Hong Kong Med J 2017 Oct;23(5):470–9 | Epub 4 Aug 2017
DOI: 10.12809/hkmj164684
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Assessment of dietary food and nutrient intake
and bone density in children with eczema
TF Leung, MD, FRCPCH1;
SS Wang, PhD1; Flora YY Kwok, MPhil1; Lesley WS Leung, BSc2; CM Chow, MB, ChB1; KL Hon, MD, FAAP1
1 Department of Paediatrics, The Chinese University of Hong Kong, Prince
of Wales Hospital, Shatin, Hong Kong
2 Faculty of Science, The University of Melbourne, Melbourne, Victoria,
Australia
Corresponding author: Dr TF Leung (tfleung@cuhk.edu.hk)
Abstract
Introduction: Dietary restrictions are common
among patients with eczema, and such practice may
lead to diminished bone mineral density. This study
investigated dietary intake and bone mineral density
in Hong Kong Chinese children with eczema.
Methods: This cross-sectional and observational
study was conducted in a university-affiliated
teaching hospital in Hong Kong. Chinese children
aged below 18 years with physician-diagnosed
eczema were recruited from our paediatric allergy
and dermatology clinics over a 6-month period in
2012. Subjects with stable asthma and/or allergic
rhinitis who were free of eczema and food allergy
as well as non-allergic children were recruited from
attendants at our out-patient clinics as a reference
group. Intake of various foods and nutrients was
recorded using a food frequency questionnaire
that was analysed using Foodworks Professional
software. Bone mineral density at the radius and the
tibia was measured by quantitative ultrasound bone
sonometry, and urinary cross-linked telopeptides
were quantified by immunoassay and corrected for
creatinine level.
Results: Overall, 114 children with eczema
and 60 other children as reference group were
recruited. Eczema severity of the patients was
classified according to the objective SCORing
Atopic Dermatitis score. Males had a higher daily
energy intake than females (median, 7570 vs 6736
kJ; P=0.035), but intake of any single food item or
nutrient did not differ between them. Compared
with the reference group, children with eczema had
a higher intake of soybeans and miscellaneous dairy
products and lower intake of eggs, beef, and shellfish.
Children with eczema also consumed less vitamin D,
calcium, and iron. The mean (standard deviation)
bone mineral density Z-score of children with
eczema and those in the reference group were 0.52
(0.90) and 0.55 (1.12) over the radius (P=0.889), and
0.02 (1.03) and –0.01 (1.13) over the tibia (P=0.886),
respectively. Urine telopeptide levels were similar
between the groups. Calcium intake was associated
with bone mineral density Z-score among children
with eczema.
Conclusions: Dietary restrictions are common
among Chinese children with eczema in Hong Kong,
who have a lower calcium, vitamin D, and iron intake.
Nonetheless, such practice is not associated with
changes to bone mineral density or bone resorptive
biomarker.
New knowledge added by this study
- Hong Kong children with moderate-to-severe eczema had a lower consumption of eggs, beef, and shellfish as well as vitamin D, calcium, and iron.
- Children with eczema had an increased intake of soybean and miscellaneous dairy products.
- These changes in dietary and nutrient intake were not associated with altered bone mineral density or urinary levels of cross-linked N-telopeptides of type 1 collagen in our children with eczema.
- Nutritional assessment and counselling should be offered to parents with children who have moderate-to-severe eczema.
- Children with eczema who have extensive food avoidance or impaired growth should undergo allergy evaluation so that their family can follow evidence-based advice about dietary modification.
- Bone mineral density assessment is unnecessary for the majority of children with eczema.
Introduction
Eczema is a chronic inflammatory skin disease
associated with cutaneous hyperreactivity to
environmental stimuli such as microbial exposure
and food ingestion that are otherwise tolerated by
unaffected subjects.1 In our community study, nearly one third of preschool children had eczema and 8.1%
had parent-reported adverse food reactions.2 Thus,
there is a significant health care burden from both
eczema and food allergy in Hong Kong. Food allergy
is believed by many patients and their family to be
a major cause of eczema. Many traditional Chinese medicine practitioners also advise extensive food
avoidance for eczema. Our previous study reported
that over half of local children with eczema practised
food avoidance.3 There are, however, limited
objective data on the intake of specific food items
and nutrients in children with eczema. Henderson
and Hayes4 reported the correlation between dietary
calcium intake and bone mineral density (BMD) in
55 children and adolescents aged 5 to 14 years with
cow’s milk sensitisation. Their calcium intake was
determined using a food frequency questionnaire
(FFQ). The bone density Z-score of their patients
with a milk allergy serially increased with increasing
calcium intake. In another study, Jensen et al5
investigated BMD in children aged 8 to 17 years
with verified cow’s milk allergy (CMA) for more
than 4 years and compared them with 343 healthy
controls. Their patient’s BMD was markedly reduced
for age, and height for age was reduced indicating
‘short’ bones. Furthermore, calcium consumption
was about 25% of that recommended. In another
study, BMD was assessed in 27 young children with
CMA.6 During bone resorption, osteoclasts secrete
a mixture of acid and neutral proteases that degrade
the collagen fibrils into molecular fragments
including C-terminal telopeptide. Its aspartic acid
changes from alpha to beta form as bone ages.
The latter form called beta-crosslaps is a specific marker for bone resorption; its concentration was
lower in CMA patients than in controls, indicating
an increased bone turnover in the former. Ten CMA
patients had a BMD Z-score of less than –1 standard
deviation (SD) value. Despite these results, there
was limited evidence of compromised bone health
in eczema patients when such results were adjusted
for confounders such as calcium intake and physical
activity. This study investigated intake of food items
and nutrients as well as BMD in Chinese children
in Hong Kong with varying degrees of eczema, and
compared these values with unaffected children.
Methods
Study population
This cross-sectional study recruited ethnic Chinese
children aged below 18 years with physician-diagnosed
eczema from our paediatric allergy and
dermatology clinics over a 6-month period in 2012.
Eczema was diagnosed using standard criteria,7 and
disease severity was assessed by SCORing Atopic
Dermatitis.8 Patients were treated with topical
mometasone furoate only. Those prescribed other
topical steroids were changed to this drug for at
least 4 weeks before the study. Patients prescribed
oral immunosuppressive drugs within 6 months
were excluded. Subjects with stable asthma and/or allergic rhinitis who were free of eczema and
food allergy as well as non-allergic children were
recruited from attendants at our out-patient clinics
as a reference group. As an inclusion criterion,
subjects in the reference group had no dietary
restrictions. Following informed written consent,
our research staff recorded subjects’ intake of a wide
spectrum of dietary components using a Chinese
FFQ. Children who attended secondary school and
beyond were assessed using the adolescent/adult
version,9 10 whereas those in primary schools and
below were assessed using the preschool version.11
Intake of individual food items and groups of major
nutrients as recorded in FFQ were quantified and
analysed by Foodworks Professional software
(version 7; Xyris, Brisbane, Australia). This software
included only certain Chinese food items commonly
found in Australia. Thus, our co-author (FYYK), who
has a dietetic qualification, added new Chinese food
data with reference to a comprehensive Chinese
food composition database published in 2004 by
the Peking University Medical Press (available
upon request). These new food items were saved in
the software as a new food composition database
so that their nutrient data could be computed.
Physical activity level of participants was assessed
using a Physical Self-Description Questionnaire.12
The study was approved by the Clinical Research
Ethics Committee of our university. All participants
provided written informed consent.
Urinary concentration of cross-linked
N-telopeptides of type 1 collagen
Urinary concentrations of cross-linked N-telopeptides
of type 1 collagen (NTx), biomarkers of
bone resorption,13 were measured by enzyme-linked
immunosorbent assay (Wampole Laboratories,
Princeton [NJ], US) with a lower limit of detection
set at 20 nM of bone collagen equivalent. Results
were corrected for creatinine that was measured by
modified Jaffe reaction (Roche Diagnostics GmbH,
Mannheim, Germany).
Bone mineral density measurement
Participants’ BMD was measured at the mid-point
of the radius in the non-dominant arm and at the left
tibia using quantitative ultrasound bone sonometry
(QUBS) to determine the velocity of ultrasound
wave, expressed as the speed of sound in m/s, using
Omnisense 7000P (BeamMed, Petach Tikva, Israel)
as described previously.14 15 This machine compared
speed of sound measurements to a built-in reference
database of a healthy urban Chinese population of
797 boys and 760 girls aged 0 to 18 years. As described
in the manufacturer’s manual, subjects with previous
osteoporotic fractures, use of medications affecting
bone health, presence of a disease known to affect
bone metabolism, recent prolonged immobilisation,
or a systemic malignant disease within 5 years
were excluded from such reference database. The
Omnisense devices were transported from place to
place, and the same group of operators performed all
measurements. Results of BMD were then expressed as age- and gender-matched Z-score.
Statistical analysis
Dietary food and nutrient intake, BMD Z-scores,
and urine NTx levels between different groups were
analysed by Student’s t test or analysis of variance
(ANOVA). The relationship between eczema and
dietary, BMD, and urinary variables that differed
significantly between children with eczema and
those in the reference group were confirmed by
multivariable stepwise binary logistic regression
adjusted for age, gender, body mass index (BMI),
physical activity level, and co-morbid allergic
diseases as covariates. The correlations between
clinical variables, BMD Z-scores, and urine NTx
levels were analysed by Pearson correlation.
Continuous variables with skewed data distribution
were transformed to achieve normal distribution
prior to analyses. All analyses were performed with
the use of SPSS (Windows version 18.0; SPSS Inc,
Chicago [IL], US). The level of significance was set at
0.05, and all P values were two-tailed.
Results
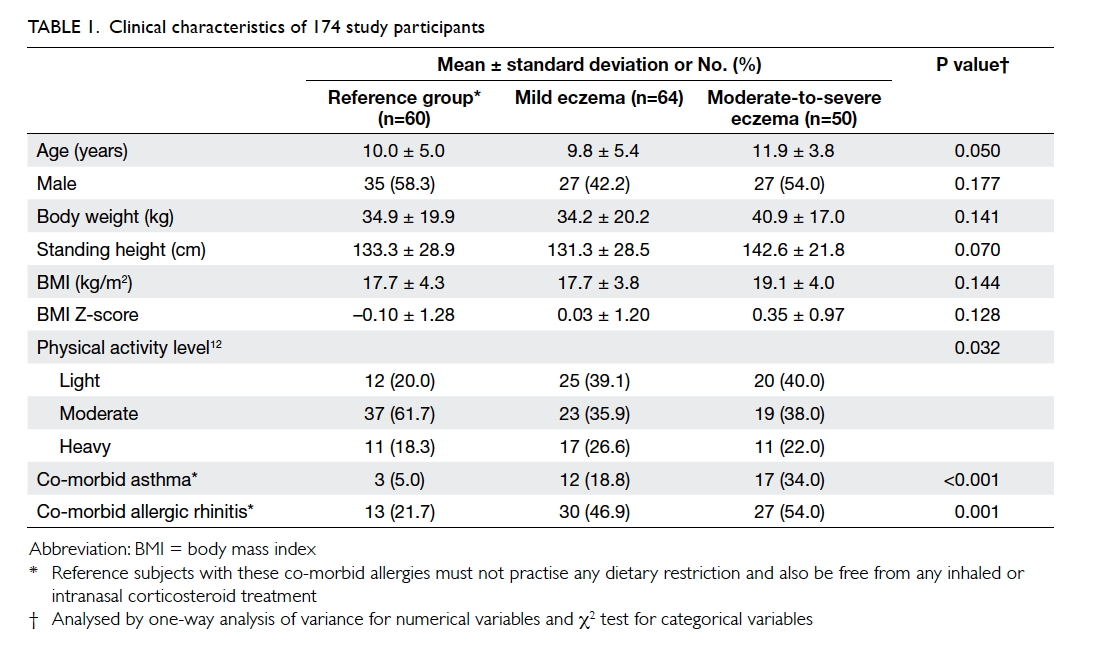
A total of 114 children with eczema and other 60
children as the reference group were recruited
(Table 1). The means (± SDs) age of children in the
reference group, children with mild eczema, and
children with moderate-to-severe eczema were 10.0
± 5.0, 9.8 ± 5.4, and 11.9 ± 3.8 years, respectively.
Age, gender, and anthropometric variables did not
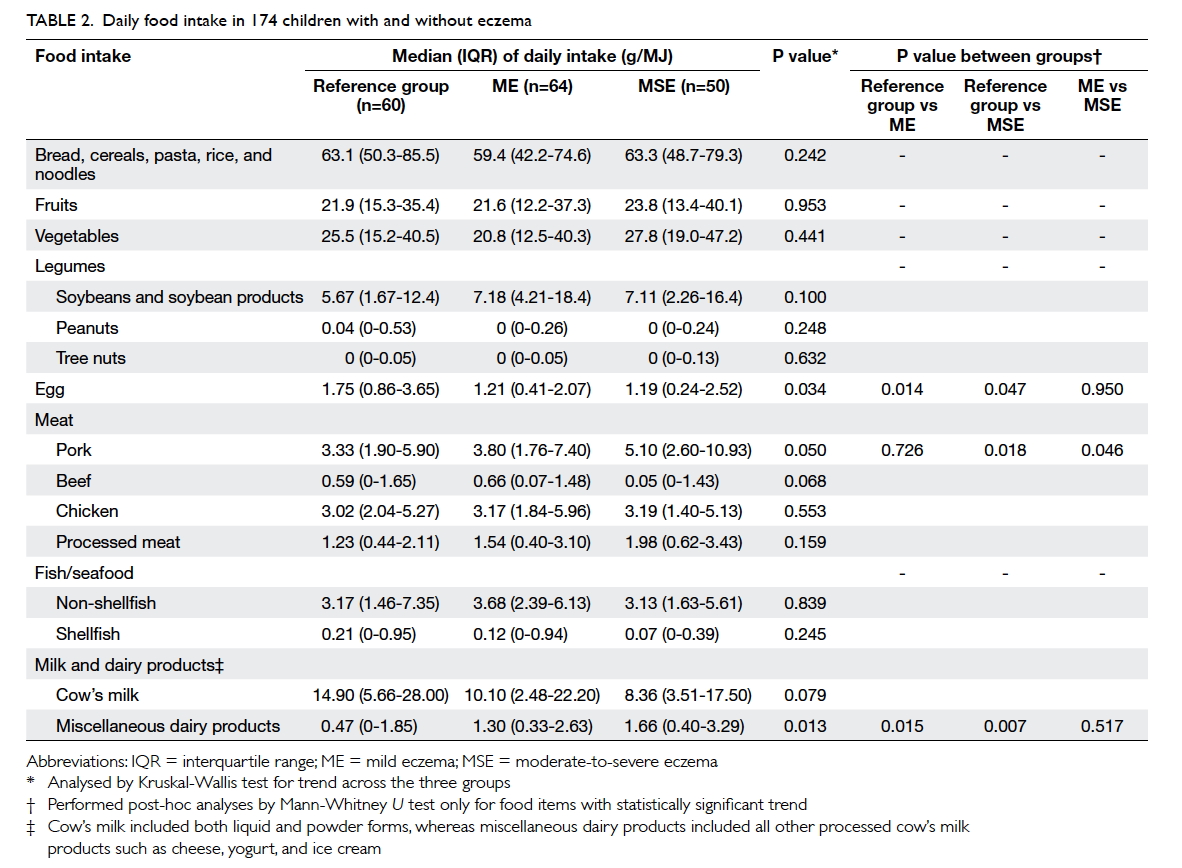
differ among children with eczema and those in the reference group. Table 2 describes the children’s daily
food intake adjusted for total energy as recorded by
FFQ. Males had a higher daily energy intake than
females (median [interquartile range]: 7570 [6267-9487] kJ vs 6736 [5438-8547] kJ; P=0.035), but intake
of any single food item or nutrient did not differ
when adjusted for daily energy intake. Multivariable
stepwise binary logistic regression analyses revealed that a
higher intake of soybeans and soybean products
as well as miscellaneous dairy products was
associated with an increased risk of eczema,
although statistically significant associations were
only found between the third tertile of soybean
intake and mild eczema, as well as between the second tertile and mild
eczema, and between the third tertile and moderate-to-severe
eczema for intake of miscellaneous dairy products.
A higher consumption of eggs, beef, and shellfish
was associated with lower risk of eczema (Table 3).
Children with eczema and those in the reference
group consumed similar supplements of cod liver oil,
fish oil, vitamins, and calcium (P>0.9 for all; data not
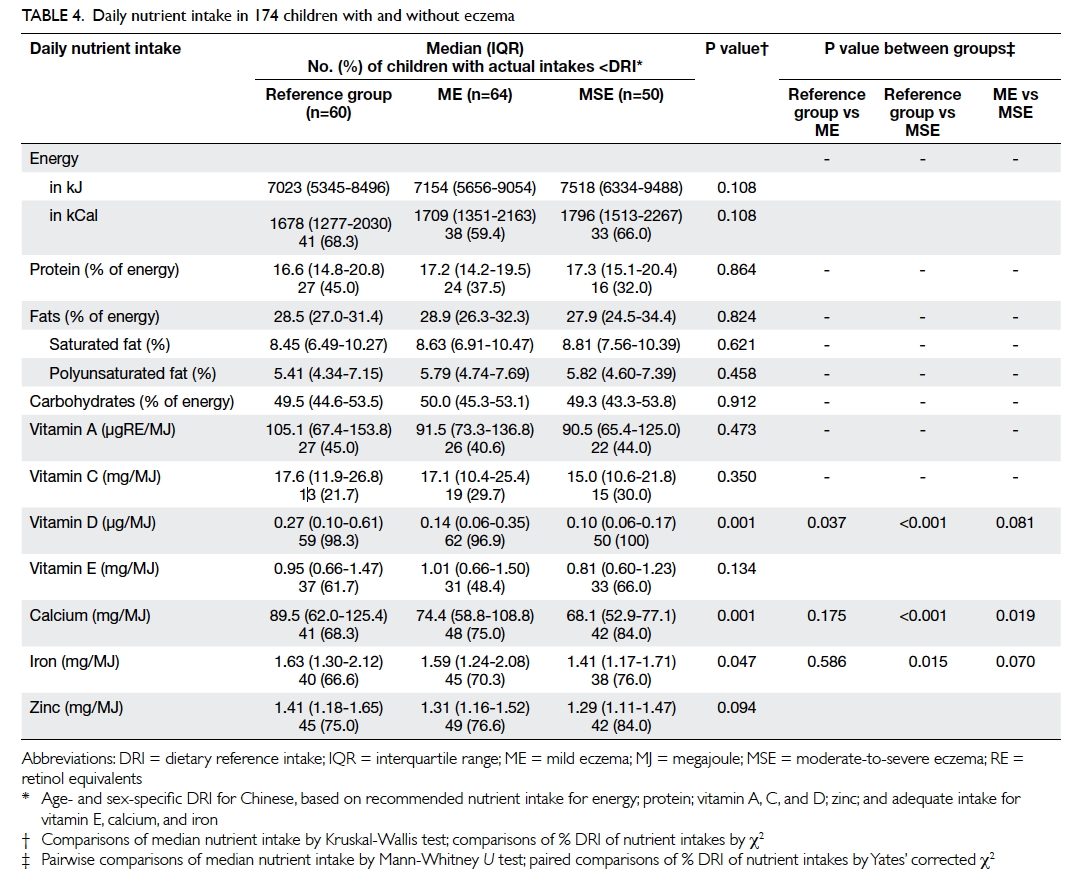
shown). Table 4 summarises their nutrient intake. Patients with moderate-to-severe eczema consumed
a lower amount of vitamin D, calcium, and iron when
compared with those with mild eczema and/or those
in the reference group. Children with the highest
tertile of intake for vitamin D (odds ratio [OR]=0.16;
95% confidence interval [CI] 0.05-0.48; P=0.001) and
calcium (OR=0.17; 95% CI, 0.06-0.51; P=0.002) had
a lower risk for moderate-to-severe eczema than
those with the lowest tertile when adjusted for age,
gender, BMI Z-score, and physical activity level as
covariates.
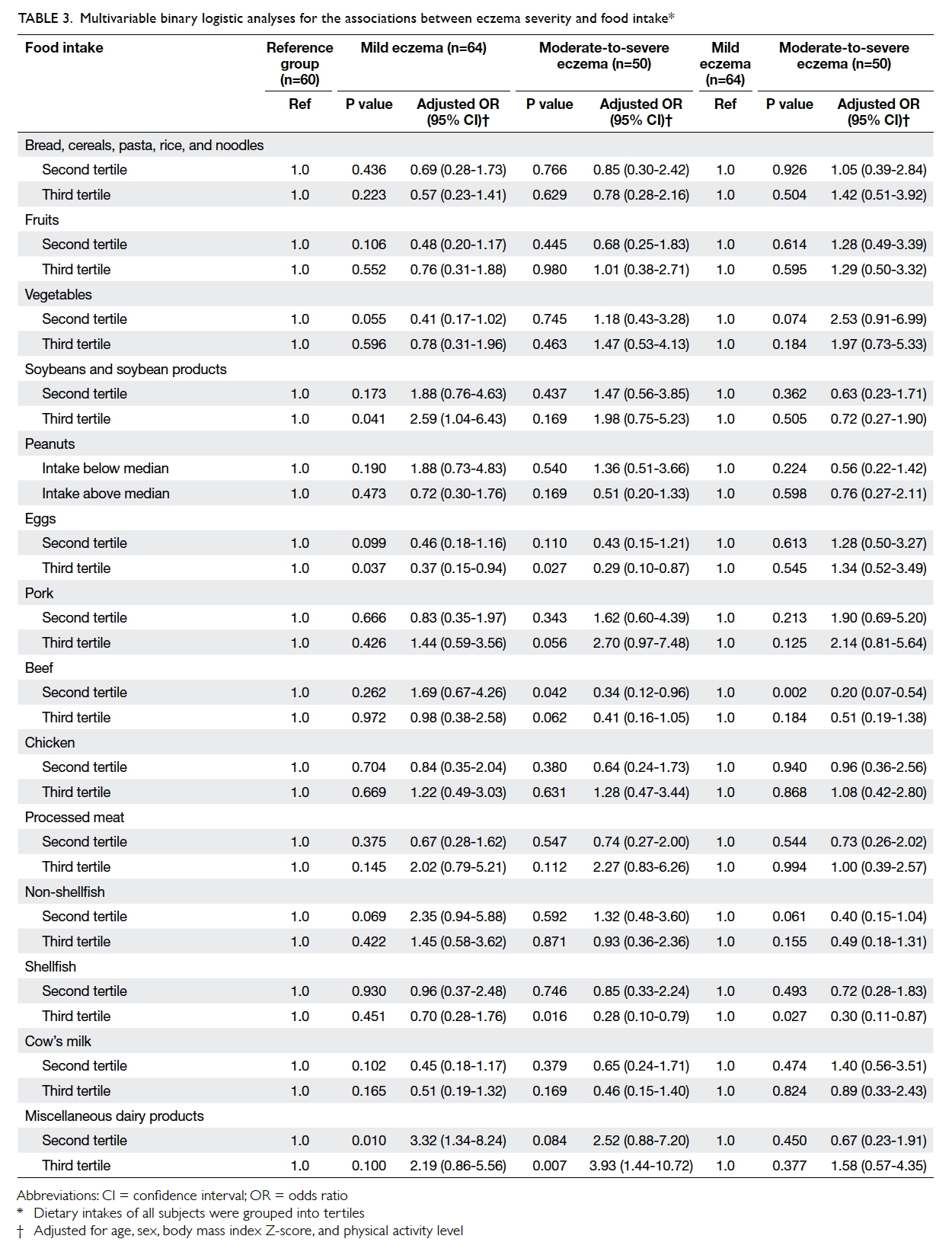
Table 3. Multivariable binary logistic analyses for the associations between eczema severity and food intake
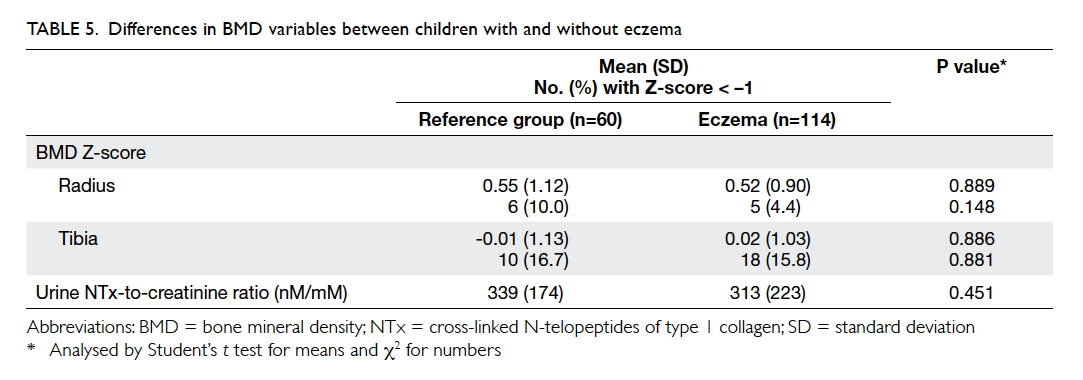
The mean BMD Z-score for children with
eczema and those in the reference group was 0.52
and 0.55 over the radius (P=0.889), and 0.02 and
–0.01 over the tibia (P=0.886) [Table 5]. The BMD
did not differ between children with mild and
moderate-to-severe eczema over these two regions
(P=0.296 and 0.661, respectively). The BMD Z-score
at the tibia correlated inversely with children’s
age (r = –0.282, P<0.001), but the Z-score at both
regions was independent of BMI Z-score. Age of
onset of eczema did not influence BMD Z-score at the radius (P=0.349) or tibia (P=0.240), nor was
it associated with physical activity level (P>0.1
for both). Urine NTx levels did not differ between
patients and reference subjects (P=0.451; Table 5), between reference subjects and those with mild or
moderate-to-severe eczema, or between those with
mild and moderate-to-severe eczema (P>0.3 for
all). This biomarker showed an inverse correlation with subject’s age (r = –0.569, P<0.001) but not BMI Z-score (r = –0.132, P=0.101) or BMD Z-score at
the radius (r = –0.133, P=0.097) or tibia (r = –0.135, P=0.093). Repeated analyses in eczema or reference
subject subgroups yielded similar results.
Regarding the possible effects of calcium
intake, the tertiles of daily intake were not associated
with BMD Z-score at either the radius or tibia among
reference subjects (Table 6). On the other hand,
patients at the second tertile of calcium intake had
a lower BMD Z-score at both the radius and tibia
than those at the first and third tertiles (P=0.016 and
0.015, respectively by one-way ANOVA). Patients
with the highest tertile of calcium intake had higher
BMD Z-score at the tibia.
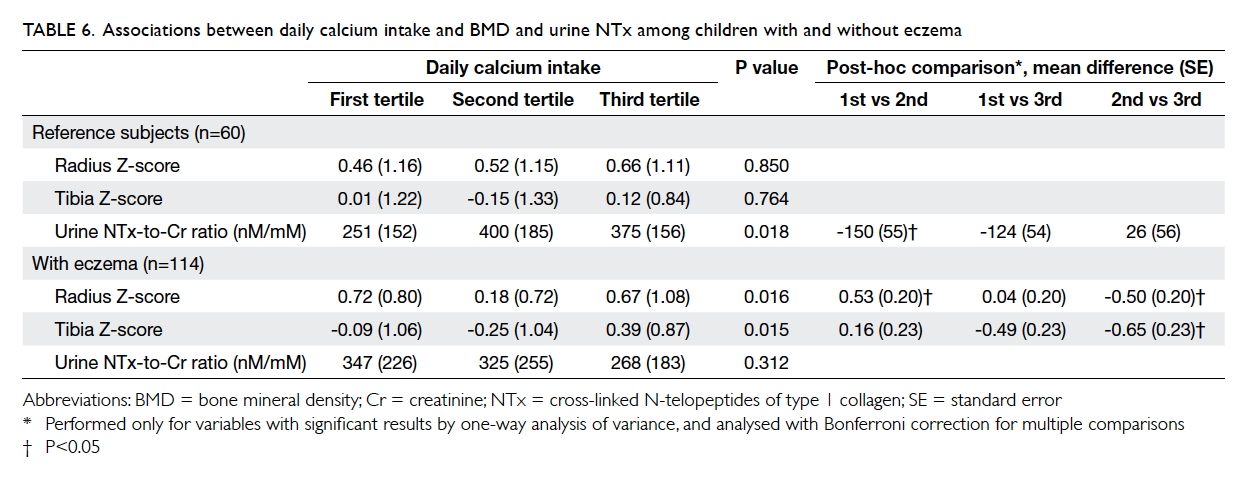
Table 6. Associations between daily calcium intake and BMD and urine NTx among children with and without eczema
Discussion
This study is the first to report the effects of eczema
and dietary intake on BMD in Chinese children in
Hong Kong. Children with eczema had a higher
intake of soybeans and miscellaneous dairy products
but lower intake of eggs, beef, shellfish, vitamin D,
calcium, and iron than the reference group. Despite
these differences, children with eczema had similar
BMD Z-scores at the radius and tibia and urinary
NTx levels. We also observed a trend for patients
with the highest tertile of calcium intake to have a
higher BMD Z-score at the tibia.
Food allergy is commonly believed to be a
major cause of eczema in the Chinese culture. Werfel
and Breuer16 supported this by the finding that atopic
dermatitis could be exacerbated by certain foods in
more than 50% of affected children. Nonetheless,
reactions induced by classic foods such as hen’s eggs
and cow’s milk were less common in adolescents and
adults than in young children. Food allergy in eczema
may be immunoglobulin (Ig) E–mediated or non–IgE-mediated, thus food-induced eczema should be diagnosed only by thorough clinical history-taking
and diagnostic work-up. Because of a possible
co-existence of eczema and food allergy, dietary
restrictions form an integral component of eczema
management in children. A systematic review of 421
participants from nine randomised controlled trials
only suggested some benefit of an egg-free diet in
infants with suspected egg allergy who had positive
specific IgE to eggs. No benefit, however, could be
detected for the use of various exclusion diets in
unselected people with atopic eczema.17 Clinicians
should also bear in mind the dynamic nature of food
allergy, and that young children might outgrow such
allergies.
Eczema severity was associated with altered
dietary intake in our children. Of Hong Kong
children aged 2 to 7 years, 8% reported adverse food
reactions, with the six leading foods being shellfish,
eggs, peanuts, beef, cow’s milk, and tree nuts.2 Such
adverse reactions to multiple foods also led to worse
quality of life.18 In the EuroPrevall study, shrimp,
mango, milk, eggs, and peanuts were the foods most
commonly reported to cause allergy among primary
schoolchildren in Hong Kong.19 20 According to the
presence of allergen-specific IgE, however, milk and
egg sensitisation was identified in over 14% of these
subjects whereas that for all other foods including
shrimp and peanuts was less than 8%. In our
hospital-based patients, skin prick testing revealed
that shellfish, peanuts, nuts, and eggs were the most
common food allergens.21 In general, allergy to
milk, eggs, beef, and seafood is a common cultural
belief in Chinese families with children having eczema. Consistently, our patients with
moderate-to-severe eczema had a lower intake
of eggs, beef, and shellfish as well as vitamin D,
calcium, and iron (Tables 2 3
4). To compensate for such dietary restrictions,
they ingested more soybeans and soybean products,
as well as miscellaneous dairy products. These
alterations were consistent with our earlier findings
that dietary restrictions to avoid high calcium foods
such as cow’s milk were common among Hong
Kong children with eczema.3 22 Milk intake was also
negatively associated with eczema diagnosis among
Spanish primary schoolchildren.23
Dietary intake of vitamin D was very low in our
children, and virtually all (both cases and controls)
ingested a lower amount than the age- and sex-specific
dietary reference intake for the Chinese
population (Table 4). Our data also suggested an
inverse relationship between eczema severity and
vitamin D intake. These results echoed those of our
recent study in which low serum vitamin D level
was highly prevalent in both Hong Kong children
with eczema and non-allergic controls.24 Vitamin D
deficiency was associated with disease severity in our
eczema children. Our study found eczema severity
to be associated with several single-nucleotide
polymorphisms of vitamin D pathway genes.25 Besides
its role in bone metabolism, vitamin D3 exerted
pluripotent effects on both cutaneous adaptive (eg
T-cell activation and dendritic cell maturation) and
innate (eg expression of antimicrobial peptides)
immunity.26 27 Our data suggested low serum levels
of LL-37, the biologically active form of cathelicidin
involved in antimicrobial defence, in children with
eczema.28 Alterations in local vitamin D3 levels also
modulated skin barrier function.29 Most children in
Hong Kong, a subtropical region, had long school
hours on weekdays and swam mainly in indoor pools.
Thus, they are not expected to produce enough
vitamin D from sunlight exposure. Together with
our dietary data, we recommend that local children
increase their outdoor activities and dietary intake
of vitamin D.
Dietary intake of our children was recorded by
FFQ. This method has been used to assess habitual
intake over extended periods of time, ranging from the
past month to the past year, by asking respondents to
report frequency of consumption and often portion
size for a defined list of foods and beverages.30 This
allows for investigation of individual dietary pattern,
ranking of usual individual intake, and examination
of associations between frequency of consumption of
certain items and individual clinical conditions. Woo
et al9 developed an FFQ for the Hong Kong Chinese
population that was later adapted and validated in
local children and adolescents.11 12 13 18 Energy intake of
two thirds of our subjects was below the Chinese-specific
dietary reference intake (Table 4). Based
on our data, FFQ would overestimate the intake of
energy and macronutrients when compared with
a 3-day food record.11 Thus, both our patients and
controls had an inadequate nutritional intake.
Skeletal problems were common in children
with food allergy and eczema. The BMD in children
aged 8 to 17 years with CMA was markedly reduced for age, and calcium consumption was only one
quarter that recommended.5 Beta-crosslaps
concentration as a biomarker of bone turnover
was lower in CMA patients than in controls.6 Osteoporosis and osteopenia were detected in 4.8%
and 32.8% of 125 adults with moderate-to-severe
eczema, respectively.31 Low BMD in these patients
did not seem to respond to calcium and/or vitamin
D supplementation.32 Another small adult study
found similar BMD between subjects with eczema
and controls.33 In childhood eczema, patients with
severe disease treated with topical corticosteroids
and cyclosporin had much lower BMD as measured
by dual energy X-ray absorptiometry (DEXA) than
those prescribed topical corticosteroids alone.34 The BMD was similar between Dutch children
with moderate-to-severe eczema and the general
population.35 Overall, there are limited data
regarding BMD in childhood eczema. In this study,
children with eczema had similar sonographically
measured BMD at the radius and tibia and urinary
level of NTx when compared with controls (Table 5). Nonetheless, BMD Z-scores were lower among
patients in the second than third tertile of dietary
calcium intake (Table 6). We do not recommend
unjustified restriction of calcium-rich foods such as
cow’s milk and soybean in children with eczema.
This study has several limitations. First, we did
not record subjects’ outdoor activities or measure
their serum vitamin D level. Second, this study
assessed subjects’ dietary intake using a FFQ instead
of a 3-day food record. The FFQ is cheap and easy
to administer. Such FFQ with parental reporting was
also a reasonably valid way to collect dietary data
on children even in situations when parents do not
observe all meals and snacks eaten by their child.36 37 38 Third, because of its cross-sectional nature, this
study did not collect information about the duration
of food avoidance. Fourth, BMD of our children was
measured by ultrasound rather than DEXA; with the latter
being the gold standard for diagnosing osteoporosis.
There is substantial concern about radiation hazard
especially in children.39 This study adopted the
radiation-free technique of QUBS that was useful
in assessing BMD in children.40 Lastly, our patients
with moderate-to-severe eczema were nearly 2 years
older than the reference group although patients as
a whole group were of similar age (Table 1). Because
of the higher energy needs of older and bigger
children, we adjusted for subjects’ age and gender
in multivariable regression analyses and compared
their dietary intake with age- and gender-specific
dietary reference intakes.
Conclusion
Dietary restrictions are common among Chinese
children with eczema in Hong Kong. These patients
had a lower calcium, vitamin D, and iron intake. Despite this, childhood eczema was not associated
with diminished BMD. Nonetheless, a significant
association was detected between calcium intake
and BMD among these patients.
Acknowledgements
This work was funded by Direct Grants for Research
(2011.1.058 and 2013.2.033) of the Chinese
University of Hong Kong. We thank Yvonne YF Ho
and Patty PP Tse for helping with patient assessment
and data collection.
Declaration
The authors have disclosed no conflicts of interest.
References
1. Leung DY, Bieber T. Atopic dermatitis. Lancet
2003;361:151-60. Crossref
2. Leung TF, Yung E, Wong YS, Lam CW, Wong GW. Parent-reported
adverse food reactions in Hong Kong Chinese
pre-schoolers: epidemiology, clinical spectrum and risk
factors. Pediatr Allergy Immunol 2009;20:339-46. Crossref
3. Hon KL, Leung TF, Kam WY, Lam MC, Fok TF, Ng PC.
Dietary restriction and supplementation in children with
atopic eczema. Clin Exp Dermatol 2006;31:187-91. Crossref
4. Henderson RC, Hayes PR. Bone mineralization in children
and adolescents with a milk allergy. Bone Miner 1994;27:1-12. Crossref
5. Jensen VB, Jørgensen IM, Rasmussen KB, Mølgaard C,
Prahl P. Bone mineral status in children with cow milk
allergy. Pediatr Allergy Immunol 2004;15:562-5. Crossref
6. Hidvégi E, Arató A, Cserháti E, Horváth C, Szabó A, Szabó
A. Slight decrease in bone mineralization in cow milk-sensitive
children. J Pediatr Gastroenterol Nutr 2003;36:44-9. Crossref
7. Hanifin JM, Rajka G. Diagnostic features of atopic
dermatitis. Acta Derm (Stockh) 1980;92:44-7.
8. Hon KL, Leung TF, Wong Y, Fok TF. Lesson from
performing SCORADs in children with atopic dermatitis: subjective symptoms do not correlate well with disease
extent or intensity. Int J Dermatol 2006;45:728-30. Crossref
9.Woo J, Leung SS, Ho SC, Lam TH, Janus ED. A food
frequency questionnaire for use in the Chinese population
in Hong Kong: description and examination of validity.
Nutr Res 1997;17:1633-41. Crossref
10. Chan RS, Woo J, Chan DC, Cheung CS, Lo DH. Estimated
net endogenous acid production and intake of bone health-related
nutrients in Hong Kong Chinese adolescents. Eur J
Clin Nutr 2009;63:505-12. Crossref
11. Kwok FY, Ho YY, Chow CM, So CY, Leung TF. Assessment
of nutrient intakes of picky-eating Chinese preschoolers
using a modified food frequency questionnaire. World J
Pediatr 2013;9:58-63. Crossref
12. Yu CC, Sung RY, Hau KT, Lam PK, Nelson EA, So
RC. The effect of diet and strength training on obese
children’s physical self-concept. J Sports Med Phys Fitness
2008;48:76-82.
13. Bollen AM, Eyre DR. Bone resorption rates in children
monitored by the urinary assay of collagen type 1 cross-linked
peptides. Bone 1994;15:31-4. Crossref
14. Jones G, Boon P. Which bone mass measures discriminate
adolescents who have fractured from those who have not?
Osteoporosis Int 2008;19:251-5. Crossref
15. Christoforidis A, Printza N, Gkogka C, et al. Comparative
study of quantitative ultrasonography and dual-energy
X-ray absorptiometry for evaluating renal osteodystrophy
in children with chronic kidney disease. J Bone Miner
Metab 2011;29:321-7. Crossref
16. Werfel T, Breuer K. Role of food allergy in atopic dermatitis.
Curr Opin Allergy Clin Immunol 2004;4:379-85. Crossref
17. Bath-Hextall F, Delamere FM, Williams HC. Dietary
exclusions for improving established atopic eczema in
adults and children: systematic review. Allergy 2009;64:258-64. Crossref
18. Leung TF, Yung E, Wong YS, Li CY, Wong GW. Quality-of-life assessment in Chinese families with food-allergic
children. Clin Exp Allergy 2009;39:890-6. Crossref
19. Wong GW, Mahesh PA, Ogorodova L, et al. The
EuroPrevall-INCO surveys on the prevalence of food
allergies in children from China, India and Russia: the
study methodology. Allergy 2010;65:385-90. Crossref
20. Wong GW, Ogorodova L, Mahesh PA, et al. Food allergy
in schoolchildren from China, Russia and India: the
EuroPrevall-INCO surveys. Allergy 2011;66 Suppl 94:27.
21. Hon KL, Wang SS, Wong WL, Poon WK, Mak KY, Leung
TF. Skin prick testing in atopic eczema: atopic to what and
at what age? World J Pediatr 2012;8:164-8. Crossref
22. Hon KL, Leung TF, Lam MC, et al. Eczema exacerbation
and food atopy beyond infancy: how should we advise
Chinese parents about dietary history, eczema severity and
skin prick testing? Adv Ther 2007;24:223-30.
23. Suárez-Varela MM, Alvarez LG, Kogan MD, et al. Diet
and prevalence of atopic eczema in 6 to 7-year-old
schoolchildren in Spain: ISAAC phase III. J Investig
Allergol Clin Immunol 2010;20:469-75.
24. Wang SS, Hon KL, Kong AP, Pong HN, Wong GW, Leung
TF. Vitamin D deficiency is associated with diagnosis and
severity of childhood atopic dermatitis. Pediatr Allergy
Immunol 2014;25:30-5. Crossref
25. Wang SS, Hon KL, Kong AP, et al. Eczema phenotypes
are associated with multiple vitamin D pathway genes in
Chinese children. Allergy 2014;69:118-24. Crossref
26. Bikle DD. What is new in vitamin D: 2006-2007. Curr Opin
Rheumatol 2007;19:383-8. Crossref
27. Schauber J, Dorschner RA, Yamasaki K, Brouha B,
Gallo RL. Control of the innate epithelial antimicrobial
response is cell-type specific and dependent on relevant
microenvironmental stimuli. Immunology 2006;118:509-19. Crossref
28. Leung TF, Ching KW, Kong AP, Wong GW, Chan JC, Hon
KL. Circulating LL-37 is a biomarker for eczema severity in
children. J Eur Acad Dermatol Venereol 2012;26:518-22. Crossref
29. Bikle DD, Chang S, Crumrine D, et al. Mice lacking 25OHD
1alpha-hydroxylase demonstrate decreased epidermal
differentiation and barrier function. J Steroid Biochem Mol
Biol 2004;89-90(1-5):347-53. Crossref
30. Thompson FE, Byers T. Dietary assessment resource
manual. J Nutr 1994;124(11 Suppl):2245S-2317S.
31. Haeck IM, Hamdy NA, Timmer-de Mik L, et al. Low bone
mineral density in adult patients with moderate to severe
atopic dermatitis. Br J Dermatol 2009;161:1248-54. Crossref
32. Haeck I, van Velsen S, de Bruin-Weller M, Bruijnzeel-Koomen C. Bone mineral density in patients with atopic dermatitis. Chem Immunol Allergy 2012;96:96-9.
Crossref
33. Aalto-Korte K, Turpeinen M. Bone mineral density
in patients with atopic dermatitis. Br J Dermatol
1997;136:172-5. Crossref
34. Pedreira CC, King E, Jones G, et al. Oral cyclosporin plus
topical corticosteroid therapy diminishes bone mass in
children with eczema. Pediatr Dermatol 2007;24:613-20. Crossref
35. van Velsen SG, Knol MJ, van Eijk RL, et al. Bone mineral
density in children with moderate to severe atopic
dermatitis. J Am Acad Dermatol 2010;63:824-31. Crossref
36. Andersen LF, Lande B, Trygg K, Hay G. Validation of a
semi-quantitative food-frequency questionnaire used
among 2-year-old Norwegian children. Public Health Nutr
2004;7:757-64. Crossref
37. Blum RE, Wei EK, Rockett HR, et al. Validation of a food frequency questionnaire in native American and
Caucasian children 1 to 5 years of age. Matern Child Health
J 1999;3:167-72. Crossref
38. Parrish LA, Marshall JA, Krebs NF, Rewers M, Norris JM.
Validation of a food frequency questionnaire in preschool
children. Epidemiology 2003;14:213-7. Crossref
39. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure
from CT scans in childhood and subsequent risk of
leukaemia and brain tumours: a retrospective cohort study.
Lancet 2012;380:499-505. Crossref
40. Mainz JG, Sauner D, Malich A, et al. Cross-sectional
study on bone density-related sonographic parameters in
children with asthma: correlation to therapy with inhaled
corticosteroids and disease severity. J Bone Miner Metab
2008;26:485-92. Crossref