Hong Kong Med J 2017 Jun;23(3):282–90 | Epub 5 May 2017
DOI: 10.12809/hkmj166096
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Clinical use of venoarterial extracorporeal membrane oxygenation
George WY Ng, FCICM, FHKAM (Medicine);
Henry J Yuen, FHKCA(Intensive Care), FHKAM (Anaesthesiology);
KC Sin, FHKCP, FHKAM (Medicine);
Anne KH Leung, FCICM, FHKAM (Anaesthesiology);
KW Au Yeung, FCICM, FHKAM (Anaesthesiology);
KY Lai, FRCP (Edin), FHKAM (Medicine)
Department of Intensive Care, Queen Elizabeth Hospital, Jordan, Hong
Kong
Corresponding author: Dr George WY Ng (georgeng77@yahoo.com)
Abstract
With advances in mechanical circulation, venoarterial
extracorporeal membrane oxygenation
has become an established technique to provide
cardiopulmonary support for patients with
cardiovascular collapse. This article reviews the
physiological principles of such extracorporeal
technique and its interaction with the native
heart. Practical aspects including equipment,
patient selection, and common complications with
their prevention and specific management are
summarised. The strategy for weaning from venoarterial
extracorporeal membrane oxygenation is
also discussed.
Introduction
Venoarterial extracorporeal membrane oxygenation
(VA-ECMO) is an extracorporeal life support system
that can temporarily provide support to the body
circulation while the pumping function of the heart
is absent or very weak. It is used in different scenarios
of severe cardiac failure to support the patient and
serves as a bridge to recovery, to implantation of a
ventricular assist device, or to transplantation.
Types of extracorporeal membrane oxygenation circuits
The basic ECMO circuit comprises a non-pulsatile
pump for blood propulsion, and a membrane
oxygenator for gas exchange. In general, an ECMO
circuit can have two configurations: venovenous
(VV) and venoarterial (VA). While VV-ECMO
provides only pulmonary support, VA-ECMO can
provide both pulmonary and cardiac support. Of
note, VA-ECMO can be categorised further into
central VA and peripheral VA.
Peripheral
The VA-ECMO technique can provide both
respiratory and cardiac support. The circuit is
connected in parallel to the heart and lungs. In
peripheral VA-ECMO, the access cannula is usually
placed in either the right internal jugular vein or
the femoral vein. Deoxygenated blood is extracted
from the right heart circulation by a motor pump
that then drives the blood through an oxygenator.
Oxygen diffuses across the oxygenator membrane
into the blood that is returned to the arterial system
via the return cannula placed either at the femoral or
axillary artery (Fig 1a).
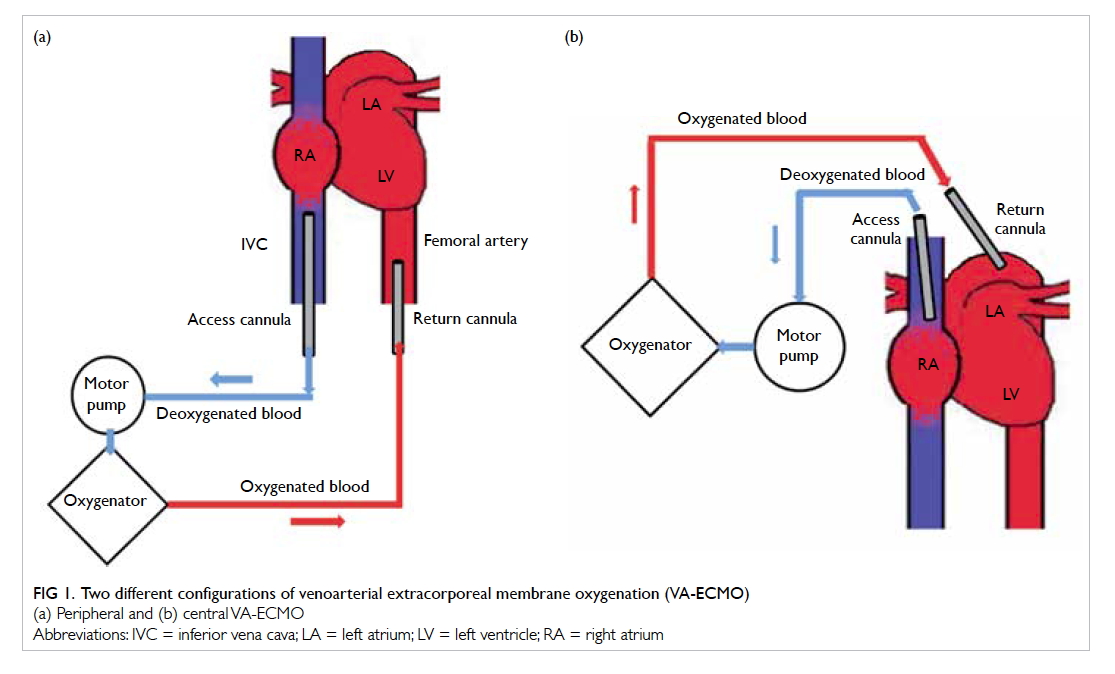
Figure 1. Two different configurations of venoarterial extracorporeal membrane oxygenation (VA-ECMO)
(a) Peripheral and (b) central VA-ECMO
Central
In central VA-ECMO, the access cannula is usually
placed in the right atrium. This requires surgical
placement with an open sternotomy (Fig 1b). The
oxygenated blood returns to the arterial system via
the return cannula placed in the ascending aorta.
Clinical indications and contra-indications for venoarterial extracorporeal membrane oxygenation
According to the Extracorporeal Life Support
Organization (ELSO) Registry, 41% of all patients
who underwent VA-ECMO survived to discharge or
transfer.1 On the contrary, the intra-aortic balloon
pump (IABP) was shown to have no survival benefit over
conventional medical treatment alone.2 3 Therefore,
VA-ECMO has emerged as the first-line treatment
to provide rapid support to patients with cardiogenic
shock, defined as a state of end-organ hypoperfusion
due to cardiac failure. Nonetheless, ECMO therapy
is not an ultimate treatment. It only helps to sustain
life for bridging to a definitive plan. Careful selection
of suitable candidates for VA-ECMO is vital for a
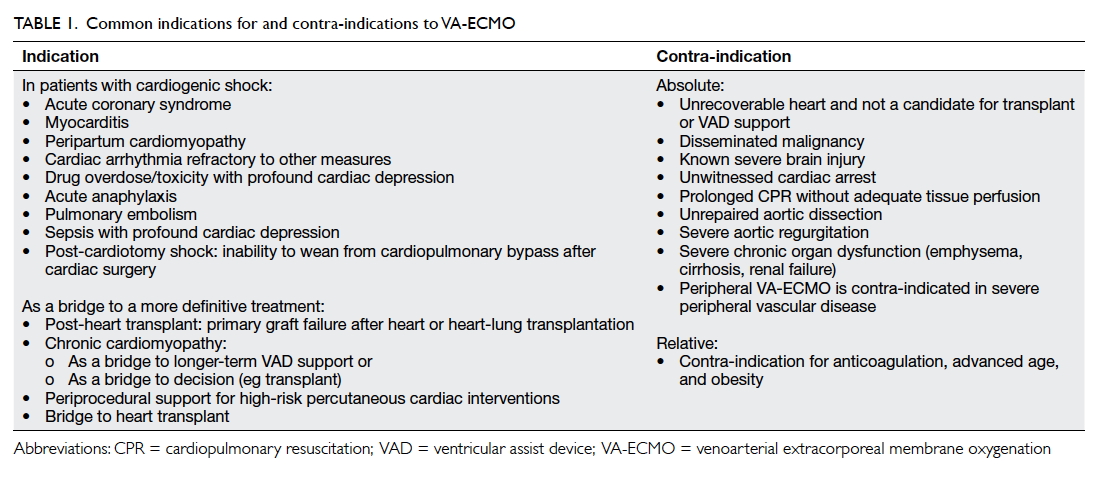
favourable outcome. Table 1 illustrates the common
indications for and contra-indications to VA-ECMO.
4 5
Physiology of the artificial heart
Support to the native heart
Unlike cardiopulmonary bypass, VA-ECMO
provides only approximately 80% of the predicted
resting cardiac output in the ideal setting. The
rest of the perfusion is still provided by the native
heart.6 Of note, VA-ECMO allows the heart to rest
by decreasing venous return and subsequent volume
work, wall tension, and oxygen consumption of the
heart.6 In addition, animal studies have shown that
the decrease in preload decreases left ventricular
end-diastolic volume and pressure, thus promoting
a better coronary perfusion pressure due to a greater
pressure gradient (coronary perfusion pressure
= aortic diastolic pressure – left ventricular end-diastolic
pressure).7 8 The return of oxygenated blood from the ECMO circuit to the arterial system,
however, may negatively affect the left ventricle (LV)
by increasing afterload and the pressure work of
the myocardium.9 The overall effect of the decrease
in volume work and the increase in pressure work
depends on the degree of ECMO support as well as
the native myocardial function and its response to
these triggers.
After commencing peripheral VA-ECMO
support, there is initially a decrease in cardiac
performance due to an increase in LV afterload. The
cardiac performance can usually return to baseline
by 72 hours.10 Right and left ventricular stroke
volumes are almost identical at baseline but vary
inversely with increasing ECMO pump flow rates.
The mean arterial pressure (MAP) is directly related
to the total aortic flow, which is the summation of the
native cardiac output and the ECMO pump flow. The
MAP should be kept above 60 mm Hg for adequate
organ perfusion:
MAP = (native CO + pump flow) x SVR
(where CO = cardiac output; SVR = systemic vascular resistance)
MAP = (native CO + pump flow) x SVR
(where CO = cardiac output; SVR = systemic vascular resistance)
The blood flow created by ECMO is nonpulsatile.
The patient with poor heart contractility
will have a narrow pulse contour and a small pulse
pressure. Absence of pulsatility in the arterial
waveform means that there is no blood being ejected
from the LV. At this point the aortic valve does not
open due to poor heart contractility, and total bypass
occurs (ECMO circuit takes over 100% of the cardiac
output).
Oxygenation
With the same blood flow and extracorporeal circuit
setting, VA-ECMO may theoretically provide better
lung support than VV-ECMO. First, the artificially
oxygenated blood returns directly to the arterial
systemic circulation to perfuse end-organs. Second,
in the presence of hypoxia, the pulmonary arteries
constrict so that blood is directed to the alveoli with
higher oxygen content. The extracorporeal circuit
of VV-ECMO is connected in series with the native
lungs. When blood with a high oxygen saturation
from the return cannula reaches the pulmonary
arteries, the shunt fraction of the native lung will
increase due to the loss of hypoxic pulmonary
vasoconstriction. In VA-ECMO, the extracorporeal
circuit is in parallel with the native lungs. The
return of oxygenated blood bypasses the venous
and pulmonary circulation so there is no loss of
oxygenation via the native lungs.11
Combined use of an intra-aortic balloon pump and extracorporeal membrane oxygenation
Peripheral VA-ECMO increases the afterload of the
LV as it directs blood into the descending aorta in
a retrograde direction. Intra-aortic balloon pump deflates in systole and inflates in diastole
and helps reduce afterload and improve coronary
perfusion, respectively. An IABP is sometimes used
concomitantly with VA-ECMO, especially for those
patients who are already on IABP but still have
refractory shock. It is believed that the concomitant
use of IABP can facilitate aortic valve opening and
achieve better LV decompression, improve aortic
diastolic pressure and thus coronary perfusion.12 A recent meta-analysis, however, found that the
concomitant use of IABP and ECMO in adult patients
with cardiogenic shock and cardiac arrest offered
no survival benefit.2 3 13 14 Special attention should
be paid to the arterial waveform when using IABP
with VA-ECMO. The pressure waveform generated
by the IABP and the arterial pulsation generated by
the native heart may have similar morphology. We
recommend examining the native arterial pulsation
with the IABP set to 2:1 assist ratio, and checking for
aortic valve opening by echocardiography with the
IABP on standby (Fig 2).
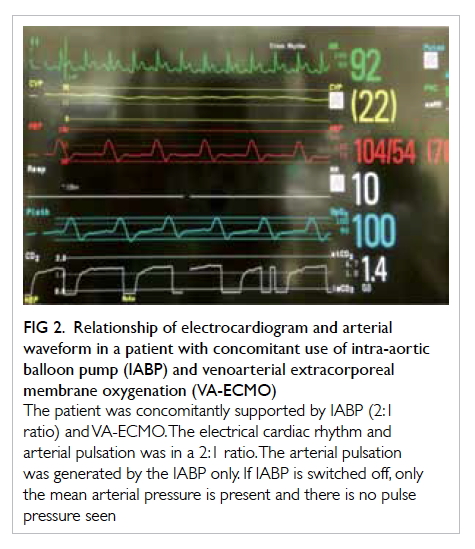
Figure 2. Relationship of electrocardiogram and arterial waveform in a patient with concomitant use of intra-aortic balloon pump (IABP) and venoarterial extracorporeal membrane oxygenation (VA-ECMO)
The patient was concomitantly supported by IABP (2:1 ratio) and VA-ECMO. The electrical cardiac rhythm and arterial pulsation was in a 2:1 ratio. The arterial pulsation was generated by the IABP only. If IABP is switched off, only the mean arterial pressure is present and there is no pulse pressure seen
Procedure
Cannulation
In peripheral VA-ECMO, the access cannula is
normally placed in the common femoral vein or
the right internal jugular vein. The return cannula
is usually placed in the common femoral artery
with the tip located in the iliac artery or abdominal
aorta. The cannulation procedure is accomplished
at the bedside via a Seldinger technique and
serial dilatation under ultrasound or fluoroscopic
guidance. Ultrasound is commonly used for
cannulation in the intensive care unit setting. The
femoral artery is round in shape and pulsatile, has a
thicker wall, is less easily compressed, and is lateral
to the femoral vein. In small patients, ultrasound
estimation of the vessel calibre is particularly useful
to select an appropriately sized cannula and predict
the need for distal limb perfusion. The guidewire of
the return cannula should preferably be visualised by
transoesophageal echocardiography or fluoroscopy
to confirm placement in the lumen of the aorta
before dilatation.
In central VA-ECMO, cannulation is done in
the operating theatre by the cardiothoracic surgical
team. The access cannula is placed in the right heart
circulation (superior vena cava or right atrium) and
the return cannula in the ascending aorta through an
open sternum. This is usually performed when the
patient fails to wean from cardiopulmonary bypass
after open heart surgery, as direct access is already
available.
Priming of circuit
The circuit, pump head, and oxygenator should be
primed with 0.9% normal saline before use so that
air inside the circuit is eliminated. The priming
procedure is done passively by gravity drainage from
the priming bag followed by active priming by the
ECMO machine.
Reperfusion cannula
The femoral artery is commonly used for cannulation
in peripheral VA-ECMO due to its ease of access. As
the return cannula is placed in a retrograde direction
in the femoral artery, perfusion of the ipsilateral
lower limb may be compromised as the direction
of blood flow from the return cannula is opposite
to that from the native heart. The risk of distal limb
ischaemia is even higher if large-size cannulas are
used. Successful distal limb perfusion in VA-ECMO
has been reported using various approaches,
including antegrade cannulation of the superficial
femoral artery, and retrograde cannulation of the
dorsalis pedis or posterior tibial artery.15 16 17 Antegrade
percutaneous cannulation of the superficial femoral
artery is the most commonly used technique in our
locality. The reperfusion cannula should be placed
using the Seldinger technique under fluoroscopic or
ultrasound guidance. If ultrasound is used, in-plane
visualisation is helpful to differentiate the superficial
femoral artery from the deep femoral artery, and to
guide the angle and depth of the puncture needle
(Fig 3a). The proximal end of the reperfusion cannula
is connected to the return cannula of the ECMO
circuit so that oxygenated blood can directly perfuse
the distal part of the lower limb (Fig 3b). Surgical
exposure for vascular access is an alternative in
difficult cases. A blood flow of 100-150 mL/min in
the superficial femoral artery is usually sufficient to
perfuse the leg.15 17 An additional flowmeter can be
used to monitor the blood flow of this limb of the
circuit.
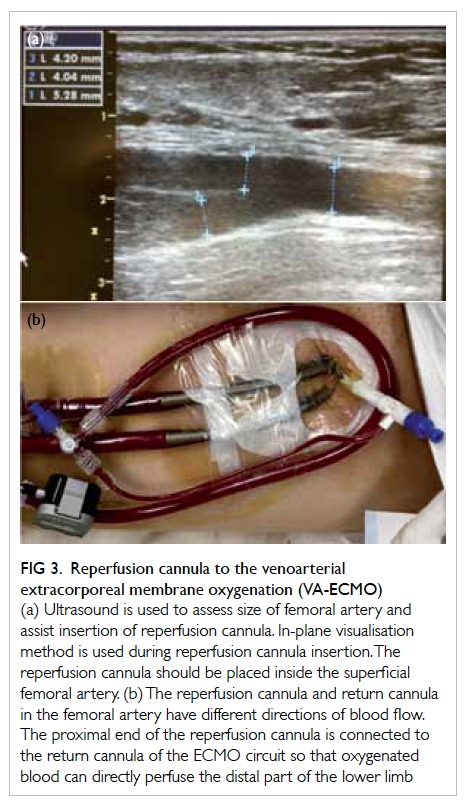
Figure 3. Reperfusion cannula to the venoarterial extracorporeal membrane oxygenation (VA-ECMO)
(a) Ultrasound is used to assess size of femoral artery and assist insertion of reperfusion cannula. In-plane visualisation method is used during reperfusion cannula insertion. The reperfusion cannula should be placed inside the superficial femoral artery. (b) The reperfusion cannula and return cannula in the femoral artery have different directions of blood flow. The proximal end of the reperfusion cannula is connected to the return cannula of the ECMO circuit so that oxygenated blood can directly perfuse the distal part of the lower limb
Possible complications related to venoarterial extracorporeal membrane oxygenation
The outcome of patients treated with VA-ECMO
is dependent on two factors: careful selection
of patients and avoidance of ECMO-related
complications. Improved understanding and
heightened awareness of known complications are
good preventive measures. Early detection and
timely treatment may prevent or mitigate harm to
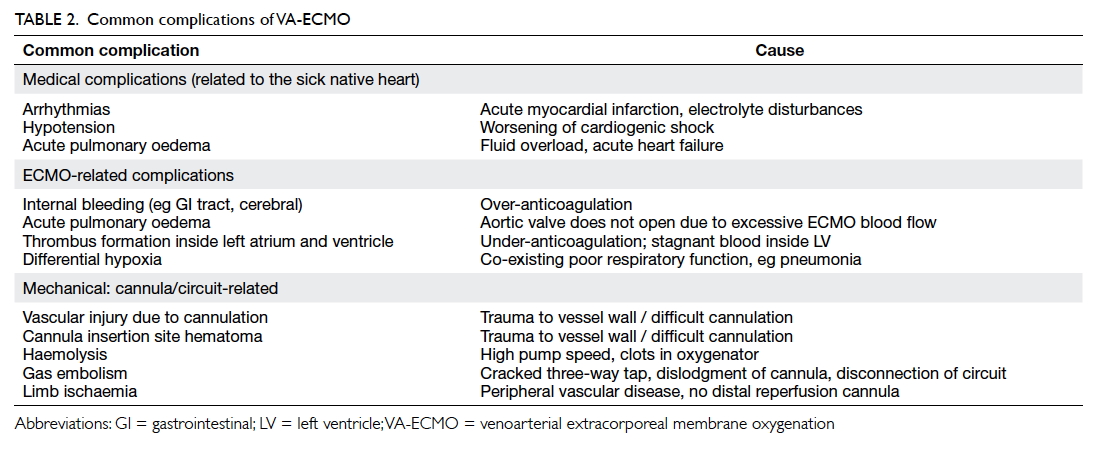
patients due to complications. Table 2 shows the common complications related to VA-ECMO.
General complications
Bleeding
Bleeding is one of the most frequent complications
and may lead to catastrophic events. Bleeding can
occur at the sternotomy wound in central VA-ECMO,
the cannulation sites in peripheral VA-ECMO,
and gastrointestinal, intra-abdominal, intrathoracic,
or intracranial areas. Risk factors for severe
bleeding include central VA-ECMO, use of dual
antiplatelet agents, platelet dysfunction, and over
anticoagulation.
Neurological complications
According to the ELSO Registry, approximately
15% of patients who received VA-ECMO developed
neurological complications. Among the 4522 adult
patients supported with VA-ECMO from 1992 to
2013, 358 (7.9%) had brain death, 161 (3.6%) had a
cerebral infarction, 83 (1.8%) developed seizures, and
80 (1.8%) were found to have cerebral haemorrhage.18
Those who developed neurological complications
had a significantly higher hospital mortality rate
than those who did not. Neurological complications
were more frequently observed in patients who
required cardiopulmonary resuscitation before
receiving VA-ECMO. Age,19 pre-ECMO cardiac
arrest, use of inotropes while on ECMO, and post-ECMO hypoglycaemia were associated with the
development of neurological complications.18
Cerebral hypoxia and hypoperfusion after
cardiac arrest, post-resuscitation reperfusion injury,
coagulopathy, and thromboembolism are usually the
underlying causes of neurological complications.
Presence of intracranial haemorrhage in patients
supported with VA-ECMO had a mortality rate
of higher than 90%. Kasirajan et al20 reported that
female gender and thrombocytopaenia, especially
with platelet counts of <50 000 cells/mm3, were
predictors of intracranial haemorrhage.
Haemolysis
The new generation of centrifugal pumps is safer and
causes less haemolysis related to blood stagnation,
heating, and thrombosis. They are, however, still
associated with some degree of haemolysis. The
haemolysis is usually caused by cavitation rather than
mechanical shearing or squeezing of red cells. When
the pump speed is more than 3000 revolutions per
minute, the negative pressure generated within the
pump head can exceed –700 mm Hg and cavitation
may occur.21 Plasma-free haemoglobin level will be
elevated when haemolysis is significant. The increase
in plasma-free haemoglobin is a risk factor for acute
kidney injury during ECMO.22
Complications specific to venoarterial extracorporeal membrane oxygenation
Thrombus formation
In patients with severe LV dysfunction, stasis of blood
can occur at sites of stagnant flow. The retrograde
flow of blood from peripheral VA-ECMO can oppose
the opening of the aortic valve if LV contractility is
very poor. Lack of cardiac ejection causes stasis of
blood inside the LV that can result in catastrophic
intracardiac thrombus formation. Rarely, thrombus
may form at the aortic root and ascending aorta
when the aortic valve does not open.23 Systemic
thromboembolism may occur when the aortic valve
opens again later in the recovery phase of the heart. A
higher anticoagulation intensity and target activated
clotting time or activated partial thromboplastin
time should be considered in patients with very poor
LV contractility.
Acute pulmonary oedema
The configuration of peripheral VA-ECMO, unlike
cardiopulmonary bypass, does not provide 100%
bypass of the cardiopulmonary circulation. Some
venous blood may still flow through the right
ventricle and pulmonary circulation and finally into
the LV. The left heart also receives blood through
collaterals of the bronchial and pulmonary arterial
circulations. In the presence of severe LV failure,
the LV fails to eject blood and the aortic valve
fails to open due to retrograde pressure from the
peripheral VA-ECMO. Distension of LV occurs
followed by hydrostatic pulmonary oedema. Daily
echocardiographic assessment is necessary to assess
spontaneous echo contrast or thrombus inside the
LV chamber. An inotrope (eg adrenaline) can be
used to increase LV contractility and promote the
emptying of blood. Vasodilators, when used in a
delicate balance with inotropes, can help to decrease
the afterload and facilitate aortic valve opening.
Percutaneous drainage of the left atrium or surgical
drainage of the LV has to be considered in refractory
cases.24 25 26
Differential hypoxia
Patients who have concomitant respiratory and
cardiac failure, who are on peripheral VA-ECMO with
the return cannula inside the femoral artery, are at
risk of developing differential hypoxia. Patients with
poor respiratory function eject poorly oxygenated
blood antegrade from the LV into the ascending
aorta where it mixes with the fully oxygenated
blood flowing retrograde from the ECMO circuit.
During the recovery phase of the heart, more poorly
oxygenated blood is pumped from the LV while the
retrograde flow from ECMO, which carries fully
oxygenated blood, is relatively weaker. At this state,
the upper body including the brain is perfused by
poorly oxygenated blood while the lower part of the
body is supplied by fully oxygenated blood. As the
left and right coronary arteries originate from the
root of the aorta, the myocardium is susceptible to
hypoxia.27 Therefore, it is important to monitor the
oxygen saturation in both hands for patients receiving
peripheral VA-ECMO. If differential hypoxia occurs,
ventilator settings are increased to maximise lung
oxygenation. In severe cases, the VA-ECMO circuit
may be converted into a venoarterial-venous (VAV)
configuration with an additional return cannula
placed in the right internal jugular vein. Oxygenated
blood will then be ejected from the LV to supply the
upper part of the body.28
Complications specific to cannulation
Vascular injury
Vessel wall injury can be catastrophic with massive
blood loss. To minimise this risk, cannulation should
be carried out by trained personnel under ultrasound
or fluoroscopic guidance. The cannula introducer
should be retracted once the cannula tip is inside the
vessel before further cannula advancement.
Limb ischaemia
Limb ischaemia is commonly seen in peripheral
VA-ECMO when the return cannula is placed in the
femoral artery. Peripheral vascular disease, young
age, and large-calibre cannula are underlying risk
factors.29 30 If the cannulation procedure is difficult
requiring multiple attempts at vessel puncture,
any haematoma formation with its mass effect will
compromise downstream perfusion of the leg. The
leg may then develop ischaemia, compartment
syndrome, or rhabdomyolysis if a reperfusion
cannula is not placed in a timely manner.31
Role of echocardiography in extracorporeal membrane oxygenation
Echocardiography is essential during the course of
VA-ECMO support. It helps select suitable patients,
assist in safe cannulation, monitor response to
ECMO support, detect complications, and assess
recovery for weaning from ECMO.32
Selection of suitable patients
Patients who require VA-ECMO support usually
have severe heart failure. Echocardiography can be
used to exclude contra-indications to VA-ECMO
such as cardiac tamponade, unrepaired aortic
dissection, and severe aortic regurgitation.
Assist safe cannulation
Echocardiography also helps assess structural heart
abnormalities that may hinder correct positioning
of the cannula. The presence of Chiari network in
the right atrium, and prominent interatrial septal
aneurysm are well-known congenital defects that
impede proper cannula position. This is particularly
important in the setting of VA-ECMO when a
second return cannula is placed inside the right
internal jugular vein to form a VAV configuration.
Ideally the tip of the access cannula, if placed inside
the IVC, should be just proximal to the entry into
the right atrium, whereas the tip of the return
cannula should be at the mid-right atrium (Fig 4a).
Transoesophageal echocardiography is especially
useful to guide correct placement of the ECMO
return cannula in the superior vena cava, whereas
transthoracic echocardiography is useful to locate
the position of the access cannula that is placed
inside the inferior vena cava.
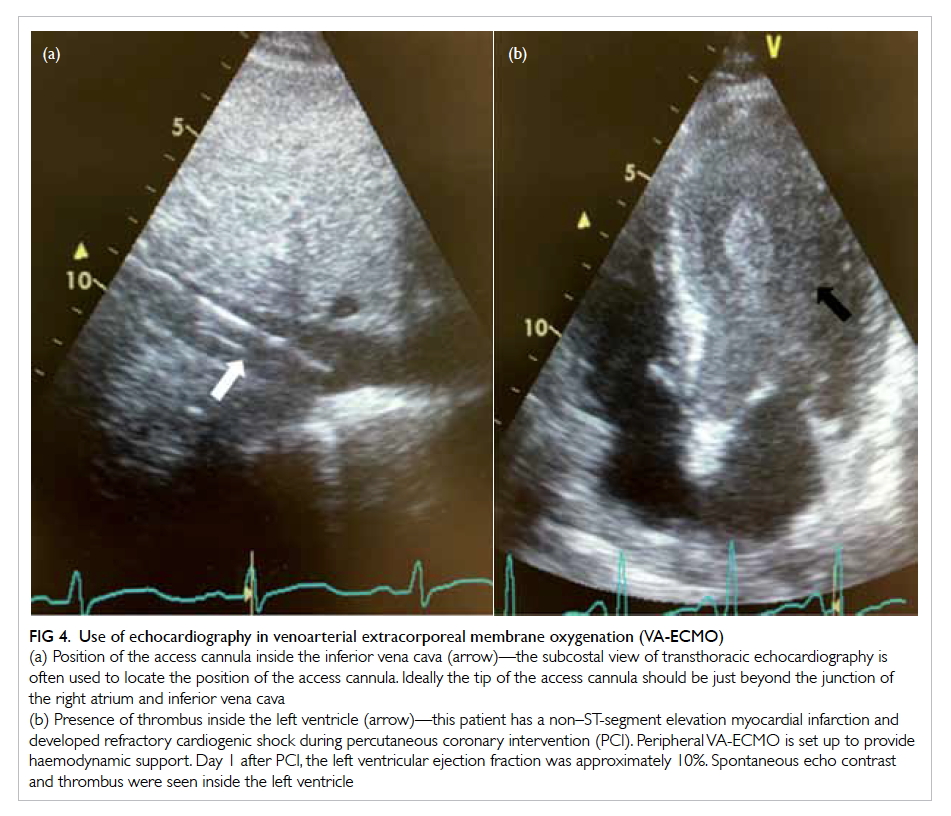
Figure 4. Use of echocardiography in venoarterial extracorporeal membrane oxygenation (VA-ECMO)
(a) Position of the access cannula inside the inferior vena cava (arrow)—the subcostal view of transthoracic echocardiography is often used to locate the position of the access cannula. Ideally the tip of the access cannula should be just beyond the junction of the right atrium and inferior vena cava
(b) Presence of thrombus inside the left ventricle (arrow)—this patient has a non–ST-segment elevation myocardial infarction and developed refractory cardiogenic shock during percutaneous coronary intervention (PCI). Peripheral VA-ECMO is set up to provide haemodynamic support. Day 1 after PCI, the left ventricular ejection fraction was approximately 10%. Spontaneous echo contrast and thrombus were seen inside the left ventricle
Monitoring response to extracorporeal membrane oxygenation support
Daily echocardiographic examination is necessary
to assess aortic valve opening, LV chamber size,
and ventricular contractility. Patients on peripheral
VA-ECMO have a lower LV preload and higher
LV afterload. The LV may become enlarged when
the aortic valve does not open due to increased
afterload. Stasis of blood inside the LV increases the
risk of thrombus formation (Fig 4b). Inotropes may
be used to increase myocardial contractility while
vasodilators may be used to decrease afterload, both
facilitating the opening of the aortic valve.
Detection of complications
Transoesophageal echocardiography is particularly
useful to locate intracardiac thrombi. Thrombus can
be present inside the LV chamber, inside the superior
vena cava, above the aortic valve, or between the
balloon of the IABP and the tip of the return cannula
inside the descending aorta. Migration of the
thrombus can cause pulmonary embolism or acute
cerebral embolic infarction.
Weaning of extracorporeal membrane oxygenation
There are no specific echocardiographic protocols
for ECMO weaning. A trial-off ECMO can be
considered, however, when the left ventricular
ejection fraction (LVEF) is >40%, left ventricular
outflow tract velocity time integral (LVOT VTI)
is >10 cm, and there is no LV dilatation or cardiac
tamponade. During the weaning trial, ECMO flow is
usually reduced by 0.5 L/min every 30-60 minutes
while vital signs (blood pressure, pulse pressure,
pulse rate) and echocardiographic parameters
(LVEF, LV dimension, aortic valve opening, LVOT
VTI) are closely monitored. Patients who are able
to wean off VA-ECMO retain stable blood pressure,
satisfactory contractility, and LVOT VTI during the
weaning process.33
Weaning from venoarterial extracorporeal membrane oxygenation
The timing of weaning from VA-ECMO is important
as premature withdrawal will expose the heart to
the stress effects of high-dose inotropes, whereas
unnecessary extension of VA-ECMO can increase
the risks of ECMO-related complications. Different
ECMO centres may have different weaning
guidelines. In general, patients who are ready to be
weaned from VA-ECMO have signs of native heart
recovery that include improving aortic pulsatility
(pulse pressure), improving myocardial contraction
on echocardiography, reduction of ECMO blood
flow with the same pump driving force, lessening
of inotrope dependence, less demand for renal
replacement therapy, decreasing pulmonary
capillary wedge pressure and central venous
pressure, and improving right ventricular function.34
Weaning from VA-ECMO is commonly done under
echocardiographic assessment. An LVEF of >40%,
LVOT VTI of >10 cm, and normal LV size all suggest
a higher chance of successful weaning.35 36 37 Even
when the trial-off ECMO is successful, some centres
will consider leaving the cannulae temporarily
(<24 hours) in place in case the patient deteriorates.
Continuous infusion of heparinised saline to these
cannulae is necessary to avoid thrombus formation.
Conclusion
Venoarterial ECMO is a proven strategy and is
being increasingly used to support patients with
cardiovascular collapse, as a bridge to recovery
or more definitive therapies. Initiation should be
carefully considered with regard to patient selection.
Understanding the physiology of and interplay
between the artificial circuit and the native heart,
together with management strategies for specific
patient care, stringent monitoring, and early
detection of complications are essential for the
success of VA-ECMO.
References
1. ECLS registry report. International summary. Ann Arbor,
MI: The Extracorporeal Life Support Organization; Jul
2016.
2. Zeymer U, Hochadel M, Hauptmann KE, et al. Intra-aortic
balloon pump in patients with acute myocardial infarction
complicated by cardiogenic shock: results of the ALKK-PCI
registry. Clin Res Cardiol 2013;102:223-7. Crossref
3. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon
support for myocardial infarction with cardiogenic shock.
N Engl J Med 2012;367:1287-96. Crossref
4. Beckmann A, Benk C, Beyersdorf F, et al. Position article
for the use of extracorporeal life support in adult patients.
Eur J Cardiothorac Surg 2011;40:676-80. Crossref
5. ELSO adult cardiac failure supplement to the ELSO general
guidelines. Version 1.3. Ann Arbor, MI: The Extracorporeal
Life Support Organization; 2013.
6. Chung M, Shiloh AL, Carlese A. Monitoring of the
adult patient on venoarterial extracorporeal membrane
oxygenation. Scientific World Journal 2014;2014:393258. Crossref
7. Bělohlávek J, Mlček M, Huptych M, et al. Coronary versus
carotid blood flow and coronary perfusion pressure in a
pig model of prolonged cardiac arrest treated by different
modes of venoarterial ECMO and intraaortic balloon
counterpulsation. Crit Care 2012;16:R50. Crossref
8. Brehm C, Schubert S, Carney E, et al. Left anterior
descending coronary artery blood flow and left ventricular
unloading during extracorporeal membrane oxygenation
support in a swine model of acute cardiogenic shock. Artif
Organs 2015;39:171-6. Crossref
9. Ostadal P, Mlcek M, Kruger A, et al. Increasing venoarterial
extracorporeal membrane oxygenation flow negatively
affects left ventricular performance in a porcine model of
cardiogenic shock. J Transl Med 2015;13:266. Crossref
10. Chauhan S, Subin S. Extracorporeal membrane
oxygenation, an anesthesiologist’s perspective: physiology
and principles. Part 1. Ann Card Anaesth 2011;14:218-29. Crossref
11. Gattinoni L, Carlesso E, Langer T. Clinical review:
Extracorporeal membrane oxygenation. Crit Care
2011;15:243. Crossref
12. Petroni T, Harrois A, Amour J, et al. Intra-aortic balloon
pump effects on macrocirculation and microcirculation
in cardiogenic shock patients supported by venoarterial
extracorporeal membrane oxygenation. Crit Care Med
2014;42:2075-82. Crossref
13. Romeo F, Acconcia MC, Sergi D, et al. Percutaneous assist
devices in acute myocardial infarction with cardiogenic
shock: Review, meta-analysis. World J Cardiol 2016;8:98-111. Crossref
14. Lin LY, Liao CW, Wang CH, et al. Effects of additional intra-aortic
balloon counter-pulsation therapy to cardiogenic
shock patients supported by extra-corporeal membranous
oxygenation. Sci Rep 2016;6:23838. Crossref
15. Madershahian N, Nagib R, Wippermann J, Strauch J,
Wahlers T. A simple technique of distal limb perfusion
during prolonged femoro-femoral cannulation. J Card Surg
2006;21:168-9. Crossref
16. Kimura N, Kawahito K, Ito S, et al. Perfusion through the
dorsalis pedis artery for acute limb ischemia secondary
to an occlusive arterial cannula during percutaneous
cardiopulmonary support. J Artif Organs 2005;8:206-9. Crossref
17. Spurlock DJ, Toomasian JM, Romano MA, Cooley E,
Bartlett RH, Haft JW. A simple technique to prevent
limb ischemia during veno-arterial ECMO using the
femoral artery: the posterior tibial approach. Perfusion
2012;27:141-5. Crossref
18. Lorusso R, Barili F, Mauro MD, et al. In-hospital neurologic
complications in adult patients undergoing venoarterial
extracorporeal membrane oxygenation: results from the
Extracorporeal Life Support Organization Registry. Crit
Care Med 2016;44:e964-72. Crossref
19. Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE,
Schears GJ, Wijdicks EF. Neurological injury in adults
treated with extracorporeal membrane oxygenation. Arch
Neurol 2011;68:1543-9. Crossref
20. Kasirajan V, Smedira NG, McCarthy JF, Casselman F,
Boparai N, McCarthy PM. Risk factors for intracranial
hemorrhage in adults on extracorporeal membrane
oxygenation. Eur J Cardiothorac Surg 1999;15:508-14. Crossref
21. Toomasian JM, Bartlett RH. Hemolysis and ECMO pumps
in the 21st century. Perfusion 2011;26:5-6. Crossref
22. Lyu L, Long C, Hei F, et al. Plasma free hemoglobin is a
predictor of acute renal failure during adult venous-arterial
extracorporeal membrane oxygenation support. J
Cardiothorac Vasc Anesth 2016;30:891-5. Crossref
23. Madershahian N, Weber C, Scherner M, Langebartels
G, Slottosch I, Wahlers T. Thrombosis of the aortic root
and ascending aorta during extracorporeal membrane
oxygenation. Intensive Care Med 2014;40:432-3. Crossref
24. Rupprecht L, Flörchinger B, Schopka S, et al. Cardiac
decompression on extracorporeal life support: a review
and discussion of the literature. ASAIO J 2013;59:547-53. Crossref
25. Alkhouli M, Narins CR, Lehoux J, Knight PA, Waits B, Ling
FS. Percutaneous decompression of the left ventricle in
cardiogenic shock patients on venoarterial extracorporeal
membrane oxygenation. J Card Surg 2016;31:177-82. Crossref
26. Soleimani B, Pae WE. Management of left ventricular
distension during peripheral extracorporeal membrane
oxygenation for cardiogenic shock. Perfusion 2012;27:326-31. Crossref
27. Cove ME. Disrupting differential hypoxia in peripheral
veno-arterial extracorporeal membrane oxygenation. Crit
Care 2015;19:280. Crossref
28. Biscotti M, Lee A, Basner RC, et al. Hybrid configurations
via percutaneous access for extracorporeal membrane
oxygenation: a single-center experience. ASAIO J
2014;60:635-42. Crossref
29. Foley PJ, Morris RJ, Woo EY, et al. Limb ischemia during
femoral cannulation for cardiopulmonary support. J Vasc
Surg 2010;52:850-3. Crossref
30. Bisdas T, Beutel G, Warnecke G, et al. Vascular
complications in patients undergoing femoral cannulation
for extracorporeal membrane oxygenation support. Ann
Thorac Surg 2011;92:626-31. Crossref
31. Roussel A, Al-Attar N, Khaliel F, et al. Arterial vascular
complications in peripheral extracorporeal membrane
oxygenation support: a review of techniques and outcomes.
Future Cardiol 2013;9:489-95. Crossref
32. Douflé G, Roscoe A, Billia F, Fan E. Echocardiography for
adult patients supported with extracorporeal membrane
oxygenation. Crit Care 2015;19:326. Crossref
33. Aissaoui N, Guerot E, Combes A, et al. Two-dimensional
strain rate and Doppler tissue myocardial velocities:
analysis by echocardiography of hemodynamic and
functional changes of the failed left ventricle during
different degrees of extracorporeal life support. J Am Soc
Echocardiogr 2012;25:632-40. Crossref
34. Pappalardo F, Pieri M, Arnaez Corada B, et al. Timing and
strategy for weaning from venoarterial ECMO are complex
issues. J Cardiothorac Vasc Anesth 2015;29:906-11. Crossref
35. Platts DG, Sedgwick JF, Burstow DJ, Mullany DV, Fraser
JF. The role of echocardiography in the management
of patients supported by extracorporeal membrane
oxygenation. J Am Soc Echocardiogr 2012;25:131-41. Crossref
36. Scherer M, Sirat AS, Moritz A, Martens S. Extracorporeal
membrane oxygenation as perioperative right ventricular
support in patients with biventricular failure undergoing
left ventricular assist device implantation. Eur J
Cardiothorac Surg 2011;39:939-44. Crossref
37. Santelices LC, Wang Y, Severyn D, et al. Development of
a hybrid decision support model for optimal ventricular
assist device weaning. Ann Thorac Surg 2010;90:713-20. Crossref