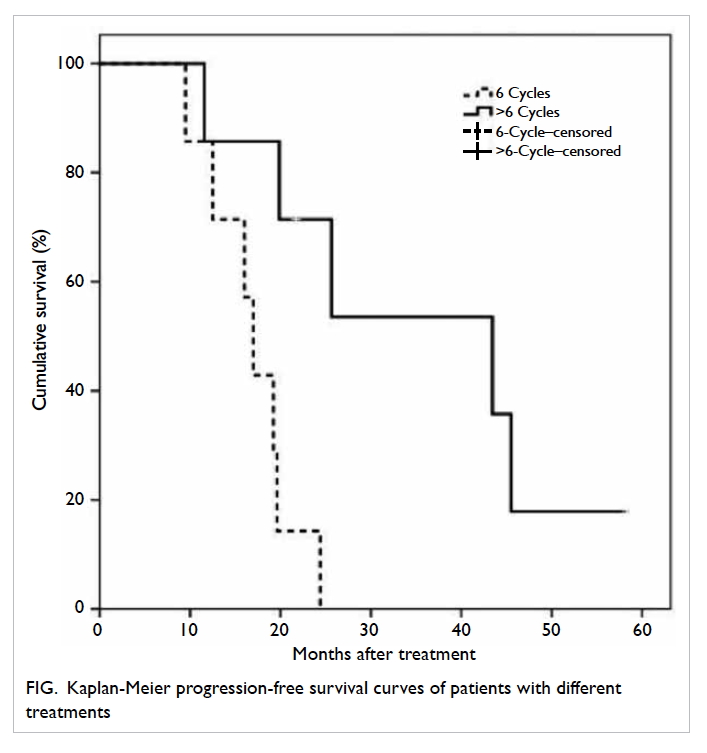
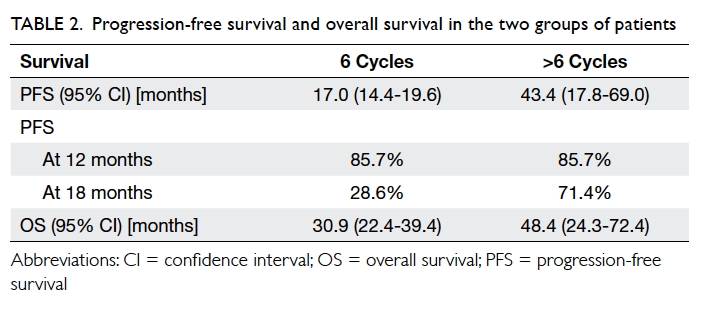
Progression-free survival differed significantly
between the two groups: 17.0 (95% confidence interval [CI], 14.4-19.6)
months for 6-cycle versus 43.4 (95% CI, 17.8-69.0) months for the
>6-cycle group (P=0.007, log-rank test; Fig).
Progression-free survival at 12 months was 85.7%
for both groups, and that at 18 months declined to only 28.6% for the
6-cycle group compared with 71.4% for the >6-cycle group (Table
2; Fig).
Three out of seven patients in the >6-cycle
group were still alive at their last follow-up. Their median overall
survival was 48.4 (95% CI, 24.3-72.4) months. In the 6-cycle group, the
median overall survival was 30.9 (95% CI, 22.4-39.4) months. No
statistical significance was observed by the end of the study (P=0.07,
log-rank).
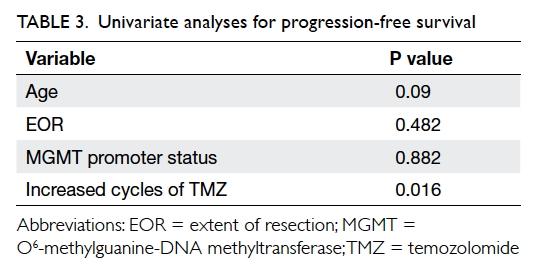
All factors including age, gender, and MGMT status
were well balanced, except for the EOR. Despite the higher proportion of
patients with subtotal resection who elected to receive extended TMZ, EOR
was not predictive of longer PFS (P=0.482, Mann-Whitney U test).
When subgrouping the cohort with MGMT promoter status, there was no
evidence to suggest that methylated MGMT promoter status favoured patients
with better PFS (P=0.882, Mann-Whitney U test). Age was also not
correlated with PFS (P=0.09, Pearson correlation). Cox regression hazard
model suggested that increased cycles of TMZ were associated with
prolonged PFS (P=0.016; hazard ratio=0.68 per cycle; 95% CI, 0.48-0.94) [Table 3].
Discussion
Despite recent advances in its therapy, GBM is
still an incurable disease, characterised by rapid and inevitable
recurrence even with intensive treatment. Ample clinical research has been
carried out with the intention of defeating the disease, but the prognosis
of GBM remains dismal. Temozolomide is the first chemotherapeutic agent
proven to be effective. The standard treatment after maximum safe
resection has two components, irradiation with concomitant TMZ and
adjuvant TMZ at a higher dosage for 6 cycles. Under this regimen, survival
is favourably improved.1 Since
then, no other encouraging milestones have been achieved.
Comparison of progression-free survival and toxicity
with other studies
Our audit review showed a significant correlation
between the number of cycles of TMZ and PFS. One patient in the
>6-cycle group showed a continuous yet prominent shrinkage of the
non-operable GBM (bilateral corpus callosum) after initiation of the
seventh cycle of TMZ and finally achieved complete response after 12
cycles. The patient had only mildly deranged ALT during the fifth cycle of
adjuvant TMZ and this subsided on its own.
Temozolomide was well-tolerated by most patients.
One of our previous studies also demonstrated its satisfying anti-tumoural
activity as well as its safety profile among ethnic Chinese population.7 Extended usage of TMZ upon
completion of standard 6-cycle adjuvant courses has become common practice
in many institutions.4 8 9
A literature search revealed only a few reports
with similar settings and conclusions. Three non-randomised retrospective
studies with decent sample sizes demonstrated an indispensable impact of
extended adjuvant TMZ. The reported PFS ranged from 13 to 24.6 months; the
overall survival was also improved.2
8 10
One very recent pooled analysis of four randomised clinical trials,
however, showed a slightly different result—PFS was the only outcome that
increased with the cumulative prescription of TMZ.11 Blumenthal et al11
reported that treatment with extended maintenance TMZ was significantly
associated with better PFS with a hazard ratio of 0.77 (6 cycles vs >6
cycles). To conclude, the positive impact of long-term use of TMZ on PFS
is supported by much evidence. Its influence on overall survival, however,
needs further clarification.
Toxicity after long-term usage of temozolomide
By sacrificing its only alkyl component to the
TMZ-induced lethal depletion of alkyl products on tumoural DNA, MGMT
serves as a suicidal DNA repair enzyme. Theoretically, this irreversible
depletion of the MGMT protein could be exploited by increasing tumoural
exposure to TMZ. The effect might be even more prominent when MGMT
promoter is hypermethylated, although the impact of MGMT promoter
methylation could not be demonstrated in the present study. Nonetheless
this mechanism also accounts for myelosuppression, the main concern of
long-term use of TMZ, since MGMT protein in normal cells can also be
depleted by TMZ. It is more common in haematopoietic stem cells
contributing to toxicity for patients using this alkylating agent.3 In a cohort that comprised 114 patients, 39 (34%) were
observed to have CTCAE grade 3 haematological toxicity during
administration of TMZ. The study included all patients who received 1 to
57 cycles of TMZ.8 The French SV3
Study also evaluated the effect of prolonged TMZ and suggested that 39.6%
of cases developed haematological toxicity beyond the second cycle.10 Toxicity to a certain degree discourages both
clinicians and patients from increasing the dosage of TMZ during adjuvant
therapy, and for extending use of TMZ beyond 6 cycles. In the current
study, only 21.4% (3/14) of our patients encountered mild side-effects.
Neuro-oncologists, however, remain reluctant to persuade patients to
receive long-term TMZ. It is generally accepted by clinicians that
long-term use of alkylating agents is unwise since they are likely to be
the eventual cause of myelosuppression and secondary cancers.
Clinical and financial situation in Hong Kong
In our institute, all patients with at least stable
disease for more than 28 days after completion of the sixth cycle of
adjuvant TMZ will be offered the option of continuing TMZ beyond 6 cycles,
after being given detailed information about possible future side-effects.
Of note, TMZ is funded in Hong Kong only for the first six adjuvant
cycles; patients need to pay thereafter, making the inherent
socio-economic bias unavoidable.
This study had several limitations. The sample size
was relatively small. The analyses presented may provide only limited and
preliminary evidence. Moreover, due to the nature of this study, only
patients with disease responsive to TMZ yet with no or mild TMZ-related
toxicity were qualified for the study.
Conclusion
Extended treatment with TMZ is safe and effective
in Chinese patients with disease that is responsive to it. Careful
assessment and consideration of continuing adjuvant TMZ is feasible for
this group of patients.
Declaration
All authors have disclosed no conflicts of
interest.
References
1. Stupp R, Mason WP, Van Den Bent MJ, et
al. Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med 2005;352:987-96. Crossref
2. Barbagallo GM, Paratore S, Caltabiano R,
et al. Long-term therapy with temozolomide is a feasible option for newly
diagnosed glioblastoma: a single-institution experience with as many as
101 temozolomide cycles. Neurosurg Focus 2014;37:E4. Crossref
3. Wick W, Platten M, Weller M. New
(alternative) temozolomide regimens for the treatment of glioma. Neuro
Oncol 2009;11:69-79. Crossref
4. Mason WP, Maestro RD, Eisenstat D, et
al. Canadian recommendations for the treatment of glioblastoma multiforme.
Curr Oncol 2007;14:110-7. Crossref
5. Common Terminology Criteria for Adverse
Events (CTCAE), Version 4.0. Available from:
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
Accessed 31 Mar 2016.
6. Macdonald DR, Cascino TL, Schold SC Jr,
Cairncross JG. Response criteria for phase II studies of supratentorial
malignant glioma. J Clin Oncol 1990;8:1277-80. Crossref
7. Chan DT, Poon WS, Chan YL, Ng HK.
Temozolomide in the treatment of recurrent malignant glioma in Chinese
patients. Hong Kong Med J 2005;11:452-6.
8. Seiz M, Krafft U, Freyschlag CF, et al.
Long-term adjuvant administration of temozolomide in patients with
glioblastoma multiforme: experience of a single institution. J Cancer Res
Clin Oncol 2010;136:1691-5. Crossref
9. Hau P, Koch D, Hundsberger T, et al.
Safety and feasibility of long-term temozolomide treatment in patients
with high-grade glioma. Neurology 2007;68:688-90. Crossref
10. Rivoirard R, Falk AT, Chargari C, et
al. Long-term results of a survey of prolonged adjuvant treatment with
temozolomide in patients with glioblastoma (SV3 Study). Clin Oncol (R Coll
Radiol) 2015;27:486-7. Crossref
11. Blumenthal DT, Stupp R, Zhang P, et
al. ATCT-08. The impact of extended adjuvant temozolomide in
newly-diagnosed glioblastoma: A secondary analysis of EORTC and NRG
Oncology/RTOG. Neuro Oncol 2015;17(Suppl 5):v2. Crossref