Hong Kong Med J 2016 Dec;22(6):563–9 | Epub 29 Jul 2016
DOI: 10.12809/hkmj154746
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Initial results of selective renal parenchymal
clamping with an adjustable kidney clamp
in nephron-sparing surgery: an easy way to
minimise renal ischaemia
KC Cheng, MB, ChB;
MK Yiu, FHKAM (Surgery);
SH Ho, FHKAM (Surgery);
TL Ng, FHKAM (Surgery);
HL Tsu, FHKAM (Surgery);
WK Ma, FHKAM (Surgery)
Department of Surgery, Li Ka Shing Faculty of Medicine, The University of
Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr WK Ma (kitkitma@yahoo.com)
Abstract
Introduction: A renal parenchymal clamp has been
used at our centre since March 2012. It is used in
position over the kidney to achieve optimal vascular
control of a tumour while minimising parenchymal
ischaemia. This study aimed to report the feasibility,
surgical outcome, and oncological control of a
kidney clamp in partial nephrectomy.
Methods: This study was conducted at a teaching
hospital in Hong Kong. Partial nephrectomies
performed from January 2009 to March 2015 were
reviewed. The tumour characteristics and surgical
outcomes of kidney clamp were studied and
compared with traditional hilar clamping.
Results: A total of 92 patients were identified during
the study period. Kidney clamps were used in 20
patients and hilar clamping in 72, with a mean follow-up
of 27 and 37 months, respectively. For patients in whom a kidney
clamp was applied, all tumours were exophytic to a
different extent and the majority (90%) were located
at the polar region. The PADUA (preoperative
aspects and dimensions used for an anatomical)
classification nephrometry score was also lower than
those in whom hilar clamping was used (7.07 vs 8.34;
P=0.002). The clamp was used in open, laparoscopic,
and robot-assisted surgery. Operating time was
shorter (207 ± 72 mins vs 306 ± 80 mins; P<0.001)
and estimated blood loss was lower (205 ± 191 mL vs 331 ± 275 mL; P=0.045) with kidney clamp. No
acute kidney injury occurred. Postoperative renal
function was comparable between the two groups.
Conclusions: Partial nephrectomy using
parenchymal clamping is safe and feasible in
selected cases. The postoperative renal function and
oncological control were satisfactory.
New knowledge added by this study
- The use of a renal parenchymal clamp is feasible and safe for vascular control during partial nephrectomy.
- Early results in terms of intra-operative blood loss, operating time, and postoperative renal function are promising.
- This clamp offers an ideal and convenient alternative to hilar clamping in selected cases.
Introduction
Nephron-sparing surgery (NSS) for renal tumour
has been proved to produce similar oncological
and superior functional outcome for stage T1
renal masses as radical nephrectomy.1 It is now
recommended as the gold standard.2 Various
vascular control methods have been described
to achieve a bloodless field for tumour dissection
and excision, with the traditional way being renal
hilar clamping (HC) that results in global renal
ischaemia. The importance of minimising ischaemia
in NSS to preserve postoperative renal function
is well documented.3 While HC is less technically
demanding, zero-ischaemia NSS with selective
segmental arterial clamping requires high levels of
surgical and anaesthetic expertise.1 Furthermore, not
all renal masses are amenable to the technique, such as
peripherally located tumours without an identifiable
feeding segmental artery on preoperative imaging.
In such cases, attaining regional renal ischaemia
is a feasible way of maintaining a clear operative
field while reducing ischaemic insults to the renal
parenchyma, and will theoretically better preserve
postoperative renal function. Different methods of
regional ischaemia have been reported including
manual compression and various parenchymal
clamps.4 We describe a method of selective renal
parenchymal clamping (SRPC) with a kidney clamp
that can be adopted in open, conventional, or robot-assisted
laparoscopic NSS. In this study, we report
the case selection, feasibility, and surgical outcomes
in our initial series of SRPC technique with respect
to traditional HC NSS.
Methods
All patients who underwent NSS for renal
tumour from January 2009 to March 2015 were
retrospectively reviewed at a tertiary centre in
Hong Kong. Since March 2012, selected patients
have been prospectively recruited for SRPC after
careful review of the computed tomographic
imaging during a preoperative planning session.
Eligible indications for renal parenchymal clamping
included small tumour size, and peripherally located
and exophytic tumour; hilar or centrally located
tumours were unsuitable. All procedures including
open, conventional, and robot-assisted laparoscopic
transperitoneal or retroperitoneal approaches
were included. Patients with NSS performed using
selective segmental artery clamping technique were
excluded from the current study. This study was done in accordance with the principles outlined in the Declaration of Helsinki.
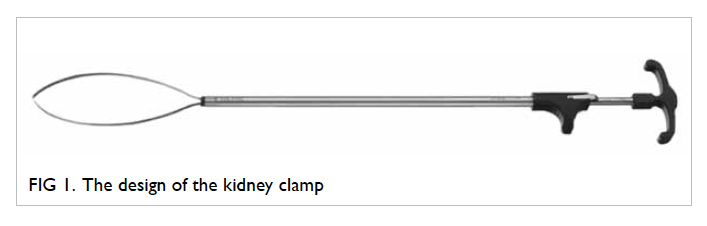
The kidney clamp
The technique of SRPC has been in use at our unit
since March 2012. The kidney clamp (Karl Storz,
Tuttlingen, Germany) is a 29-cm long, 10-mm wide
instrument. It consists of a 120-mm long distal snare
comprising nitinol, an outer sheath, and a handle
with ratchet (Fig 1). It is reusable and can be inserted
through a 10-mm laparoscopic port. It can be used
in laparoscopic, robot-assisted, or open NSS in
both transperitoneal and retroperitoneal approach.
Tightening of the snare across the renal parenchyma
surrounding the tumour occludes the blood flow with
the ratchet preventing accidental loosening of the
snare during dissection. The clamp can be released
by rotating the handle 90 degrees. The fine ratchet
mechanism of the clamp allows easy fine-tuning of
tightness on the parenchymal tissue according to the
bleeding encountered during tumour excision, as
the ratchet can be further tightened one gear tooth
at a time. Furthermore, the clamp release is gradual
and can be easily tightened again. This allows further
haemostasis procedures to be performed in case
bleeding is encountered on clamp release.
The nephron-sparing surgery with selective renal parenchymal clamping
The partial nephrectomy procedure was carried out
in a standardised way via an open, pure laparoscopic,
or robot-assisted laparoscopic approach. Intra-operative
ureteric cannulation and catheterisation
was performed after general anaesthesia in selected
patients whose tumours were considered by the
operating surgeon to be closer to the collecting
system. With the patient positioned in a lateral
bridged position, an open oblique loin or subcostal
incision along the 12th rib tip was made or
laparoscopic ports were inserted in the standard
manner according to the selected approach. After
creation of the operative field and exposure of the
kidney, the Gerota’s fascia was incised and perirenal
fat was dissected from the renal capsule. Hilar
dissections were performed in all cases in order
to prepare for HC in case excessive bleeding was
encountered. The renal tumour was exposed with
its circumferential and deep margins confirmed by
intra-operative ultrasonography. The perirenal fat
was dissected adequately to allow positioning of the
parenchymal clamp over the kidney at about 1 to 1.5
cm from the tumour edge so as to achieve optimal
vascular control while avoiding slipping of the
clamp during repair of the parenchymal defect after
tumour excision. In laparoscopic procedures, the
clamp was inserted through a 10-mm assistant port
directed towards the planned axis of clamping across
the polar region (Fig 2). The hilar area and the ureter
were always spared from clamping. The snare was
then tightened gradually until bluish discolouration
of the parenchyma was noted, and the tightness
adjusted according to the degree of bleeding during
tumour excision. A thermal excision of tumour
was performed. Breaching of the collecting system
was checked in those patients with retrograde
stenting by slightly releasing the clamp and injecting
methylene blue dye through the ureteric catheter,
and any area of leakage repaired with polydioxanone
sutures. Renorrhaphy was done by using a barbed
suture in a continuous manner, with reinforcement
by sliding polymer clips. Fibrin glue was applied
to aid haemostasis in selected patients, and the
kidney clamp was loosened once major oozing had
stopped, but kept in place providing stability for
the renorrhaphy before its final removal, such that
regional ischaemic time could be minimised.
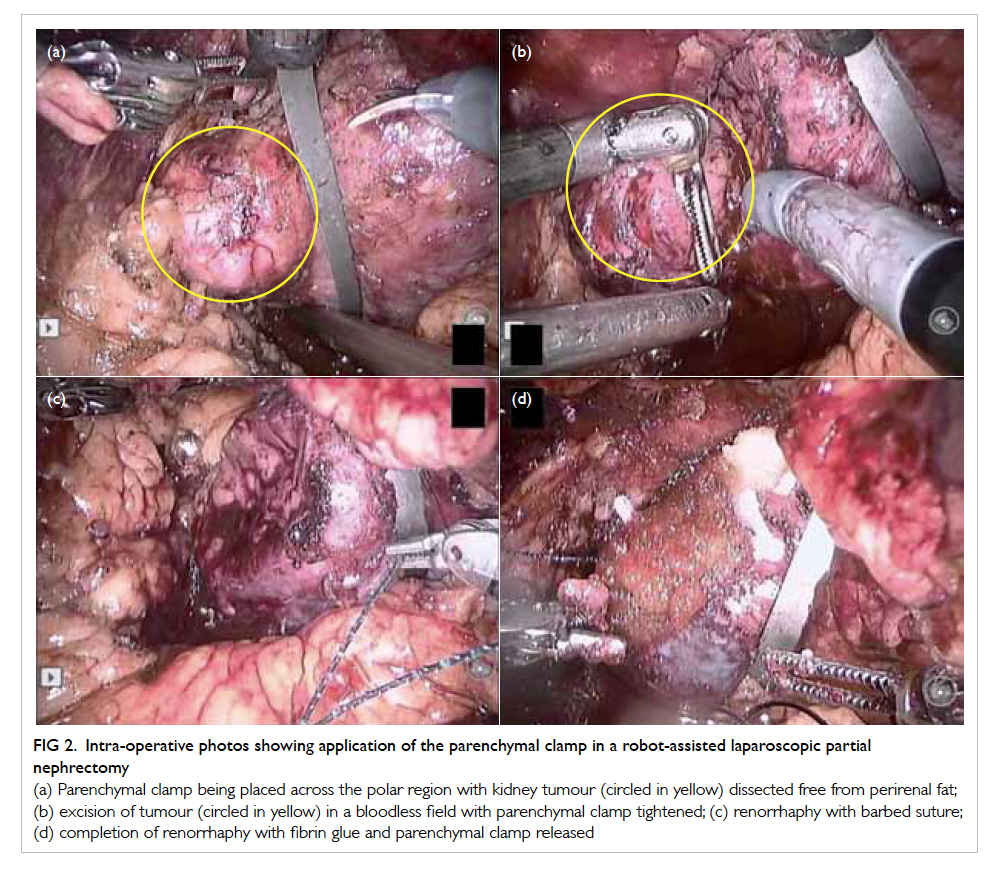
Figure 2. Intra-operative photos showing application of the parenchymal clamp in a robot-assisted laparoscopic partial nephrectomy
(a) Parenchymal clamp being placed across the polar region with kidney tumour (circled in yellow) dissected free from perirenal fat; (b) excision of tumour (circled in yellow) in a bloodless field with parenchymal clamp tightened; (c) renorrhaphy with barbed suture; (d) completion of renorrhaphy with fibrin glue and parenchymal clamp released
Data collection and analysis
Patient demographics (age, gender, Charlson
Comorbidity Index score, baseline renal function,
co-existing diabetes or hypertension), tumour
characteristics (radiological maximum tumour
diameter, PADUA [preoperative aspects and
dimensions used for an anatomical] nephrometry
score), intra-operative data (operating time,
ischaemic time, estimated blood loss), and
postoperative outcomes (complications, hospital
stay, renal function) were assessed for all patients.
Estimated glomerular filtration rate (eGFR) using
Modification of Diet in Renal Disease study equation
was used to measure postoperative renal function.5
Acute kidney injury was defined as either two-fold
increase in serum creatinine or 50% reduction
in eGFR within the postoperative hospital stay
when compared with preoperative baseline, or any
requirement for renal replacement therapy. The renal
function at 7, 30, 60, and 90 days after operation was
assessed. The PADUA nephrometry score6 was used
as an objective measure of tumour characteristics
and its individual parameters were also analysed.
Complications were graded as per the Clavien-Dindo
classification system.7 The proportion of high-grade
complications (grades 3-5) was reported.
Clinical data were compared between the
SRPC series and a larger cohort of conventional HC
series. Continuous variables were compared using
Mann-Whitney U test while categorical variables
were compared using Chi squared test or Fisher’s
exact test. Statistical significance was defined as
P<0.05. Analysis was performed using the Statistical
Package for the Social Sciences (Windows version
20.0; SPSS Inc, Chicago [IL], US).
Results
From January 2009 to March 2015, a total of 93
patients were identified. After excluding one patient
who had an infected upper moiety in a duplex
system with upper pole nephrectomy, 92 cases with
NSS procedures performed with conventional HC
(n=72) or SRPC (n=20) techniques were included for
analysis. The mean follow-up duration was 27 and
37 months for SRPC and HC groups, respectively.
Patient demographics and co-morbidity were similar
between the two groups, as was baseline renal
function (mean ± standard deviation of eGFR: 79.1 ±
25.9 mL/min vs 76.1 ± 26.5 mL/min; P=0.751; Table 1). Regarding the tumour complexity (Table 2), there were no differences in the mean radiological tumour
size (26.1 ± 10.6 mm vs 30.6 ± 15.2 mm; P=0.371),
while the mean PADUA nephrometry score was
significantly lower in the SRPC group (7.07 vs 8.34;
P=0.002). All tumours in the SRPC series were
significantly more exophytic to varying extents
(P=0.032), laterally located (P=0.015), and over the
polar region (P=0.016). Apparently more NSS procedures in the SRPC group were performed by laparoscopic approach (with or without robot assistance), though not reaching statistical significance compared with HC group. (Table 3).
Operating time was significantly shorter (207 ± 72
mins vs 306 ± 80 mins; P<0.001) and estimated
blood loss was lower (205 ± 191 mL vs 331 ± 275
mL; P=0.045) with SRPC. No open conversions were
needed in minimally invasive approaches. The mean
length of hospital stay was 6.6 days and complication
rate with Clavien-Dindo grade 3 or above was 5%
(n=1) for SRPC group.
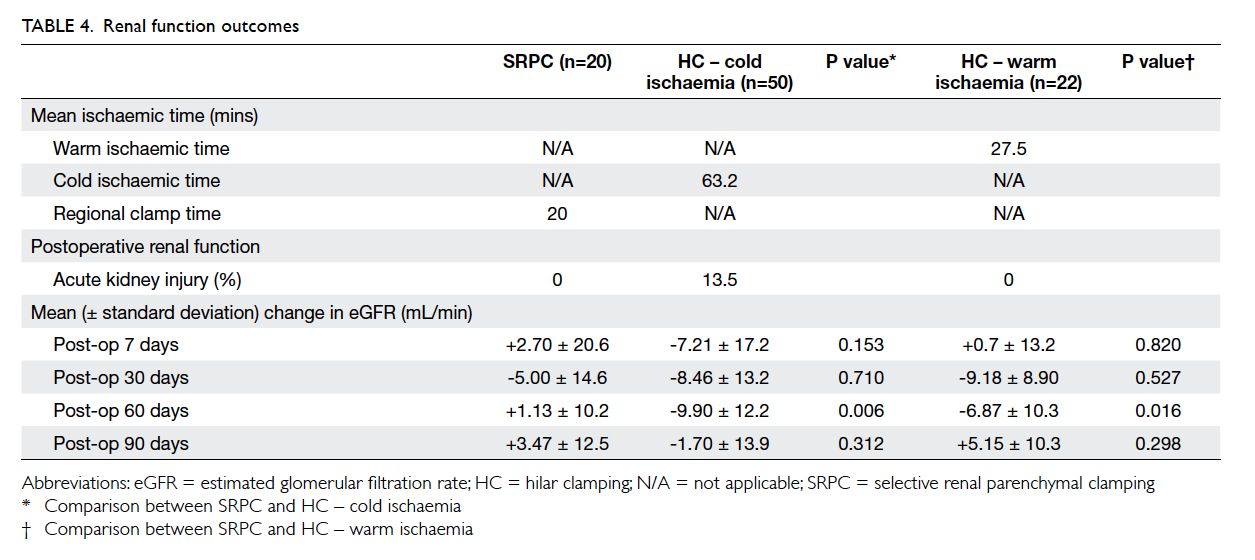
Overall postoperative renal function was
satisfactory in both groups and the changes between
preoperative and postoperative eGFR are shown
in Table 4. Cold and warm ischaemia was adopted in 50 (69.4%) and 22 (30.6%) patients in the HC
group, respectively. The mean clamping time for
SRPC was 20 minutes. The reduction in eGFR was
significantly more for HC at postoperative day 60 for
both cold (P=0.006) and warm ischaemia (P=0.016) when compared with SRPC. No acute kidney injury
occurred during the early postoperative period
after parenchymal clamping, while there were seven
(9.7%) cases of acute kidney injury in HC.
Overall 70% were renal cell carcinoma in
SRPC. All tumours were pathological T1 disease
with 92.9% in stage T1a. The mean pathological
tumour size was 26.8 mm. No patients in the SRPC
group had a positive surgical margin or developed
local recurrence or metastasis.
Discussion
This was a feasibility and safety study that showed
the initial promising result of regional ischaemia
achieved by SRPC using an adjustable kidney
clamp. The concept of regional ischaemia is indeed
not new and different instruments have been used
successfully in other centres for partial nephrectomy.
It was first described by Semb8 in 1956 using
manual compression. A self-made clamp with two
remodelled malleable retractors4 and use of Rumel
tourniquet have also been reported.9 More recently
open10 and laparoscopic Satinsky vascular clamps11 have been used. A Nussbaum clamp was used by
Simon et al in 200812 in open partial nephrectomy,
normally intended for intestinal clamping during
general surgery. They later modified this to the
laparoscopic Simon clamp with a ratchet mechanism
similar to ours.13 It consists of a pair of jaws 100-mm
long, one straight and the other one curved. Blood
loss was minimal and no complications occurred
in three cases. Recently the first study comparing
parenchymal clamping with HC for robot-assisted
laparoscopic partial nephrectomy was published.14 It
showed that parenchymal clamping was associated
with a shorter operating time and better preserved
immediate postoperative renal function. Our
method of SRPC using an adjustable kidney clamp
has the merit of allowing flexible control with
different degrees of tightness on the parenchyma
during tumour excision, collecting system repair, and
renorrhaphy. The degree of regional ischaemia can
thus be minimised. Furthermore, the clamp serves
as a mount for controlling the kidney’s position
during the procedure, mimicking the surgeon’s hand
directly holding the kidney and keeping it in a stable
position during tumour excision and renorrhaphy,
which is particularly useful in a laparoscopic setting.
Tumour characteristics
Utilisation of the kidney clamp is feasible for tumours
with certain characteristics. As illustrated in Table
2, favourable tumour features include laterally
located, polar region, and exophytic. Use
in tumours that are located near the hilum, mid
pole, or medial side is generally contra-indicated.13
Recently, different nephrometry scores have been
increasingly used to describe tumour complexity in
partial nephrectomies.6 The PADUA nephrometry
score takes several variables into account, including
the longitudinal location, the rim location, the
relationship with the renal sinus and collecting
system, percentage of tumour extending into the
kidney, the anterior or posterior location, and the
tumour diameter. A higher score is associated with
greater risk of complications,6 externally validated by
other study.15 The PADUA nephrometry score in the
SRPC group was significantly lower in this study. It
may have been that these tumours were technically
less challenging, and reflects the limitations of the
clamp as the hilum and ureter have to be spared
during clamp application, therefore not all tumour
locations are feasible. Nonetheless in selected cases,
SRPC offers an easy and safe way of performing NSS.
Currently no particular cut-off value of nephrometry
score is used to determine the method of vascular
control. Future studies are needed to deduce the
selection criteria in which SRPC could be feasible
and safely performed. This would be more objective
and could facilitate the widespread adoption of the
technique.
Versatility in surgical approaches
The kidney clamp is reusable and can be used
regardless of the surgical approach. In the current
study, seven patients had open surgery, nine had
robot-assisted laparoscopic surgery, and four had
pure laparoscopic surgery. In our experience, no
adjustment to its application is required, regardless
of surgical approach. This versatility allows the
surgeon to be flexible when deciding the surgical
approach.
Avoid global renal ischaemia
Another obvious benefit with the kidney clamp was
the avoidance of whole kidney ischaemia.16 This was
especially true for laparoscopic or robot-assisted
cases in which only warm ischaemia was permitted.
While cold ischaemia can be up to 3 hours, warm
ischaemia is classically limited to 30 minutes.17 This
is undoubtedly one of the important stresses for the
surgeons during partial nephrectomy. Recent studies
have even shown that every minute of ischaemia can
have a significant impact on postoperative renal
function. Longer warm ischaemia is associated with
acute kidney failure, with an odds ratio of 1.05 for
each 1-minute increase.3 Regional ischaemia spares
most of the non–tumour baring area from ischaemic
injury. Animal study has reported the change in
serum creatinine and intra-operative oxygenation
profiles to be improved with parenchymal clamping
or partial renal artery clamping, compared with
complete renal artery clamping.18 In this study, early
postoperative renal function was satisfactory after
parenchymal clamping with minimal changes in
eGFR. No patients experienced acute kidney injury
during the early postoperative period. Postoperative
renal function was mostly comparable between the
groups (Table 4). There was significant deterioration
in eGFR for HC (P=0.006 and 0.016 for cold and
warm ischaemia, respectively) at postoperative 60
days when compared with SRPC. This benefit in
SRPC, however, failed to translate into a long-term
renal function improvement as shown by 90-day
renal function. The significance of this apparent
transient benefit was not clear and might have been
confounded by different factors in this retrospective
study. By accumulating more cases and a longer
follow-up, we believe the renal parenchymal clamp
will be shown to better preserve renal function for
partial nephrectomy in the long term.
Safety
There was no slippage or accidental loosening of
the parenchymal clamp during tumour dissection
and renorrhaphy. No cases required any additional
hilar control. In terms of the oncological control, the
parenchymal clamps did not lead to a higher positive
margin rate, local recurrence rate, or metastases.
On the contrary, we expect it to be lower, as the
parenchymal clamp allows a more comfortable
tumour dissection with less time constraints. This
may improve the dissection result and lead to
reduced positive surgical margin and better tumour
control as the surgeon becomes more experienced.
Surgical outcomes
The mean operating time was reasonably short at
207 minutes. This was mainly attributed by the
time saved for the tedious and sometimes risky
hilar dissection.19 Renal cooling with ice was
also unnecessary and further reduced the overall
operating time. The lower estimated blood loss could
be explained by the avoidance of HC, as vascular
injury during HC can lead to profuse bleeding10 or
even renal artery dissection.
The study result of a shortened operating
time and lower estimated blood loss needs to
be interpreted with caution in view of several
limitations of our study. First, it was a retrospective
study and the sample size for SRPC was small.
Moreover parenchymal clamping was done for less
complex tumours. This difference in complexity
might have contributed to the smaller blood loss and
shorter operating time, as well as the preservation of
renal function as less volume of renal parenchyma
was removed. Another significant limitation was the
lack of volumetric analysis, which rendered the renal
function comparison between two groups difficult.
A randomised, prospective study is required to truly
compare the two methods after accumulating more
clinical experience.
Road to zero ischaemia
Recently partial nephrectomy with zero ischaemia
was reported, combining the use of selective arterial
clamping and controlled hypotension.20 Outcomes
were favourable with a mean absolute and percentage change in preoperative and 4-month postoperative
eGFR of -11.4 mL/min/1.73 m2 and 13%, respectively.
Nonetheless, this technique is technically demanding
and requires a steep learning curve. Its utilisation
is also limited to robot-assisted or laparoscopic
surgery as a magnified view is essential for the
meticulous vascular dissection. On the contrary,
the kidney clamp in our study provides a relatively
simpler way to perform partial nephrectomy without
HC, with a reasonable postoperative renal function
outcome. This kidney clamp is undoubtedly an
important addition to the surgical armamentarium
in the evolvement of partial nephrectomy with
ultimately zero ischaemia.
Conclusions
Partial nephrectomy using parenchymal clamping
as a means of vascular control is safe and feasible
in selected cases with peripherally located and
exophytic tumours. It could be used in various
surgical approaches to achieve regional ischaemia.
The postoperative renal function and oncological
control in this initial experience were satisfactory.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Uzzo RG, Novick AC. Nephron sparing surgery for renal
tumors: indications, techniques and outcomes. J Urol
2001;166:6-18. Crossref
2. Campbell SC, Novick AC, Belldegrun A, et al. Guideline
for management of the clinical T1 renal mass. J Urol
2009;182:1271-9. Crossref
3. Thompson RH, Lane BR, Lohse CM, et al. Every minute
counts when the renal hilum is clamped during partial
nephrectomy. Eur Urol 2010;58:340-5. Crossref
4. Selikowitz SM. A simple partial nephrectomy clamp. J Urol
1995;154:489-90. Crossref
5. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D.
A more accurate method to estimate glomerular filtration
rate from serum creatinine: a new prediction equation.
Modification of Diet in Renal Disease Study Group. Ann
Intern Med 1999;130:461-70. Crossref
6. Ficarra V, Novara G, Secco S, et al. Preoperative aspects and
dimensions used for an anatomical (PADUA) classification
of renal tumours in patients who are candidates for
nephron-sparing surgery. Eur Urol 2009;56:786-93. Crossref
7. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year
experience. Ann Surg 2009;250:187-96. Crossref
8. Semb C. Partial resection of the kidney: anatomical,
physiological and clinical aspects. Ann R Coll Surg Engl
1956;19:137-55.
9. Gill IS, Munch LC, Clayman RV, McRoberts JW, Nickless
B, Roemer FD. A new renal tourniquet for open and
laparoscopic partial nephrectomy. J Urol 1995;154:1113-6. Crossref
10. Denardi F, Borges GM, Silva W Jr, et al. Nephron-sparing
surgery for renal tumours using selective renal parenchymal
clamping. BJU Int 2005;96:1036-9. Crossref
11. Verhoest G, Manunta A, Bensalah K, et al. Laparoscopic
partial nephrectomy with clamping of the renal
parenchyma: initial experience. Eur Urol 2007;52:1340-6. Crossref
12. Simon J, dePetriconi R, Rinnab L, Hautmann RE, Kurtz F.
Optimizing selective renal clamping in nephron-sparing
surgery using the Nussbaum clamp. Urology 2008;71:1196-8. Crossref
13. Simon J, Bartsch G Jr, Finter F, Hautmann R, de Petriconi
R. Laparoscopic partial nephrectomy with selective control
of the renal parenchyma: initial experience with a novel
laparoscopic clamp. BJU Int 2009;103:805-8. Crossref
14. Hsi RS, Macleod LC, Gore JL, Wright JL, Harper JD.
Comparison of selective parenchymal clamping to hilar
clamping during robotic-assisted laparoscopic partial
nephrectomy. Urology 2014;83:339-44. Crossref
15. Tyritzis SI, Papadoukakis S, Katafigiotis I, et al.
Implementation and external validation of Preoperative
Aspects and Dimensions Used for an Anatomical (PADUA)
score for predicting complications in 74 consecutive partial
nephrectomies. BJU Int 2012;109:1813-8. Crossref
16. George AK, Herati AS, Srinivasan AK, et al. Perioperative
outcomes of off-clamp vs complete hilar control
laparoscopic partial nephrectomy. BJU Int 2013;111:E235-41. Crossref
17. Novick AC. Renal hypothermia: in vivo and ex vivo. Urol
Clin North Am 1983;10:637-44.
18. Raman JD, Bensalah K, Bagrodia A, et al. Comparison
of tissue oxygenation profiles using 3 different methods
of vascular control during porcine partial nephrectomy.
Urology 2009;74:926-31. Crossref
19. Mejean A, Vogt B, Cazin S, Balian C, Poisson JF, Dufour
B. Nephron sparing surgery for renal cell carcinoma using
selective renal parenchymal clamping. J Urol 2002;167:234-5. Crossref
20. Gill IS, Patil MB, Abreu AL, et al. Zero ischemia
anatomical partial nephrectomy: a novel approach. J Urol
2012;187:807-14. Crossref