Hong Kong Med J 2017 Apr;23(2):140–9 | Epub 24 Feb 2017
DOI: 10.12809/hkmj164939
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
A hospital-wide screening programme to control an outbreak of vancomycin-resistant enterococci in a large tertiary hospital in Hong Kong
Christopher KC Lai, MB, ChB, FHKCPath1,2;
Stephenie YN Wong, MB, BS, FHKCPath1,2;
Shirley SY Lee, BSc (Nursing), MSC (Nursing)2;
HK Siu, BSc (Statistics), MPhil (Social Medicine)3;
CY Chiu, BSc (Biomedical Sciences), MSc (Medical Laboratory Sciences)4;
Dominic NC Tsang, MB, BS, FHKCPath1,2,3;
Margaret PY Ip, FRCP, FRCPath4;
CT Hung, FANZCA, FHKAM (Anaesthesiology)5
1 Department of Pathology, Queen Elizabeth Hospital, Hong Kong
2 Infection Control Team, Queen Elizabeth Hospital, Hong Kong
3 Chief Infection Control Officer’s Office, Hospital Authority, Hong Kong
4 Department of Microbiology, The Chinese University of Hong Kong, Hong Kong
5 Queen Elizabeth Hospital, Hong Kong
Corresponding authors: Dr Christopher KC Lai (laikcc@ha.org.hk)
Abstract
Introduction: Apart from individual small-scale
outbreaks, infections with vancomycin-resistant
enterococci are uncommon in Hong Kong. A major
outbreak of vancomycin-resistant enterococci,
however, occurred at a large tertiary hospital in 2013.
We describe the successful control of this outbreak
and share the lessons learned.
Methods: In 2013, there was an abnormal increase
in the incidence of vancomycin-resistant enterococci
carriage compared with baseline in multiple clinical
departments at Queen Elizabeth Hospital. A
multipronged approach was adopted that included a
10-week hospital-wide active screening programme,
which aimed to identify and isolate hidden
vancomycin-resistant enterococci carriers among all
in-patients. The identified carriers were completely
segregated in designated wards where applicable.
Other critical infection control measures included
directly observed hand hygiene and environmental
hygiene. A transparent and open disclosure approach
was adopted throughout the outbreak.
Results: The infection control measures were
successfully implemented. The active screening of
vancomycin-resistant enterococci was conducted
between 30 September and 10 November 2013.
A total of 7053 rectal swabs were collected from
patients in 46 hospital wards from 11 departments.
The overall carriage rate of vancomycin-resistant
enterococci was 2.8% (201/7053). Pulsed-field gel
electrophoresis showed a predominant outbreak
clone. We curbed the outbreak and kept the
colonisation of vancomycin-resistant enterococci
among patients at a pre-upsurge low level.
Conclusions: We report the largest cohesive effort to
control spread of vancomycin-resistant enterococci
in Hong Kong. Coupled with other infection control
measures, we successfully controlled vancomycin-resistant
enterococci to the pre-outbreak level. We
have demonstrated that the monumental tasks can
be achieved with meticulous planning, and thorough
communication and understanding between all
stakeholders.
New knowledge added by this study
- This is the largest vancomycin-resistant enterococci control study ever conducted in Hong Kong.
- We have demonstrated the infection control measures required in controlling a large outbreak in a Hong Kong public hospital setting.
- The key infection control measures are active case finding followed by case-cohorting, directly observed hand hygiene, and equipment and environmental hygiene.
- Control of large infectious disease outbreaks and effective implementation of infection control measures can be achieved with meticulous planning, thorough communication, and understanding between all stakeholders.
Introduction
Vancomycin-resistant enterococci (VRE) is
an important cause of health care–associated
infection and is known to prolong hospital stay,
increase treatment cost, and patient morbidity
and mortality.1 2 3 4 5 A VRE carrier was defined as any patient with VRE isolated from a clinical or
surveillance specimen. The first case of VRE in Hong
Kong was identified at Queen Elizabeth Hospital
(QEH) in 1997.6 In 2010, VRE constituted 0.4% of all
Enterococcus isolates. Apart from individual small-scale
outbreaks,7 8 VRE had not gained a foothold in Hong Kong. Queen Elizabeth Hospital is the largest
public acute general tertiary hospital under the
administration of the Hospital Authority (HA) with
1800 beds. There are more than 160 000 admissions
with 104 000 in-patients treated annually. A major
VRE outbreak occurred in QEH in 2013. There
was an abnormal increase in the incidence of VRE
carriage in multiple clinical departments compared
with baseline. Prior to this outbreak, VRE control
measures were stipulated by the HA Guideline on
Control of VRE. Active screening was not mandatory
but was usually performed in contact investigations
after VRE was recovered from clinical specimens.
The baseline incidence of VRE never exceeded five
per week prior to December 2012. Nonetheless, the
incidence crept up and by March 2013, a total of 34
VRE carriers were identified in week 13 alone. This study aimed to describe in detail the
approach to rapidly control VRE in our hospital.
Methods
Multipronged infection control measures for vancomycin-resistant enterococci
The hospital’s control measures can be divided into
two phases based on the intensity of measures with
the triggering event of the constitution of QEH VRE
Task Group.
Emerging phase (1 January 2012 to 13 May 2013)
(1) Find and confine—active case finding by
admission screening in high prevalence wards
with additional weekly screening for outbreak
wards. Carriers of VRE were cohorted in either a
single room or designated cubicles with a mobile
curtain as segregation. Signage for contact
precautions was posted at the entrance to the
cohort area and at the patients’ bedside. Gloves
and gowns were worn when in contact with the
patient or patient environment. All VRE cases
and their contacts were tagged in the corporate
electronic Clinical Management System.
(2) Hand hygiene—chlorhexidine-alcohol hand rub was used in clinical areas with high VRE prevalence. Only two visitors were allowed per VRE patient with their hand hygiene compliance monitored.
(3) Nursing care—all patients in Intensive Care Unit were bathed with chlorhexidine daily. Wards were advised that excreta and tube feeding should be handled by separate teams.
(4) Equipment and environment—we introduced colour-coding to all clinical wards. Two-in-one disinfectants and disposable wipes were provided to clinical wards to improve two-step cleaning. Dedicated non-critical patient care equipment was provided for all VRE cases. Hydrogen peroxide vaporisation sessions were used to disinfect non-critical patient care equipment. Cleaners were coached by infection control nurses and their performance was gauged by environmental sampling and fluorescence markers.
(5) Open disclosure—all outbreaks were disclosed through press release.
(2) Hand hygiene—chlorhexidine-alcohol hand rub was used in clinical areas with high VRE prevalence. Only two visitors were allowed per VRE patient with their hand hygiene compliance monitored.
(3) Nursing care—all patients in Intensive Care Unit were bathed with chlorhexidine daily. Wards were advised that excreta and tube feeding should be handled by separate teams.
(4) Equipment and environment—we introduced colour-coding to all clinical wards. Two-in-one disinfectants and disposable wipes were provided to clinical wards to improve two-step cleaning. Dedicated non-critical patient care equipment was provided for all VRE cases. Hydrogen peroxide vaporisation sessions were used to disinfect non-critical patient care equipment. Cleaners were coached by infection control nurses and their performance was gauged by environmental sampling and fluorescence markers.
(5) Open disclosure—all outbreaks were disclosed through press release.
Intensive control phase (13 May 2013 to 10 November 2013)
(1) Command and control—a VRE Task Group
was formed with clear administrative mandates
from the Hospital Chief Executive, head of
nursing, and head of administrative services.
The Task Group included senior representatives
from clinical departments, human resources,
laboratories, and infection control teams.
Weekly meetings were held. Local experts from
HA Head Office, Centre for Health Protection,
and a local university were also invited to jointly
devise an intensive VRE control programme.
(2) Active screening—the pan-hospital VRE screening was the hallmark of this period; it exemplified the determination of the hospital administration. Rectal swabs were collected to identify VRE carriers in different stages. Each ward performed a point prevalence screening followed by 2 weeks of admission and discharge screening. The screening of 46 hospital wards from 11 departments was to be completed within 10 weeks.
(2) Active screening—the pan-hospital VRE screening was the hallmark of this period; it exemplified the determination of the hospital administration. Rectal swabs were collected to identify VRE carriers in different stages. Each ward performed a point prevalence screening followed by 2 weeks of admission and discharge screening. The screening of 46 hospital wards from 11 departments was to be completed within 10 weeks.
Carriage of VRE is associated with additional
length of stay.1 A sudden surge in VRE cases
would result in blockage of admissions, resulting
in redirection of emergency admissions to other
hospitals. Based on prevalence figures from contact
investigations in previous localised VRE outbreaks
(range, 0%-20%), bed status and occupancy rates,
126 VRE cases would be identified on the first day
of screening alone, 566 cases would be identified at
the end of the screening, assuming 10% of our in-patients
were VRE carriers. To avoid overwhelming
the hospital services due to inadequate isolation
facilities, a modified risk-based pan-hospital
screening was adopted with consideration of the
following parameters: daily number of specimens,
daily number of VRE carriers identified, consequent
additional length of stay, and designated cohort ward
capacity. The final schedule had exacted the number
of specimens to be taken by ward and date over a
10-week period and was agreed by all stakeholders.
To segregate VRE carriers, a VRE ward was
created to avert cross-transmission. Bed capacity
was ‘created’ by rescheduling elective procedures
from both medical and surgical teams.
To avoid inadvertently overloading the
hospital’s capacity during active screening, two
‘brake points’ were set, namely number of patients
waiting at the emergency department at 7 am each
morning for emergency hospital admission should
not exceed 30, and total VRE cases identified should
not exceed 25 per day. When these points were
met, screening on that particular day would stop. A
real-time close monitoring communication group
using instant messaging (WhatsApp) was formed to
connect all key stakeholders on a 24/7 basis.
Other additional measures included:
- Hand hygiene—audit results of hand hygiene compliance were reported to department and hospital administration on a weekly basis. Alcoholic hand rub dispensers were installed in patient toilets. Hand hygiene before meals and medications in all conscious hospitalised patients were directly observed.
- Nursing care—disposable disinfection wipes were provided to optimise disinfection of commodes, bedpans, and urinals. On-site coaching was provided by infection control nurses about contamination-prone procedures, particularly napkin change and care for nasogastric tube.
- Equipment and environment—we increased cleaning staff manpower by recruiting additional external cleaning staff and instigating an overtime allowance for existing staff. The frequency of changing privacy curtains was shortened from monthly to biweekly for all VRE carriers. Cleaning efficacy was monitored by regular environmental sampling using Polywipe (Medical Wire & Equipment/Wiltshire, United Kingdom) in wards where the outbreak was detected.
- Staff engagement, education, and communication—staff forums were organised so all parties would understand the importance of VRE and their role as health care workers, with dedicated sessions in Cantonese for supporting staff.
- Open disclosure—the result from the pan-hospital screening was released to hospital administration and HA head office on a daily basis.
Laboratory protocol
Rectal swabs and stool specimens were inoculated
onto chromID VRE agar (bioMérieux, France)
and incubated at 35°C ± 2°C according to the
manufacturer’s recommendations. The agar plates
were examined daily for 2 days. Suspected colonies
were identified to be Enterococcus species by
both MALDI-TOF (Vitek-MS, bioMérieux) and
conventional microbiological methods of Gram
stain and biochemical reactions. Vancomycin
resistance was confirmed by E-test (bioMérieux,
France) according to Clinical Laboratory Standards
Institute breakpoints.9 Detection of vancomycin
resistance genes was performed by the local
reference laboratory. Strains were typed by pulsed-field
gel electrophoresis (PFGE) and patterns of
SmaI-restricted chromosomal DNA analysed by
unweighted pair group method with arithmetic mean (UPGMA) using the BioNumerics software
(Applied Maths).10
Hand hygiene compliance audit
We adopted the World Health Organization (WHO)
hand hygiene observation tools by directly observing
compliance with the WHO five moments. The
observation was conducted by infection control
nurses using a WHO standardised audit form.
Nurses, supporting staff, doctors, and allied health
personnel were included for observation.
Antibiotics consumption data
The consumption of vancomycin, ceftazidime, and
ceftriaxone in QEH between week 1 of 2012 and
week 39 of 2015 was retrieved from the Clinical Data
Analysis and Reporting System. Consumption data
were presented in defined daily dose.
Statistical analysis
The relationship between VRE carriage, a binary
dependent variable, and five independent variables
related to patients’ demographic background and
hospitalisation history were analysed by univariate
methods (Chi squared test supplemented with
measurement of the association [odds ratio for
binary variables and Spearman’s correlation for
ordinal variables]) and the significant independent
variables were included in the subsequent multiple
logistic regression model. The 30-day mortality
between groups was analysed by Chi squared test.
In multiple logistic regression, one category of
each independent variable was selected as ‘reference
category’ to compare with other categories in the
variable and the odds ratio calculated. Likelihood
ratio test was used to compare the final model with
null model and Hosmer-Lemeshow test was used to
evaluate the goodness-of-fit of the final model. The
Statistical Package for the Social Sciences (SPSS Windows version 21.0; IBM Corp, Armonk [NY], United States) was used
for data analysis.
Results
Our multipronged infection control measures
successfully brought down VRE to pre-outbreak
level. Prior to screening, 150 non-emergency
procedures were rescheduled. The screening was
conducted between 30 September and 10 November
2013. A total of 7053 specimens from 4966 patients
were collected—1422 from point prevalence, 4225
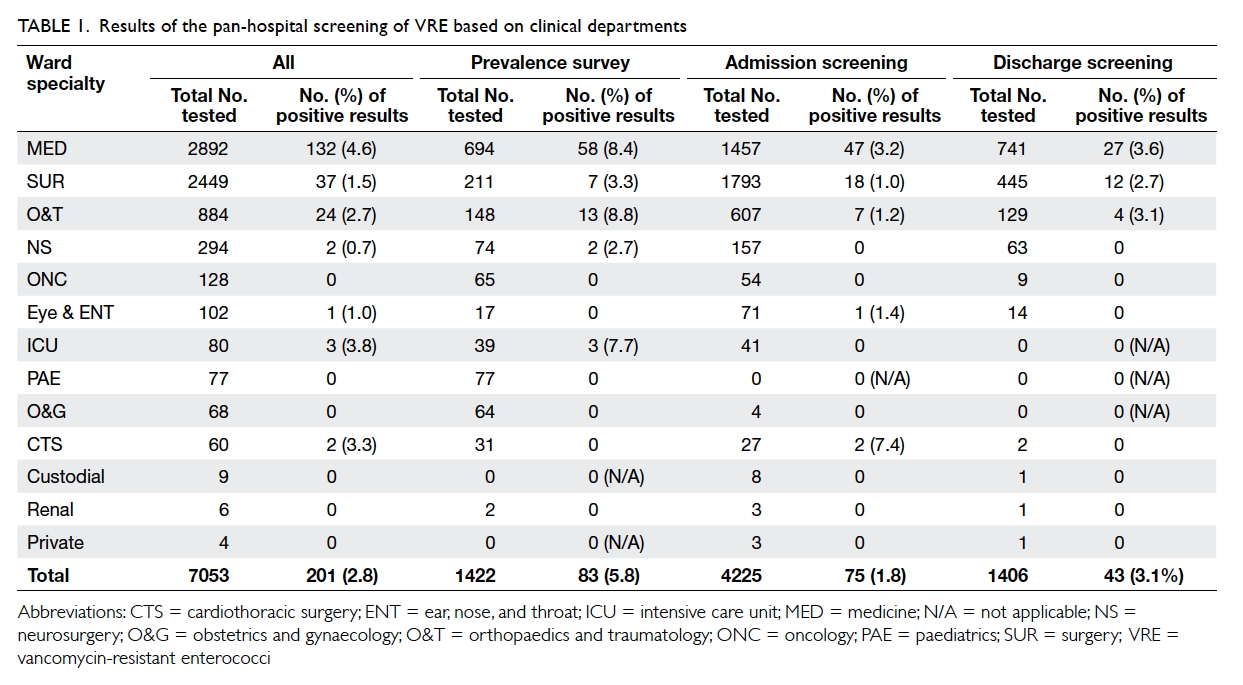
from admission, and 1406 on discharge (Table 1).
We managed to complete the screening schedule
without meeting the brake points.
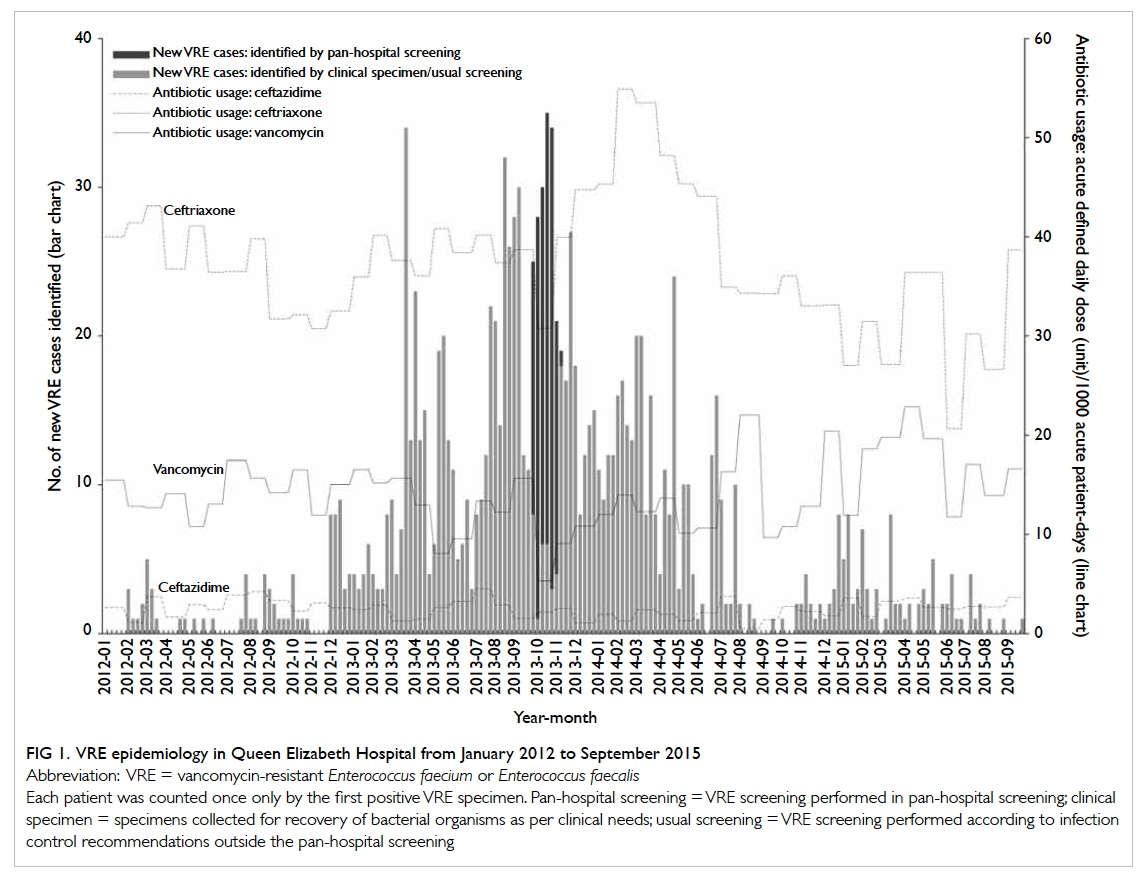
The baseline incidence of VRE never exceeded
five per week prior to the current outbreak. After
December 2012, it crept up and peaked at week 13
of 2013 with 34 new VRE cases identified. After the
pan-hospital screening, the incidence dropped to no
more than five cases per week after March 2015 (Fig 1).
Of all the specimens screened, 2.8% (201/7053)
were positive for VRE—65.7% (132/201) of VRE
came from the specialty of medicine, 19.9% (40/201)
from the surgical stream (all surgical subspecialties
except neurosurgery and orthopaedics). The point
prevalence of VRE was 5.8% (83/1422), admission
prevalence was 1.8% (75/4225), and discharge
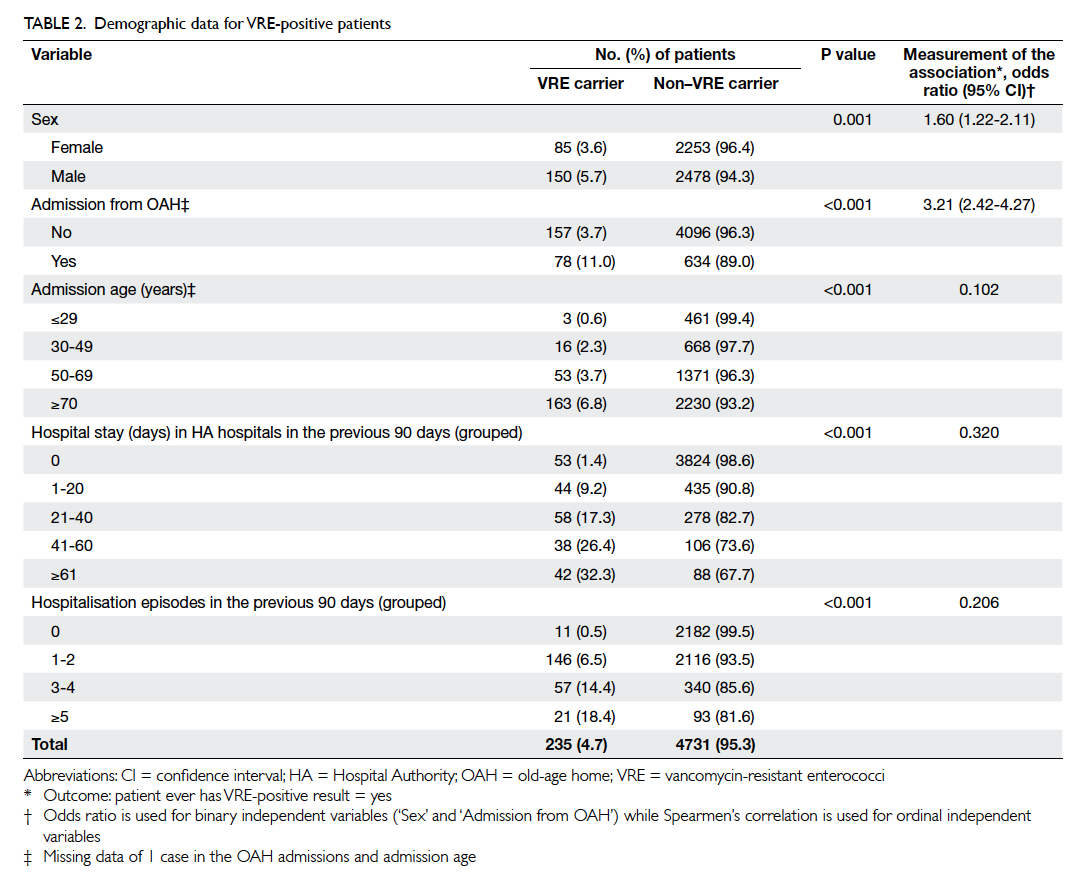
prevalence was 3.1% (43/1406). Risk factors for
VRE carriage included male gender, residence in a
home for the elderly, older age, longer hospital stay,
and more hospitalisation episodes in the previous
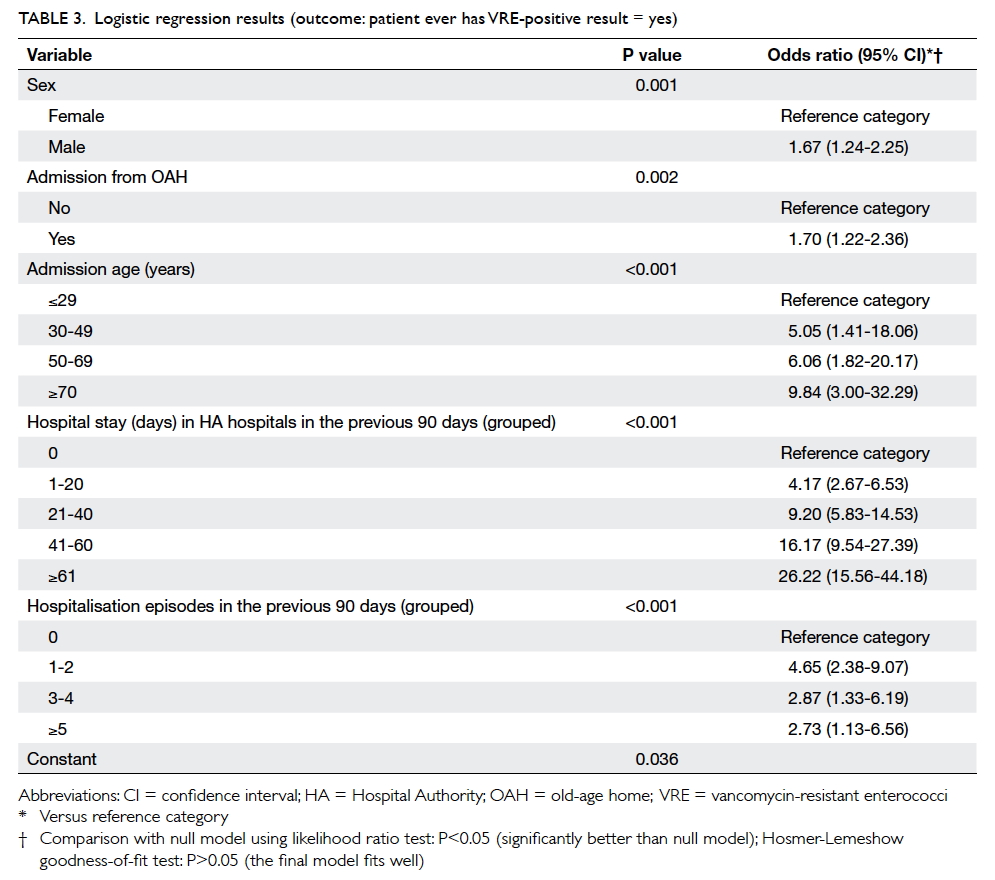
90 days prior to screening (Table 2). From logistic
regression results compared with the reference
group, there was a progressive increase in the risk
of VRE carriage with increasing age, and increase
in days of hospitalisation in the previous 90 days
prior to screening, but not with increasing episodes
of hospitalisation in the previous 90 days prior to
screening (Table 3).
Infection control measures
A total of 28 588 hand hygiene observations were
made in 2013. The compliance rate improved from
37% in the first quarter of 2013 to 73% in the fourth
quarter of 2013. The improvement was seen across
all departments and all staff groups. A total of 30
sessions of on-site education about napkin change,
nasogastric tube care, and environmental cleaning
were provided with 88 napkin care procedures
observed in 28 wards. Furthermore, 37 hydrogen
peroxide vapour sessions were offered to disinfect
non-critical equipment; and 15 staff forums
dedicated to VRE control were held with a total of
1339 attendances.
Microbiology
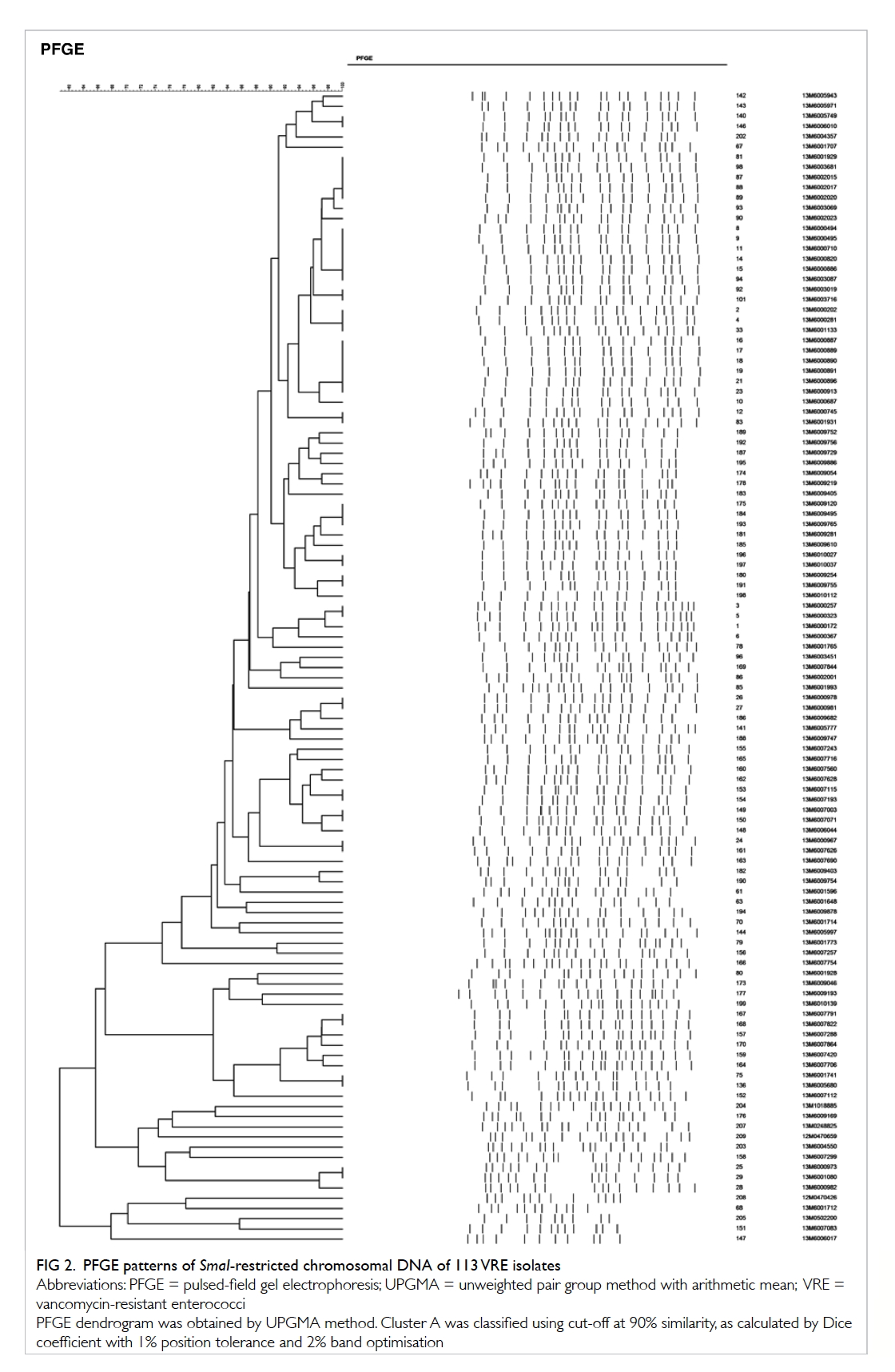
During the screening period, 105 VRE isolates
recovered from the pan-hospital screening were all
vanA gene carrying Enterococcus faecalis. They were
analysed with eight unrelated archived VRE strains.
The PFGE patterns of SmaI-restricted chromosomal
DNA of 113 VRE isolates are shown in Figure 2. Dendrogram of PFGE patterns was obtained
by UPGMA method. A predominant cluster A
was classified using a cut-off at 90% similarity, as
calculated by Dice coefficient with 1% position
tolerance and 2% band optimisation. Cluster A
comprised 49 strains from the current pan-hospital
screening and one unrelated archived strain from
another hospital.
Carriage of vancomycin-resistant enterococci and 30-day mortality
During the pan-hospital screening period, the 30-day
all-cause mortality of all VRE carriers identified in
the pan-hospital screening and non-VRE carriers
were 20.5% and 6.1%, respectively. The odds ratio
was 3.93 (95% confidence interval, 2.68-5.78). When
compared with previously known VRE carriers but
with negative VRE screening results in the same
period (13.6%), the 30-day all-cause mortality were
20.5% and 13.6%, respectively. The odds ratio was
1.64 (95% confidence interval, 0.71-3.76).
Antibiotic consumption
There was no significant change in consumption
of vancomycin or ceftazidime during the emerging
phase or during and beyond the intensive
control phase. There was an apparent increase in
consumption of ceftriaxone noted after the intensive
phase in the first half of 2014 (Fig 1).
Discussion
Identification of VRE carriers, segregation of primary
sources, hand hygiene, and environmental hygiene
are the critical success factors in controlling the VRE
outbreak. The territory-wide effort to control the
emergence of VRE in public hospitals in Hong Kong
has been discussed elsewhere.11 Our study revealed
the critical elements involved in controlling a multi-sourced
VRE outbreak in a major tertiary hospital.
We believe our failure to contain VRE in the emerging
phase was in part due to the lack of perceived need of
staff for VRE control as well as skepticism about the
effectiveness of infection control measures. Senior
clinicians may be ambivalent towards our approach
due to perceived loss of autonomy. Frontline staff
rebuffed the screening programme as they sensed
extra work and doubted its effectiveness. Overseas
experience has shown that once VRE becomes
hospital endemic, eradication is difficult despite the
best efforts.12 13 14
We faced an additional challenge of an absence
of facilities to completely segregate VRE carriers.
Our hospital faces overcrowding on a daily basis
with bed occupancy often exceeding 100%, and
reaching as high as 130% during influenza seasons.
Studies have shown that bed occupancy, isolation
room availability, and staffing have a direct impact
on ease of VRE control.15 16 Our difficulties were compounded by lack of inter-bed spacing and
limited toilet facilities as the hospital was designed
more than 60 years ago, and the need to keep the
hospital functioning at all times.
In the intensive control phase, commitment
from hospital administration became visible as a
result of the pan-hospital screening. Close liaison
between departments, careful and extensive planning
with input from the frontline at every step, effective
communication, and staff engagement were also key
to our success. Some researchers have questioned
the effectiveness of active surveillance cultures in
reducing VRE transmission.17 Others have suggested
that VRE will not be successfully controlled if the
policy excludes asymptomatic VRE colonisation.18 19 20 21
We believed it was necessary to take drastic action
and perform active screening of the whole hospital.
Our planning took reference from similar
overseas experiences. Christiansen et al18
successfully controlled VRE by screening 19 658
patients and found 169 patients from 23 wards to
be colonised with vanB-containing Enterococcus faecium in 6
months. Their experience was different from ours
as they had fewer cases. Moretti et al19 reported
their extensive active surveillance with enhanced
infection control measures in a Brazilian teaching
hospital. They performed 8692 rectal swabs for
VRE (mean, 300 swabs/month), with an overall
positive rate of 3.7%. In their 2.5-year intervention,
their VRE positive rate decreased from 7.2% in
2007 to 1.5% in 2009. Kurup et al20 reported their
experience in a large Singaporean hospital. They
performed a large-scale screening of 4924 patients
over 2 months and successfully reduced the positive
rate from 11.4% at the peak of the outbreak to
4.2% at the end of screening. We did not observe a
decline over the pan-hospital screening period as
in the Singaporean experience. It was because we
deliberately spaced out the departments with a high
VRE prevalence throughout the 10-week period to
avoid overwhelming the hospital’s facilities.
Rapid laboratory turnaround time is another
key element.22 It was soon evident that the hospital
laboratory could not handle the additional specimens
alone. Assistance from three HA microbiology
laboratories was sought. A huge amount of liaison
work with extensive communication between
laboratory directors, senior medical technologists,
and scientific officers followed to ensure the smooth
running of this unprecedented inter-laboratory
cooperation. A unified set of logistics was established,
governing the tiniest details. Procurement of key
reagents like chromogenic agar was coordinated
centrally with support from the HA head office.
Hygiene management has been shown to be
important in controlling VRE in endemic areas.16 23 24
Contamination of the hospital environment by
VRE, and occurrence of cross-contamination, either
through the hands of health care workers, equipment,
or surfaces is well known.25 26 27 The association of
environmental contamination and the occurrence of
an outbreak has also been well established.18 28 29 30
The improvement in hand hygiene compliance
from approximately 37% to 73% was remarkable.
Several explanations are postulated: (1) the VRE Task
Group escalated the need for urgent improvement.
The weekly reporting of hand hygiene compliance
rate via the VRE workgroup created a driving force at
an administrative level; (2) we implemented directly
observed hand hygiene before meals and when taking
medications in all conscious hospitalised patients; (3)
we actively engaged infection control link nurses,
creating a collective learning opportunity that has
facilitated collaboration and system thinking; and
(4) making the hand hygiene compliance data visible
(and comparing with other wards/departments)
might change the behaviour of many.31
All the VRE recovered in the pan-hospital
screening was vanA-containing Enterococcus faecium.
The PFGE patterns showed 49 out of the 105 pan-hospital
screening isolates belonged to a single
cluster (cluster A), signifying the possibility of clonal
spread of a dominant strain, with co-circulation
of various less dominant strains. Some clones may
have developed de novo. Further analysis of these
strains will allow a more thorough understanding of
the transmission dynamics within the hospital, and
whether the outbreak clone has a survival advantage
over other clones.
We identified residents of homes for the
elderly, advanced age, and prolonged hospitalisation
as risk factors for VRE carriage. This is most likely
due to their associated co-morbidities rather than
the individual factors per se. It is unknown why men
were at a higher risk than women. It might have been
a chance finding since more outbreaks occurred in
male wards before and during the study period than
female wards.
Antibiotics, especially vancomycin and
third-generation cephalosporins like ceftazidime
and ceftriaxone, were known to be a risk for VRE
colonisation. We did not observe significant changes
in the consumption of vancomycin or ceftazidime
throughout the study period. Nonetheless, an
increase in consumption of ceftriaxone was observed
in the first half of 2014. We hypothesise the increase
might be a squeeze-the-balloon effect by actively
avoiding big gun antibiotics, or an artefact due to
irregularities in returning ward antibiotic stock to
the hospital pharmacy.
We observed a significant increase in 30-day
mortality in VRE carriers identified in the pan-hospital
screening when compared with those who
tested negative for VRE during the same period.
However, when we compared the VRE carriers
identified in pan-hospital screening with those who
tested VRE negative but were known to have had
previous VRE carriage, they were not significantly
different. Confounding factors like length of
hospitalisation and co-morbidities are likely causes
of this observation. Further analysis of these factors
is required to give a more definitive answer.
The pan-hospital screening was immediately
followed by the 10-week HA-wide targeted
surveillance screening. Any patient with a history of
admission to any one of the hospitals in Hong Kong
within 3 months, or on haemodialysis, were actively
screened for VRE on admission. The VRE level
continues to be maintained at a low level, 3 years
after the intensive period that ended in 2013. This is
important because a one-time effort is often difficult
and does not always result in a lasting effect unless a
system and culture change has been brought about.
A limitation of this study was that the analysis
was performed retrospectively. We retrospectively
studied the odds ratio after both the exposure and the
outcomes had already occurred. It is in contrast to prospective cohort studies where participants are
enrolled and then followed over time to identify the
occurrence of VRE carriage. In addition, sustained
control of VRE is multifactorial and not dependent
on any one isolated intervention. Although there
were no large-scale outbreaks or VRE control
programmes in other hospitals, interdependence
among hospitals and other health care facilities are
well described.
Conclusions
We have successfully controlled a multiple-sourced
hospital-wide VRE outbreak in a tertiary hospital
with multipronged infection control measures.
The need to establish a close working relation
between all stakeholders in the hospital cannot be
overemphasised. Our experience is useful to other
hospitals challenged by VRE or other multidrug-resistant
bacteria.
Acknowledgements
We are grateful to all the medical, nursing, and
supporting staff in Queen Elizabeth Hospital who
assisted in VRE control. We thank the microbiology
laboratories of Princess Margaret Hospital, United
Christian Hospital, and Queen Mary Hospital for
their excellent support in handling VRE specimens
during pan-hospital screening.
Declaration
We would like to acknowledge the Food and Health
Bureau, Hong Kong SAR for supporting the typing of
the VRE strains under Health and Medical Research
Fund (Commissioned HMRF Project No. CU-15-B5).
References
1. Cheah AL, Spelman T, Liew D, et al. Enterococcal
bacteraemia: factors influencing mortality, length of
stay and costs of hospitalization. Clin Microbiol Infect
2013;19:E181-9. Crossref
2. Vergis EN, Hayden MK, Chow JW, et al. Determinants of
vancomycin resistance and mortality rates in enterococcal
bacteremia: A prospective multicenter study. Ann Intern
Med 2001;135:484-92. Crossref
3. Lloyd-Smith P, Younger J, Lloyd-Smith E, Green H, Leung V,
Romney MG. Economic analysis of vancomycin-resistant
enterococci at a Canadian hospital: assessing attributable
cost and length of stay. J Hosp Infect 2013;85:54-9. Crossref
4. Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. Health
and economic outcomes of vancomycin-resistant
enterococci. Arch Intern Med 2002;162:2223-8. Crossref
5. Muto CA, Giannetta ET, Durbin LJ, Simonton BM, Farr
BM. Cost-effectiveness of perirectal surveillance cultures
for controlling vancomycin-resistant Enterococcus. Infect
Control Hosp Epidemiol 2002;23:429-35. Crossref
6. Chuang VW, Tsang DN, Lam JK, Lam RK, Ng WH.
An active surveillance study of vancomycin-resistant
Enterococcus in Queen Elizabeth Hospital, Hong Kong.
Hong Kong Med J 2005;11:463-71.
7. Cheng VC, Tai JW, Ng ML, et al. Extensive contact tracing
and screening to control the spread of vancomycin-resistant
Enterococcus faecium ST414 in Hong Kong. Chin
Med J 2012;125:3450-7.
8. Cheng VC, Chan JF, Tai JW, et al. Successful control of
vancomycin-resistant Enterococcus faecium outbreak in a
neurosurgical unit at non-endemic region. Emerg Health
Threats J 2009;2:e9. Crossref
9. Clinical and Laboratory Standards Institute (CLSI).
Performance standards for antimicrobial susceptibility
testing: twenty-third informational supplement M100-S23.
Wayne, PA: CLSI; 2013.
10. Miranda AG, Singh KV, Murray BE. DNA fingerprinting
of Enterococcus faecium by pulsed-field gel electrophoresis
may be a useful epidemiologic tool. J Clin Microbiol
1991;29:2752-7.
11. Cheng VC, Tai JW, Chau PH, et al. Successful control of
emerging vancomycin-resistant enterococci by territory-wide
implementation of directly observed hand hygiene in
patients in Hong Kong. Am J Infect Control 2016;44:1168-71. Crossref
12. Willems RJ, Top J, van Santen M, et al. Global spread
of vancomycin-resistant Enterococcus faecium from
distinct nosocomial genetic complex. Emerg Infect Dis
2005;11:821-8. CrossRef
13. Arias CA, Murray BE. The rise of the Enterococcus: beyond
vancomycin resistance. Nat Rev Microbiol 2012;10:266-78. Crossref
14. Werner G, Coque TM, Hammerum AM, et al. Emergence
and spread of vancomycin resistance among enterococci in
Europe. Euro Surveill 2008;13.pii:19046.
15. Arias CA, Mendes RE, Stilwell MG, Jones RN, Murray
BE. Unmet needs and prospects for oritavancin in the
management of vancomycin-resistant enterococcal
infections. Clin Infect Dis 2012;54 Suppl 3:S233-8. Crossref
16. Aumeran C, Baud O, Lesens O, Delmas J, Souweine
B, Traoré O. Successful control of a hospital-wide
vancomycin-resistant Enterococcus faecium outbreak in
France. Eur J Clin Microbiol Infect Dis 2008;27:1061-4. Crossref
17. Huskins WC, Huckabee CM, O’Grady NP, et al.
Intervention to reduce transmission of resistant bacteria in
intensive care. N Engl J Med 2011;364:1407-18. Crossref
18. Christiansen KJ, Tibbett PA, Beresford W, et al. Eradication
of a large outbreak of a single strain of vanB vancomycin-resistant
Enterococcus faecium at a major Australian
teaching hospital. Infect Control Hosp Epidemiol
2004;25:384-90. Crossref
19. Moretti ML, de Oliveira Cardoso LG, Levy CE, et al.
Controlling a vancomycin-resistant enterococci outbreak
in a Brazilian teaching hospital. Eur J Clin Microbiol Infect
Dis 2011;30:369-74. Crossref
20. Kurup A, Chlebicki MP, Ling ML, et al. Control of a
hospital-wide vancomycin-resistant Enterococci outbreak.
Am J Infect Control 2008;36:206-11. Crossref
21. Lee SC, Wu MS, Shih HJ, et al. Identification of vancomycin-resistant
enterococci clones and inter-hospital spread
during an outbreak in Taiwan. BMC Infect Dis 2013;13:163. Crossref
22. Delmas J, Robin F, Schweitzer C, Lesens O, Bonnet R.
Evaluation of a new chromogenic medium, ChromID VRE,
for detection of vancomycin-resistant Enterococci in stool
samples and rectal swabs. J Clin Microbiol 2007;45:2731-3. Crossref
23. Nolan SM, Gerber JS, Zaoutis T, et al. Outbreak of
vancomycin-resistant enterococcus colonization among
pediatric oncology patients. Infect Control Hosp Epidemiol
2009;30:338-45. Crossref
24. Morris-Downes M, Smyth EG, Moore J, et al. Surveillance
and endemic vancomycin-resistant enterococci: some
success in control is possible. J Hosp Infect 2010;75:228-33. Crossref
25. Ramsey AM, Zilberberg MD. Secular trends of
hospitalization with vancomycin-resistant enterococcus
infection in the United States, 2000-2006. Infect Control
Hosp Epidemiol 2009;30:184-6. Crossref
26. Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline
for preventing nosocomial transmission of multidrug-resistant
strains of Staphylococcus aureus and enterococcus.
Infect Control Hosp Epidemiol 2003;24:362-86.
27. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci
resistant to multiple antimicrobial agents, including
vancomycin. Establishment of endemicity in a university
medical center. Ann Intern Med 1995;123:250-9. Crossref
28. Rossini FA, Fagnani R, Leichsenring ML, et al. Successful
prevention of the transmission of vancomycin-resistant
enterococci in a Brazilian public teaching hospital. Rev Soc
Bras Med Trop 2012;45:184-8. Crossref
29. Boyce JM. Environmental contamination makes an
important contribution to hospital infection. J Hosp Infect
2007;65 Suppl 2:50-4. Crossref
30. Harris AD. How important is the environment in the
emergence of nosocomial antimicrobial-resistant bacteria?
Clin Infect Dis 2008;46:686-8. Crossref
31. Kirkland KB, Homa KA, Lasky RA, Ptak JA, Taylor EA,
Splaine ME. Impact of a hospital-wide hand hygiene
initiative on healthcare-associated infections: results of an
interrupted time series. BMJ Qual Saf 2012;21:1019-26. Crossref